This report describes the clinical trial results that led to the U.S. FDA approval of bevacizumab in combination with interferon for the treatment of renal cell carcinoma.
Abstract
On July 31, 2009, the U.S. Food and Drug Administration granted approval for the use of bevacizumab (Avastin®; Genentech, Inc., South San Francisco, CA) in combination with interferon (IFN)-α2a for the treatment of patients with metastatic renal cell carcinoma.
The approval was primarily based on results from a randomized, double-blind, placebo-controlled clinical trial. The primary efficacy endpoint, progression-free survival (PFS), was assessed by investigators and by an independent review committee (IRC) blinded to treatment assignment.
In total, 649 patients (bevacizumab plus IFN, 327; placebo plus IFN, 322) were enrolled. The median PFS times, by investigator determination, were 10.2 months for the bevacizumab plus IFN arm and 5.4 months for the placebo plus IFN arm (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.49–0.72; p < .0001). The IRC analysis of 569 patients with available radiographs yielded similar results (median PFS time, 10.4 months versus 5.5 months; HR, 0.57; 95% CI, 0.45–0.72; p < .0001). There was no survival advantage (HR, 0.86; 95% CI, 0.72–1.04; p = .13).
Support for the above results was provided by summarized results of a North American cooperative group study of bevacizumab plus IFN-α2b versus IFN-α2b alone. The median PFS times were 8.4 months versus 4.9 months in favor of the bevacizumab combination. There was no survival advantage.
In the reviewed trial, serious adverse events and National Cancer Institute Common Terminology Criteria for Adverse Events grade ≥3 adverse events were reported more frequently in bevacizumab-treated patients (31% versus 19% and 63% versus 47%, respectively). The most common bevacizumab-related toxicities were bleeding/hemorrhage, hypertension, proteinuria, and venous or arterial thromboembolic events.
Introduction
Historically, metastatic renal cell cancer (RCC) has demonstrated resistance to chemotherapy and modest responsiveness to cytokine therapy [1]. Before the year 2000, high-dose interleukin-2 was the only drug that was approved by the U.S. Food and Drug Administration (FDA) for this indication, with an approximately 10% longer duration of complete remission [2, 3]. Although not FDA approved, interferon (IFN)-α regimens have been used to treat metastatic RCC based on a survival advantage demonstrated in some, but not all, studies [4–8].
In more recent years, an understanding of the molecular pathogenesis of clear cell RCC, the most common histologic variant, involving von Hippel-Lindau (VHL) tumor suppressor gene inactivation, has occurred. Normally VHL encodes a protein that is a component of a ligase for hypoxia-inducible factor (HIF). Under normal oxygen tension, ligase action inactivates HIF. Under hypoxic conditions, or with VHL inactivation, HIF upregulates the transcription of multiple hypoxia-inducible genes, including those encoding vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), epidermal growth factor receptor (EGFR), transforming growth factor-α, and others that promote angiogenesis and cellular proliferation [9–11]. Other non-VHL pathways leading to RCC may share aberrant activation of the hypoxic response [12].
Based on the above, considerable recent effort has been devoted to develop and test therapies that block HIF targets [13]. Approved therapies include inhibitors of VEGF and PDGF receptors (sunitinib and sorafenib) and mammalian target of rapamycin (mTOR) inhibitors (temsirolimus and everolimus). mTOR is a kinase protein in the phosphoinositide 3-kinase–AKT pathway that, when activated by mutation, acts on proliferation and angiogenesis by stimulating HIF [13–16]. Most recently, the anti-VEGF antibody bevacizumab in combination with IFN was approved and is the subject of this communication [17–20].
Patients and Methods
Two trials to support the efficacy of bevacizumab in clear cell RCC were submitted. The pivotal trial, for which complete data were available, was a “randomized, double-blind phase 3 study to evaluate the efficacy and safety of bevacizumab in combination with interferon alfa-2a (Roferon®) versus interferon alfa-2a and placebo as first-line treatment administered to nephrectomized patients with metastatic clear cell renal cell carcinoma.” That study was conducted outside the U.S. [19]. The second, supportive trial, submitted in summary form, was a published Cancer and Leukemia Group B (CALGB) study of bevacizumab plus IFN-α2b (Intron®; Schering Corporation, Kenilworth, NJ) compared with IFN-α2b alone [18]. The design of that clinical trial was similar to that of the pivotal trial except for the IFN used and the fact that the trial was not blinded.
A third clinical study, a “phase II, multicenter, randomized, double-blind clinical trial to evaluate the efficacy and safety of erlotinib hydrochloride (Tarceva®) in combination with bevacizumab versus bevacizumab alone for treatment of metastatic renal cell carcinoma” provided safety information for single-agent bevacizumab treatment [21].
In the pivotal trial, eligible patients were randomized 1:1 to receive either IFN-α2a, 9 MIU s.c. three times a week, until disease progression for a maximum period of 52 weeks plus placebo equivalent i.v. every 2 weeks until disease progression (IFN–Pla) or the same IFN dose and schedule plus bevacizumab, 10 mg/kg i.v. every 2 weeks until disease progression (IFN–Bev).
Randomization was stratified by geographic region (Western Europe, Eastern Europe, other) and by Motzer prognostic score [22, 23]. Motzer score was provided in two data sets. One data set listed Motzer scores used to stratify patients at the time of randomization and the other provided scores from case report forms (CRFs). The former data set had no missing values whereas 52 (8%) of the scores derived from the CRFs were missing, and 29 (4%) of the scores derived from the CRFs were discordant with the score reported at the time of randomization. The Motzer score from the CRF was the variable used for study analyses.
Treatment was given until disease progression, unacceptable toxicity, or death. After 1 year of therapy, IFN was stopped and the investigator was given the option to stop or continue the experimental agent (bevacizumab or placebo).
The study population comprised patients aged ≥18 years with metastatic RCC (>50% clear cell), status postnephrectomy or partial nephrectomy with clear surgical margins, Karnofsky performance status score ≥70%, and a life expectancy >4 months. Absence of proteinuria at baseline (<0.5 g in 24 hours), an international normalized ratio (INR) ≤1.5, an activated partial thromboplastin time (aPTT) ≤1.5× the upper limit of normal, a serum creatinine level ≤2.0 mg/dl or ≥177 μmol/l, and adequate hematologic and hepatic laboratory values were also required, as was signed written informed consent, pregnancy testing, and adequate contraception.
Exclusion criteria included prior systemic treatment for metastatic RCC (including neoadjuvant therapy), major surgical procedure, open biopsy or significant traumatic injury within 28 days prior to the start of study treatment, anticipation of the need for major surgical procedure during the course of the study, serious nonhealing wound, ulcer or bone fracture, evidence of current central nervous system metastases, evidence of bleeding diathesis or coagulopathy, need for a full therapeutic dose of oral or parenteral anticoagulants or chronic daily treatment with aspirin (>325 mg/day), uncontrolled hypertension (≥160 mm Hg systolic and/or ≥90 mm Hg diastolic) while receiving chronic antihypertensive medication, and clinically significant (i.e., active) cardiovascular disease, for example, a cerebrovascular accident ≤6 months before randomization, a myocardial infarction ≤6 months before randomization, unstable angina, New York Heart Association grade ≥2 congestive heart failure, or serious cardiac arrhythmia requiring medication and chronic treatment with corticosteroids (dose ≥10 mg/day methylprednisolone equivalent), excluding inhaled steroids.
The primary efficacy endpoint, in the initial protocol submission, was overall survival (OS), defined as the time between the date of randomization and the date of death resulting from any cause. Progression-free survival (PFS), defined as the time between the date of randomization and the first date of documented progression or date of death resulting from any cause, was a secondary efficacy endpoint. In October 2006, approximately 2 years after study onset, the FDA agreed that a statistically robust and clinically important effect on PFS that was confirmed by an independent review committee (IRC) masked to treatment assignment could serve as a basis for label expansion.
In the PFS analysis, patients without an event were censored at the date of last follow-up for progression or date of last available tumor measurement if no follow-up assessment for progression was performed. For patients who had not experienced disease progression at the time of the statistical analysis, PFS was censored at the date of their last tumor assessment (or if no tumor assessments were performed after the baseline visit, at the time of randomization). For patients who experienced their first documented disease progression or died >84 days after the last dose of study treatment, data were censored at the time of the last tumor assessment that occurred prior to the last dose of study treatment plus 84 days. For patients who received nonprotocol-specified antineoplastic therapy prior to experiencing documented disease progression, PFS was censored at the time of their last tumor assessment prior to that treatment.
Other endpoints were time to progression, defined as the time between the date of randomization and the date of documented progression, and objective response, defined as a complete or partial response, per the Response Evaluation Criteria in Solid Tumors, confirmed by a repeat assessment performed by the investigator ≥4 weeks after the criteria for response were first met [24]. Duration of objective response was defined as the time from when a response, complete or partial, was first documented to the first documented disease progression or death, whichever occurred first. The censoring method for duration of objective response was the same as that for the primary definition of PFS.
Tumor measurements, using computed tomography (CT) scans, magnetic resonance imaging (MRI) scans, x-rays, and clinical examination, were performed every 8 weeks up to week 32 and every 12 weeks up to week 56. Bone scans were only required at screening or during the study as clinically indicated. Bone lesions noted on a screening scan were to be confirmed and followed by plain x-ray. At screening, a brain CT scan or MRI was mandatory. During the study, these studies were obtained only if clinically indicated.
Safety assessments were performed every 2 weeks until week 7 and then every 4 weeks until week 51. An additional visit on week 57 was performed in cases in which the patient completed the scheduled 52 weeks of trial treatment. Adverse events (AEs) were graded using the Common Terminology Criteria for Adverse Events (version 3.0). Safety data sets were coded according to the Medical Dictionary for Regulatory Activities (version 10.1).
Laboratory assessments included CBCs, measurement of INR, prothrombin time (PT), and aPTT, serum chemistry, liver function tests and creatinine evaluation, a urinalysis, a 24-hour urine collection for protein determination at baseline, and a dipstick test for erythrocytes at baseline. Thereafter, a urine dipstick test for protein only was to be performed before each administration of bevacizumab.
Specific bevacizumab toxicities were also assessed. If grade 3 or 4 hypertension occurred, additional blood pressure measurements were required; if a urine protein ≥1+ on the dipstick test was detected in the first 80 patients, a 24-hour urine collection was required before the subsequent bevacizumab infusion. For all subsequent patients, a 24-hour urine collection was required in cases when there was a protein ≥2+ dipstick result. For grade 3 or 4 thrombosis, a blood sample was to be taken for INR, aPTT, PT, D-dimer, and antithrombin III evaluation. For grade ≥2 hemorrhagic events, a blood sample was to be taken for platelet count, INR, aPTT, and PT evaluation.
The North American CALGB cooperative group trial was similar in design to the pivotal study but differed in the following ways: (a) The cooperative group study was not a blinded, placebo-controlled study; (b) the IFN studied in the CALGB study was Schering-Plough's Intron® (IFN-α2b); and (c) eligible patients in the cooperative group study were diagnosed with metastatic RCC with a clear cell component. Eligible patients in the pivotal study were diagnosed with metastatic RCC, status postnephrectomy or, if partial nephrectomy was performed, with a negative surgical margin and the majority component of the tumor being clear cell carcinoma.
IFN and bevacizumab doses and schedules were identical to those in the pivotal trial. Randomization was stratified by nephrectomy status (yes or no) and number of adverse prognostic factors [22, 23]. The primary study objective was to compare OS between arms, and secondary objectives were to compare PFS and objective response rates.
Results
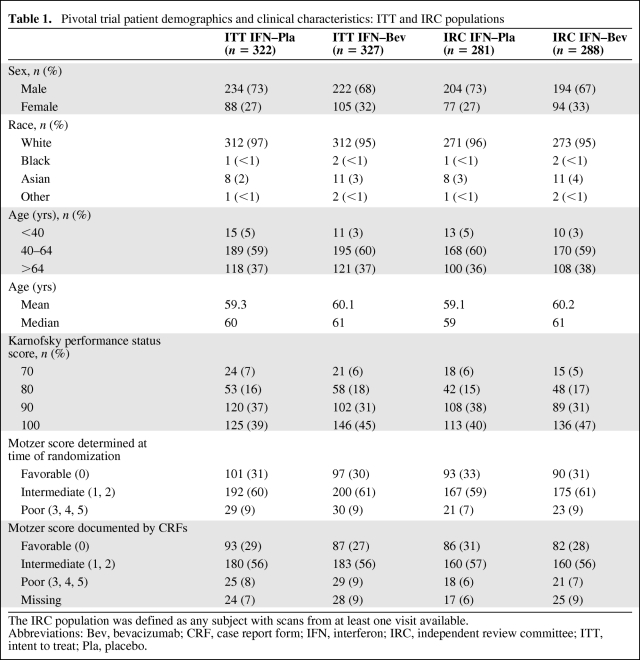
The pivotal trial opened on June 29, 2004 and was closed to subject entry on September 8, 2006. Demographics and clinical characteristics of the intent-to-treat and independent review committee (IRC) patient populations are summarized in Table 1. There were no imbalances in the demographic characteristics in the study arms that precluded meaningful analysis of trial results.
Table 1.
Pivotal trial patient demographics and clinical characteristics: ITT and IRC populations
The IRC population was defined as any subject with scans from at least one visit available.
Abbreviations: Bev, bevacizumab; CRF, case report form; IFN, interferon; IRC, independent review committee; ITT, intent to treat; Pla, placebo.
Among the 649 patients randomized, 139 (21%) were censored early, that is, they were not followed until progression, death, or the end of the PFS follow-up period (September 8, 2006). Of these, 4.8% progressed or died beyond 84 days from the last treatment, 6.8% were treated with a new therapy, and 9.8% were lost to follow-up.
The investigator and IRC PFS analysis resulted in similar findings. In both analyses, the median PFS time was approximately 5 months longer for patients receiving IFN–Bev. The concordance rate between the investigator and IRC determinations of the date of progression was approximately 80%.
The PFS time, based on investigator assessment, was 5.4 months for the IFN–Pla arm and 10.2 months for the IFN–Bev arm, (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.49–0.72; p < .0001). Two hundred thirty-five patients (73%) in the IFN–Pla arm and 195 patients (59.6%) in the IFN–Bev arm experienced a PFS event.
The PFS duration, based on IRC assessment, was 5.5 months for the IFN–Pla arm and 10.4 months for the IFN–Bev arm (HR, 0.57; 95% CI, 0.45–0.72; p < .0001). One hundred fifty-four patients (55%) in the former arm and 138 patients (48%) in latter arm experienced a PFS event.
The objective response rate was 30% for IFN–Bev treatment versus 12% for IFN–Pla treatment (p < .0001). Nearly all responses were partial (29% versus 10%). The median response durations were 12 months versus 11 months, respectively.
The final analysis of OS was performed when 444 deaths had occurred. The median OS time was not significantly different between patients randomized to IFN–Pla and those randomized to IFN–Bev, 21.3 months versus 23.3 months (HR, 0.86; 95% CI, 0.72–1.04; p = .128).
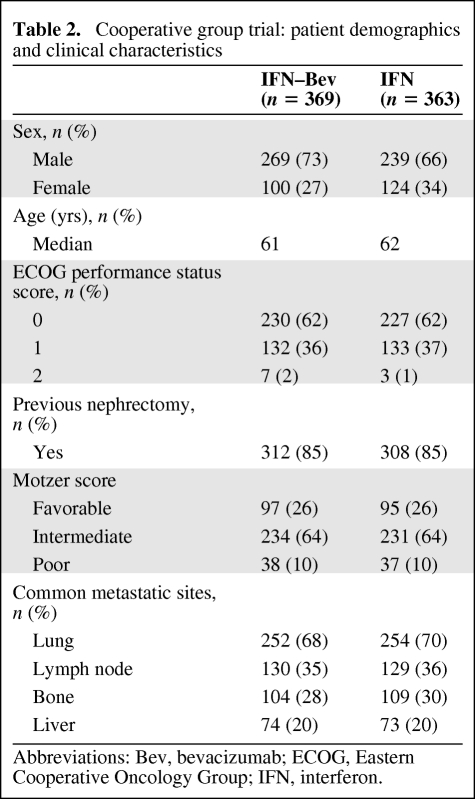
Demographics and clinical characteristics of patients entered into the CALGB cooperative group trial are summarized in Table 2. Primary data were not provided for this study.
Table 2.
Cooperative group trial: patient demographics and clinical characteristics
Abbreviations: Bev, bevacizumab; ECOG, Eastern Cooperative Oncology Group; IFN, interferon.
At the time of the PFS analysis, 561 patients had progressed (265 patients in the IFN–Bev arm and 296 patients in the IFN monotherapy arm). The median PFS interval was 9.6 months (95% CI, 8.3–11.2), compared with 5.5 months (95% CI, 4.2–6.0 months). In the stratified analysis, the HR was 0.67 (95% CI, 0.56–0.79). An updated PFS analysis was recently presented [24]. The median PFS duration was 8.4 months in the IFN–Bev arm, compared with 4.9 months in the IFN monotherapy arm.
The final analysis of OS was also presented [25]. The median OS times were 18.3 months (95% CI, 16.5–22.5) for the IFN–Bev arm and 17.4 months (95% CI, 14.4–20.0) for the IFN monotherapy arm (unstratified log rank p = .097). The stratified HR was 0.86 (95% CI, 0.73–1.01) for the IFN–Bev arm, compared with IFN alone (stratified log-rank p = .069).
Primary data for the phase II trial comparing bevacizumab plus placebo with bevacizumab plus erlotinib were not submitted. One hundred four patients were enrolled, with 53 receiving the former regimen and 51 receiving the latter. Demographic characteristics were generally similar, except for gender and older age—the bevacizumab plus placebo arm included more male patients (75% versus 65%) and the bevacizumab plus erlotinib arm had more patients aged ≥65 years (55% versus 34%).
That study was discontinued after a landmark analysis conducted in October 2005 indicated that the PFS times were similar in the two study arms. The median PFS times were 8.5 months (bevacizumab plus placebo) versus 9.9 months (bevacizumab plus erlotinib). The HR was 0.86 (95% CI, 0.50–1.49; p = .58).
Safety
Data sets from the pivotal trial were used to evaluate the safety of IFN–Bev treatment in patients with metastatic RCC. Data sets from the randomized phase II trial, comparing bevacizumab with and without erlotinib or placebo, provided additional supporting safety information.
The safety-evaluable population consisted of 304 patients in the IFN–Pla arm and 337 patients in the IFN–Bev arm. The IFN–Bev arm included 12 patients randomized to placebo who received at least 1 dose of bevacizumab.
Any AE was experienced by 95% of IFN–Pla treated patients and by 98% of IFN–Bev treated patients. Serious AEs occurred in 19% and 38% of patients, respectively. AEs leading to death occurred in 3% and 4% of patients, and discontinuation of any study drug occurred in 13% and 29% of patients, respectively.
The median (range) durations of exposure to placebo or bevacizumab were 155 (1–190) days and 295 (1–1,121) days. The median (range) dose intensities for placebo or bevacizumab were 95% (39%–110%) and 92% (24%–122%). The median numbers of doses were 12 (1–69) and 16 (1–80). The median (range) durations of exposure to IFN in the IFN–Pla arm and the IFN–Bev arm were 140 (5–388) days and 236 (1–391) days, respectively. The median (range) dose intensities were 96% (28%–120%) and 91% (4%–150%). The median numbers of doses were 57 (2–165) and 95 (1–167).
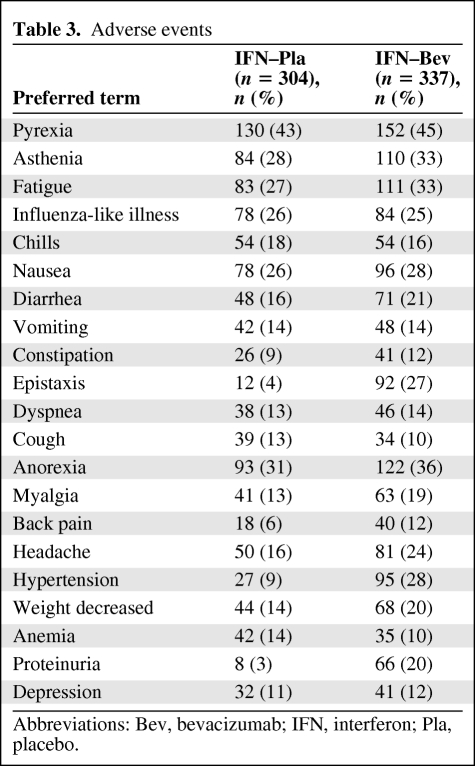
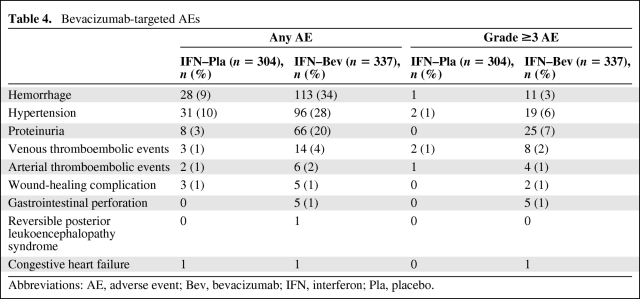
Table 3 lists AEs occurring in >10% of patients in either arm. The majority of the AEs identified by this analysis are recognized AEs associated with bevacizumab. Targeted bevacizumab-associated AEs were also evaluated (Table 4). The incidence and severity of bevacizumab-associated AEs in RCC patients were comparable in the pivotal trial and the supporting phase II trial.
Table 3.
Adverse events
Abbreviations: Bev, bevacizumab; IFN, interferon; Pla, placebo.
Table 4.
Bevacizumab-targeted AEs
Abbreviations: AE, adverse event; Bev, bevacizumab; IFN, interferon; Pla, placebo.
There were three deaths in IFN–Bev treated patients that were probably treatment related. In one case, a patient developed a gastric perforation 8 days after receiving a dose of bevacizumab. The second death was a patient with a medical history of an aortic aneurysm who died as a result of a ruptured aortic aneurysm 7 days after a dose of bevacizumab. The third was a patient with progressive pulmonary metastases who developed a fatal pulmonary hemorrhage 7 days after receiving a dose of bevacizumab.
Discussion
Two randomized, multicenter studies support an expanded bevacizumab labeling claim. The pivotal trial was conducted outside the U.S. and was reviewed, in detail, by the FDA. Only a summary report was provided for the CALGB cooperative group trial conducted in North America. Both of these studies demonstrated a clinically important and, in the pivotal trial, a statistically significant longer PFS duration among patients receiving IFN and bevacizumab than among those receiving IFN plus placebo or IFN alone. Both trials were designed, but failed, to demonstrate a difference in OS.
Issues considered during the evaluation of this application were as follows: (a) whether the observed longer PFS time, in the absence of an effect on survival, outweighs the risks of bevacizumab treatment; (b) the appropriateness of extrapolation of data from non-U.S. sites to the U.S. population; (c) use of a single study to support a new indication, because the FDA did not review individual patient data in the cooperative group trial; and (d) the contribution of IFN-α to the bevacizumab-containing combination regimen.
With regard to the acceptability of PFS as the major efficacy outcome measure, the FDA considers effects on PFS to be direct evidence of clinical benefit if the magnitude of the difference is substantial and reasonable in light of drug-induced toxicity and if PFS is determined by a blinded, independent review committee [25]. In this application, the longer median PFS time of approximately 5 months in the pivotal study is clinically meaningful. The effect on PFS appears to have been replicated in the cooperative group study; however, because this was a secondary endpoint in a “failed” survival study, one cannot assign a level of statistical significance to the PFS effects seen in that trial.
The FDA's decision to accept PFS as the primary efficacy endpoint was based on the following considerations: (a) that postprogression treatment with newly approved drugs could confound determination of bevacizumab-treatment effects on survival, (b) that determination of PFS events based on a blinded IRC review would likely minimize bias in the assessment of this endpoint [25], and (c) the prior acceptance by the FDA of PFS as a measure of direct clinical benefit in metastatic RCC when the magnitude of the effect was large and toxicities were determined to be tolerable.
With regard to extrapolation of data from non-U.S. sites, there is no evidence that the underlying disease (clear cell RCC) or patterns of care differ from that in the U.S. population. The similar outcomes reported for the North American study also support the appropriateness of extrapolation of results to the U.S. population.
The results of a single study were considered sufficient to support the proposed new labeling claim because these data are supported by the summary results of a second study in metastatic RCC and by earlier trials demonstrating the efficacy of bevacizumab in colon, lung, breast, and brain cancer.
Finally, the treatment effects of bevacizumab are clearly isolated in the design of both randomized phase III studies. However, the contribution, if any, of IFN-α to the efficacy of the combination regimen remains unclear, as does the question of the acceptability of the toxicity of IFN-α in light of its benefits. No IFN-α product is approved in the U.S. for the treatment of RCC patients, and the inability of the study design to address the role of IFN-α was previously raised by the FDA in 2004, following submission of the CALGB cooperative group study to the National Cancer Institute–held Investigational New Drug application for bevacizumab. Justification for IFN use was based on a Cochrane meta-analysis suggesting that treatment with IFN-α led to a longer survival time [26] and the general acceptance of IFN-α by the international medical community. The FDA did not find these justifications compelling; however, requests to modify the CALGB study to include a bevacizumab monotherapy arm were unsuccessful and the pivotal trial was nearly completed at the time the trial design was discussed with the FDA. Additional studies evaluating the risks and benefits of IFN-α as part of this combination regimen should be undertaken.
Although the toxicity of the IFN–Bev combination is greater than that of IFN alone, the overall risk–benefit ratio of the combination is acceptable to allow extension of the bevacizumab indication to include treatment of metastatic clear cell RCC in combination with IFN-α.
Acknowledgment
The views expressed are the result of independent work and do not necessarily represent the views and findings of the U.S. Food and Drug Administration.
Author Contributions
Conception/Design: Jeff Summers, Martin H. Cohen, Patricia Keegan, Richard Pazdur
Provision of study material or patients: Jeff Summers, Martin H. Cohen, Patricia Keegan, Richard Pazdur
Collection and/or assembly of data: Jeff Summers, Martin H. Cohen, Patricia Keegan, Richard Pazdur
Data analysis and interpretation: Jeff Summers, Martin H. Cohen, Patricia Keegan, Richard Pazdur
Manuscript writing: Jeff Summers, Martin H. Cohen, Patricia Keegan, Richard Pazdur
Final approval of manuscript: Jeff Summers, Martin H. Cohen, Patricia Keegan, Richard Pazdur
References
- 1.Molina AM, Motzer RJ. Current algorithms and prognostic factors in the treatment of metastatic renal cell carcinoma. Clin Genitourin Cancer. 2008;6(suppl 1):S7–S13. doi: 10.3816/CGC.2008.s.002. [DOI] [PubMed] [Google Scholar]
- 2.Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6(suppl 1):S55–S57. [PubMed] [Google Scholar]
- 3.McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 4.Courtney KD, Choueiri TK. Optimizing recent advances in metastatic renal cell carcinoma. Curr Oncol Rep. 2009;11:218–226. doi: 10.1007/s11912-009-0031-5. [DOI] [PubMed] [Google Scholar]
- 5.Negrier S, Perol D, Ravaud A, et al. Medroxyprogesterone, interferon alfa-2a, interleukin 2, or combination of both cytokines in patients with metastatic renal carcinoma of intermediate prognosis: Results of a randomized controlled trial. Cancer. 2007;110:2468–2477. doi: 10.1002/cncr.23056. [DOI] [PubMed] [Google Scholar]
- 6.Bukowski RM. What role do combinations of interferon and targeted agents play in the first-line therapy of metastatic renal cell carcinoma? Clin Genitourin Cancer. 2008;6(suppl 1):S14–S21. doi: 10.3816/cgc.2008.s.003. [DOI] [PubMed] [Google Scholar]
- 7.Reeves DJ, Liu CY. Treatment of metastatic renal cell carcinoma. Cancer Chemother Pharmacol. 2009;64:11–25. doi: 10.1007/s00280-009-0983-z. [DOI] [PubMed] [Google Scholar]
- 8.McDermott DF. Immunotherapy of metastatic renal cell carcinoma. Cancer. 2009;115(10 suppl):2298–2305. doi: 10.1002/cncr.24236. [DOI] [PubMed] [Google Scholar]
- 9.Choueiri TK, Vaziri SA, Jaeger E, et al. von Hippel-Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol. 2008;180:860–865. doi: 10.1016/j.juro.2008.05.015. discussion 865–866. [DOI] [PubMed] [Google Scholar]
- 10.Rini BI. Vascular endothelial growth factor-targeted therapy in metastatic renal cell carcinoma. Cancer. 2009;115(10 suppl):2306–2312. doi: 10.1002/cncr.24227. [DOI] [PubMed] [Google Scholar]
- 11.Sosman J, Puzanov I. Combination targeted therapy in advanced renal cell carcinoma. Cancer. 2009;115(10 suppl):2368–2375. doi: 10.1002/cncr.24234. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury S, Larkin JM, Gore ME. Recent advances in the treatment of renal cell carcinoma and the role of targeted therapies. Eur J Cancer. 2008;44:2152–2161. doi: 10.1016/j.ejca.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Costa LJ, Drabkin HA. Renal cell carcinoma: New developments in molecular biology and potential for targeted therapies. The Oncologist. 2007;12:1404–1415. doi: 10.1634/theoncologist.12-12-1404. [DOI] [PubMed] [Google Scholar]
- 14.Schmidinger M, Zielinski CC. Novel agents for renal cell carcinoma require novel selection paradigms to optimise first-line therapy. Cancer Treat Rev. 2009;35:289–296. doi: 10.1016/j.ctrv.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Bastien L, Culine S, Paule B, et al. Targeted therapies in metastatic renal cancer in 2009. BJU Int. 2009;103:1334–1342. doi: 10.1111/j.1464-410X.2009.08454.x. [DOI] [PubMed] [Google Scholar]
- 16.Rini BI, Flaherty K. Clinical effect and future considerations for molecularly-targeted therapy in renal cell carcinoma. Urol Oncol. 2008;26:543–549. doi: 10.1016/j.urolonc.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Bukowski RM. Targeted therapies: Bevacizumab and interferon-alpha in metastatic renal-cell carcinoma. Nat Rev Clin Oncol. 2009;6:253–254. doi: 10.1038/nrclinonc.2009.45. [DOI] [PubMed] [Google Scholar]
- 18.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 20.Eskens FA, Sleijfer S. The use of bevacizumab in colorectal, lung, breast, renal and ovarian cancer: Where does it fit? Eur J Cancer. 2008;44:2350–2356. doi: 10.1016/j.ejca.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 21.Bukowski RM, Kabbinavar FF, Figlin RA, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. J Clin Oncol. 2007;25:4536–4541. doi: 10.1200/JCO.2007.11.5154. [DOI] [PubMed] [Google Scholar]
- 22.Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 23.Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23:832–841. doi: 10.1200/JCO.2005.05.179. [DOI] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Ford R, Schwartz L, Dancey J, et al. Lessons learned from independent central review. Eur J Cancer. 2009;45:268–274. doi: 10.1016/j.ejca.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 26.Coppin C, Porzsolt F, Awa A, et al. Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev. 2005;(1):CD001425. doi: 10.1002/14651858.CD001425.pub2. [DOI] [PubMed] [Google Scholar]