This review presents an overview of the most recent data using the novel agents thalidomide, bortezomib, and lenalidomide in the treatment of multiple myeloma and summarizes European treatment practices incorporating these novel agents.
Keywords: Multiple myeloma, Thalidomide, Bortezomib, Lenalidomide
Abstract
The treatment of multiple myeloma (MM) has undergone significant developments in recent years. The availability of the novel agents thalidomide, bortezomib, and lenalidomide has expanded treatment options and has improved the outcome of patients with MM. Following the introduction of these agents in the relapsed/refractory setting, they are also undergoing investigation in the initial treatment of MM. A number of phase III trials have demonstrated the efficacy of novel agent combinations in the transplant and nontransplant settings, and based on these results standard induction regimens are being challenged and replaced. In the transplant setting, a number of newer induction regimens are now available that have been shown to be superior to the vincristine, doxorubicin, and dexamethasone regimen. Similarly, in the front-line treatment of patients not eligible for transplantation, regimens incorporating novel agents have been found to be superior to the traditional melphalan plus prednisone regimen. Importantly, some of the novel agents appear to be active in patients with high-risk disease, such as adverse cytogenetic features, and certain comorbidities, such as renal impairment. This review presents an overview of the most recent data with these novel agents and summarizes European treatment practices incorporating the novel agents.
Introduction
Multiple myeloma (MM) is the second most frequent hematological malignancy. It is characterized by malignant plasma cell infiltration of the bone marrow and is associated with an increased level of monoclonal protein in the blood and/or urine. The uncontrolled growth of myeloma cells has many consequences, including skeletal destruction, bone marrow failure, suppression of normal immunoglobulin production, and renal insufficiency. Although the disease remains incurable, outcomes have improved substantially over recent years as a result of advances in therapy, including high-dose therapy and the availability of novel agents, as well as improvements in supportive care strategies [1, 2].
In parallel with advances in treatment options, the goals of therapy have also evolved. Although prolongation of disease-free survival and overall survival (OS) times remain the ultimate goal, newer, effective therapies are making it possible to aim for a complete response (CR) in a larger proportion of patients than previously possible. The importance of achieving a CR for overall outcome has recently been the subject of discussion. In the transplant setting, the association between best response and OS has been noted in a number of analyses [3, 4]. A study by the Spanish myeloma group in patients undergoing high-dose therapy showed that, within the group of patients who achieve disappearance of monoclonal protein, a distinction between CR and near-CR (nCR) may be important because the event-free survival (EFS) and OS times were significantly longer for patients in CR than for patients achieving a nCR or a partial response (PR) [5]. Moreover, it is not only the achievement of a CR, per se, but the maintenance of a durable CR that appears to influence outcome, as demonstrated in a study by Barlogie et al. [6], which showed that survival was significantly longer in patients who had a durable CR than in those who did not achieve a CR or in those who achieved CR but subsequently lost their CR status. In addition, achievement of a CR was found to be particularly important for patients with high-risk disease by gene-expression profiling. Interestingly, in a number of trials, a higher CR rate was not found to correlate with longer survival [7, 8]. Similarly, CR was not a surrogate marker for survival in patients whose disease had evolved from monoclonal gammopathy of undetermined significance (MGUS) or smoldering MM [9], although newer data indicate that all or almost all myelomas arise from a MGUS precursor state. These results highlight the fact that other considerations need to be taken into account, such as toxicity, and that CR may not be the goal in all patients. Although an association between CR and OS has not been observed in all trials, achievement of a durable complete remission is an important treatment goal that has to be balanced with acceptable toxicity.
This review aims to provide a summary of recent data with the novel agents, thalidomide (Thalomid®; Celgene Corporation, Warren, NJ), bortezomib (Velcade®; Millennium Pharmaceuticals, Inc., Cambridge, MA), and lenalidomide (Revlimid®; Celgene Corporation, Warren, NJ), as well as an overview of current European treatment strategies, with a focus on these novel agents. There are substantial differences in treatment practices as well as approval status of these novel agents in the U.S. and Europe (Table 1), and even within Europe the availability of the different novel agents varies substantially. The manuscript is therefore focused on the review of recent data and includes a discussion of off-label use of these novel agents.
Table 1.
Approved therapeutic indications for the novel agents thalidomide, bortezomib, and lenalidomide
Front-Line Treatment
Transplant-Eligible Patients
Induction
For young patients, high-dose therapy with autologous stem cell support is still considered the standard treatment following the results of several randomized studies that demonstrated a survival advantage for patients given this treatment, compared with conventional chemotherapy [10–12]. During the 1990s, vincristine, doxorubicin, and dexamethasone (the VAD regimen) was considered the standard induction chemotherapy for MM patients undergoing stem cell transplantation in most European centers [13]. Responses to VAD are in the range of 55%–60%; however, CRs are achieved in only a small number of patients [14], and moreover, the response to VAD induction has no impact on the outcome after autologous stem cell transplantation (ASCT) and CRs are typically achieved only post-transplant.
Recent efforts have focused on improving response rates, and in particular CR rates, by including novel agents in induction treatments. Increasing the rate of CRs pretransplant may result in higher rates of CR post-transplant and superior long-term outcomes. A number of studies, which will be summarized in the following, are investigating induction regimens incorporating novel agents.
Thalidomide.
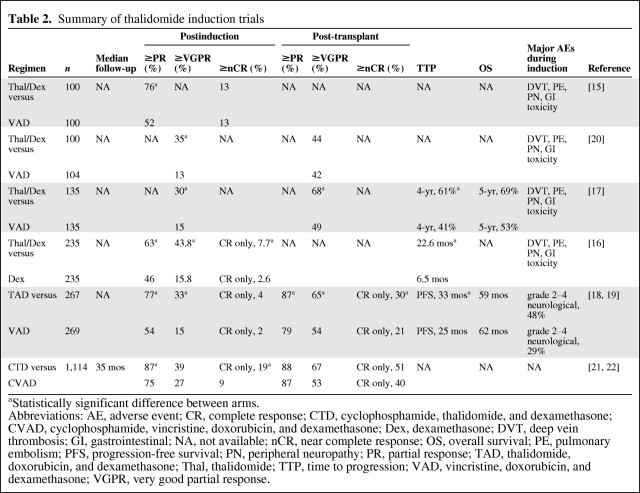
Thalidomide as short upfront therapy (4-month duration) was initially administered in combination with dexamethasone (TD) and was found to be superior to VAD or dexamethasone alone in terms of the overall response rate (ORR); however, the CR rate with the combination is low, at 4%–10% [15, 16] (Table 2). In addition, TD has been administered until second ASCT, after initial treatment with the combination during the induction phase. A recently reported case-matched analysis found that patients who underwent this treatment had better clinical outcomes in terms of a higher CR plus very good partial response (VGPR) rate, longer time to progression (TTP), and longer progression-free survival (PFS) time than those assigned to receive VAD induction plus double ASCT [17] (Table 2). Thalidomide has also been investigated as part of three-drug regimens. The Dutch-Belgian Cooperative Trial Group for Hematology-Oncology (Stichting Hemato-Oncologie voor Volwassenen Nederland [HOVON]) investigated thalidomide in combination with doxorubicin and dexamethasone (TAD) and found that TAD resulted in significantly higher response rates than VAD postinduction [18, 19]. In addition, in contrast to the results described by Macro et al. [20] for TD, the CR+VGPR rate remained significantly higher for TAD after stem cell transplantation. Furthermore, there were significantly longer EFS and PFS times in the TAD arm; however, there was no difference in terms of OS between the two arms (Table 2).
Table 2.
Summary of thalidomide induction trials
aStatistically significant difference between arms.
Abbreviations: AE, adverse event; CR, complete response; CTD, cyclophosphamide, thalidomide, and dexamethasone; CVAD, cyclophosphamide, vincristine, doxorubicin, and dexamethasone; Dex, dexamethasone; DVT, deep vein thrombosis; GI, gastrointestinal; NA, not available; nCR, near complete response; OS, overall survival; PE, pulmonary embolism; PFS, progression-free survival; PN, peripheral neuropathy; PR, partial response; TAD, thalidomide, doxorubicin, and dexamethasone; Thal, thalidomide; TTP, time to progression; VAD, vincristine, doxorubicin, and dexamethasone; VGPR, very good partial response.
Another thalidomide-containing three-drug regimen was investigated in the Medical Research Council (MRC) Myeloma IX trial, which was designed to compare cyclophosphamide, thalidomide, and dexamethasone (CTD) with cyclophosphamide, vincristine, doxorubicin, and dexamethasone (CVAD) as induction therapy, followed by a second randomization step between thalidomide maintenance and no maintenance [21, 22]. CTD treatment resulted in a significantly higher ORR and CR rate than CVAD both pre- and post-transplant (Table 2).
Taken together, the results suggest that the combination TD is suboptimal, but that the addition of another chemotherapy agent, such as cyclophosphamide or an anthracycline, may improve the outcome.
Bortezomib.
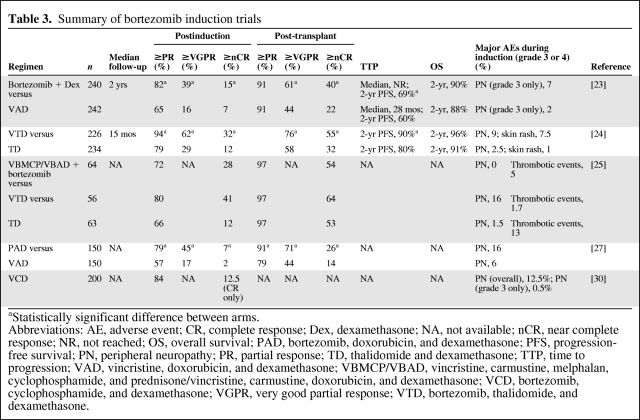
Bortezomib has been investigated as part of a number of different induction regimens. A randomized phase III study by the French Myeloma Study Group (Intergroupe Francophone du Myélome [IFM]) examined the combination of bortezomib plus dexamethasone and found this to be significantly superior to the comparator arm, which consisted of VAD, with respect to response rates postinduction and post-transplant, as well as the 2-year PFS rate (Table 3) [23].
Table 3.
Summary of bortezomib induction trials
aStatistically significant difference between arms.
Abbreviations: AE, adverse event; CR, complete response; Dex, dexamethasone; NA, not available; nCR, near complete response; NR, not reached; OS, overall survival; PAD, bortezomib, doxorubicin, and dexamethasone; PFS, progression-free survival; PN, peripheral neuropathy; PR, partial response; TD, thalidomide and dexamethasone; TTP, time to progression; VAD, vincristine, doxorubicin, and dexamethasone; VBMCP/VBAD, vincristine, carmustine, melphalan, cyclophosphamide, and prednisone/vincristine, carmustine, doxorubicin, and dexamethasone; VCD, bortezomib, cyclophosphamide, and dexamethasone; VGPR, very good partial response; VTD, bortezomib, thalidomide, and dexamethasone.
In addition, a number of studies are examining bortezomib as part of three-drug induction regimens. For example, the Italian Myeloma Network (Gruppo Italiano Malattie Ematologiche dell'Adulto [GIMEMA]) is investigating bortezomib in combination with thalidomide and dexamethasone (VTD) compared with TD given before and after double ASCT. At an interim analysis, VTD was found to be significantly superior to TD in terms of the CR+nCR and CR+VGPR rates pre- and post-transplant, as well as the PFS rate [24] (Table 3). The combination VTD as induction therapy was also found to be superior in terms of the postinduction CR rate in a phase III trial investigating the combination in comparison with TD or VBMCP/VBAD (vincristine, carmustine, melphalan, cyclophosphamide; prednisone/vincristine, carmustine, doxorubicin, and dexamethasone) plus two cycles of bortezomib, which is being conducted by the Spanish Myeloma Group (Programa para el Estudio y la Terapéutica de las Hemopatías Malignas y Grupo Español de Mieloma [PETHEMA/GEM]) [25] (Table 3).
The Arkansas group pioneered the Total Therapy approach, and a recent report of long-term follow-up of the Total Therapy 3 (TT3) regimen, which consists of tandem transplant with melphalan (200 mg/m2), induction and consolidation with bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide, and maintenance with VTD or bortezomib, lenalidomide, and dexamethasone (VRD), demonstrated encouraging results [26]. With a median follow-up of 39 months, the 4-year EFS rate was 71% and the 4-year OS rate was 78%. Comparison of the TT3 regimen with that of the predecessor trial, TT2, in which patients were randomized to receive thalidomide throughout or not, revealed that TT3 was significantly superior in terms of CR and nCR duration, EFS, and OS.
Another three-drug combination incorporating bortezomib is being examined by the HOVON and the German-speaking Myeloma Multicenter Group (GMMG). The ongoing phase III HOVON 65 MM/GMMG-HD4 trial is comparing bortezomib, doxorubicin, and dexamethasone (PAD) induction therapy with VAD followed by either bortezomib or thalidomide maintenance treatment post-ASCT [27]. In a first analysis of the trial, the PAD combination was found to be significantly superior to VAD in terms of the ≥VGPR and ≥PR rates (Table 3). Finally, an ongoing phase II/III trial by the German Myeloma Group (Deutsche Studiengruppe Multiples Myelom [DSMM]) is investigating bortezomib, cyclophosphamide, and dexamethasone (VCD) as an induction regimen based on positive results with the combination in earlier studies in the relapsed/refractory and upfront settings [28, 29]. Results of an interim analysis of the ongoing trial demonstrated positive results for the combination [30] (Table 3).
In summary, a number of bortezomib induction regimens are now available. The results of the IFM trial indicate that the combination of bortezomib and dexamethasone is an appropriate regimen that is superior to the traditional VAD regimen. The addition of thalidomide may further improve response rates, especially CR and VGPR rates, and possibly the PFS interval. Mature results of the ongoing studies incorporating anthracyclines and alkylating agents are eagerly anticipated and will further define the role for bortezomib-containing induction regimens.
Lenalidomide.
A large phase III Eastern Cooperative Oncology Group (ECOG) trial is investigating lenalidomide in combination with two different doses of dexamethasone in the upfront setting. Patients were randomized to receive lenalidomide at 25 mg on days 1–21 plus high-dose dexamethasone (40 mg on days 1–4, 9–12, and 17–20 every 28 days [RD]) or low-dose dexamethasone (40 mg on days 1, 8, 15, and 22 every 28 days [Rd]) [8]. The primary study analysis was to compare the two regimens over four cycles and showed that the RD regimen was associated with a superior ORR and VGPR rate versus Rd (ORR, 79% versus 68%; p = .008 and ≥VGPR, 42% versus 24%; p = .008). Best responses, including the ORR (81% versus 70%; p = .009) and ≥VGPR rate (50% versus 40%; p < .0001), were also significantly better for RD. However, the median PFS time and 2-year OS rate were higher for Rd (median PFS, 25.3 months versus 19.1 months; p = .026 and 2-year OS, 87% versus 75%; p = .0002 for Rd versus RD, respectively), whereas the 3-year OS rate was 75% in both arms [31]. Among patients who underwent transplantation after four cycles of primary treatment, the 3-year OS rate was 92%, compared with 55% in those patients who did not undergo transplantation. In addition, among patients who received treatment with RD or Rd beyond 4 months, the 3-year OS rate was 79%. However, it has to be noted that this analysis was not a randomized comparison and that the trial was not designed to evaluate the combination of lenalidomide and dexamethasone as an induction regimen prior to ASCT. The RD regimen was associated with more toxicities than the Rd regimen [8]: Grade ≥3 venous thromboembolisms (VTEs) occurred in 26% versus 12% of patients (p = .0003), grade ≥3 infection/pneumonia occurred in 16% versus 9% of patients (p = .04), grade ≥3 nonhematological adverse events (AEs) were seen in 65% versus 48% of patients (p = .0002), and early deaths (<4 months) were observed in 5% and 0.5% of patients, respectively. Until now, no randomized study has evaluated the combination of lenalidomide and dexamethasone as an induction regimen prior to ASCT.
A number of phase I/II and phase II studies are ongoing that are investigating lenalidomide in different combinations in the upfront setting. Lenalidomide plus bortezomib and dexamethasone is being investigated by Richardson et al. [32] and has been found to result in high response rates: the ORR was 98%, with 71% of patients achieving a ≥VGPR and 36% of patients achieving a CR/nCR. An ongoing phase II trial is examining the combination of lenalidomide and cyclophosphamide [33], whereas the phase I/II Study of Velcade® in Combination with Other Drugs to Treat Previously Untreated Multiple Myeloma Patients (EVOLUTION, Evaluation of Velcade®, dexamethasone and lenalidomide with or without cyclophosphamide using targeted innovative oncology strategies in the treatment of frontline MM) study is exploring the combination of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide [34]. Initial results suggest that these combinations are active in the setting of newly diagnosed disease, and results from prospective, randomized studies are needed to further examine the role of these combinations.
Impact of Novel Agents on Stem Cell Collection
Stem cell mobilization and collection are generally not negatively influenced by thalidomide treatment [35, 36]. Lower stem cell yield has been observed following lenalidomide-containing induction therapy [36, 37], and it has been recommended that stem cell collection be carried out within 6 months of initiating lenalidomide, or after cyclophosphamide plus G-CSF mobilization, for which no impairment was seen [36–39]. Bortezomib is not long-term myelotoxic and does not negatively impact stem cell yield or stem cell mobilization [40]. Adequate collection of peripheral blood stem cells has been reported.
Post-ASCT Therapy: A Role for Consolidation and Maintenance?
There are currently no guidelines on post-ASCT therapy [41, 42]. Thalidomide post-ASCT has been investigated in a number of randomized trials and has demonstrated benefit in terms of EFS and PFS; however, the OS time was improved in only some studies (Table 4) [7, 19, 43–49]. Although thalidomide maintenance resulted in significantly longer survival in patients with cytogenetic abnormalities in the TT2 setting, it has to be noted that, in that study, conventional cytogenetics were used for the definition of poor risk and this may have led to the identification of a subgroup of patients that was not detected in other maintenance studies [46].
Table 4.
Summary of thalidomide maintenance studies
aSignificant difference in patients with cytogenetic abnormalities.
Abbreviations: ASCT, autologous stem cell transplantation; CTDa, attenuated cyclophosphamide, thalidomide, and dexamethasone; EFS, event-free survival; IFN interferon; MP, melphalan plus prednisone; NA, not available; OS, overall survival; PFS, progression-free survival; Thal, thalidomide.
Collectively, these results indicate that, although thalidomide consolidation/maintenance results in longer EFS/PFS times, particularly among patients failing to achieve high-quality responses after ASCT [43, 44, 47], the effect on OS is ambiguous, and many open questions remain [50]. The shorter OS duration observed in several studies appears to be a result of a shorter survival time after relapse, which may be caused by different factors, such as the duration of maintenance treatment, the possible selection of more resistant clones, the age of patients, toxicities from previous treatments, and the availability of salvage treatments. Future studies should be aimed at identifying patients who may benefit from thalidomide maintenance and establishing the appropriate dose and optimal duration of therapy.
Bortezomib was investigated in the maintenance and consolidation setting in two small studies [51, 52]. Preliminary data suggest that consolidation with VTD may induce molecular remission in a number of patients [51]. Ongoing randomized studies by several European study groups are further investigating bortezomib as consolidation and maintenance therapy. For example, the DSMM is investigating the use of bortezomib as consolidation treatment following induction therapy with VCD plus high-dose therapy. The phase III GIMEMA trial also includes a consolidation randomization. Following induction treatment with VTD or TD and tandem transplantation, patients are randomized to receive VTD or TD consolidation therapy. In the HOVON 65 MM/GMMG-HD4 trial, bortezomib versus thalidomide maintenance therapy is being examined following initial randomization between PAD and VAD induction.
Patients Not Eligible for Transplantation
Outside the clinical trial setting, treatment for patients who are not eligible for transplantation has been restricted to the combination of melphalan plus prednisone (MP) or cyclophosphamide plus prednisone, which leads to responses in approximately 50% of patients [14]; however, patients rarely achieve a CR, and long-term outcomes are disappointing, with a median relapse-free survival duration of about 18 months and a median OS time of about 3 years.
Thalidomide
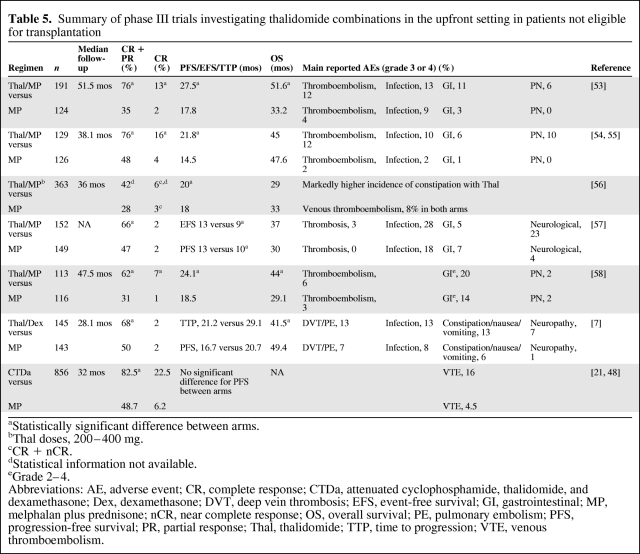
Recently, a number of studies have investigated the addition of novel agents to the traditional MP regimen. The combination of MP plus thalidomide has been investigated in five randomized trials [53–58]. In all studies, the addition of thalidomide to MP resulted in a significantly greater ORR, as well as a longer TTP, PFS time, or EFS time (Table 5). A significant benefit in terms of OS, however, was only seen in the two studies conducted by the IFM [53, 58]. There were some substantial differences in study design, such as the dose of thalidomide and duration of treatment, which included maintenance thalidomide in some of the studies [54–57], but not others [53, 58].
Table 5.
Summary of phase III trials investigating thalidomide combinations in the upfront setting in patients not eligible for transplantation
aStatistically significant difference between arms.
bThal doses, 200–400 mg.
cCR + nCR.
dStatistical information not available.
eGrade 2–4.
Abbreviations: AE, adverse event; CR, complete response; CTDa, attenuated cyclophosphamide, thalidomide, and dexamethasone; Dex, dexamethasone; DVT, deep vein thrombosis; EFS, event-free survival; GI, gastrointestinal; MP, melphalan plus prednisone; nCR, near complete response; OS, overall survival; PE, pulmonary embolism; PFS, progression-free survival; PR, partial response; Thal, thalidomide; TTP, time to progression; VTE, venous thromboembolism.
Other combinations have been examined in an attempt to improve outcomes in the elderly patient group. For example, the combination CTD was investigated in a large trial by the MRC group [21, 48]. Patients received an attenuated CTD regimen (CTDa: cyclophosphamide, 500 mg; thalidomide, 100 mg daily; dexamethasone, 20 mg), which was compared with MP. Although the CTDa regimen was superior in terms of the ORR and CR rate, the PFS time was comparable between the two arms, at 32 months [48].
Dexamethasone-based combinations have also been investigated in elderly patients with newly diagnosed MM. Ludwig et al. [7] investigated the combination TD in comparison with MP and found that although TD resulted in higher a response rate than MP, it was associated with a shorter OS time and resulted in a higher incidence of toxicity (Table 5), which was observed particularly in patients >75 years old with a poor performance status.
Lenalidomide
Lenalidomide has also been examined in the nontransplant setting for the treatment of elderly patients with newly diagnosed MM. In a phase I/II trial, the combination of lenalidomide with MP (MPR) was found to result in an ORR of 81% and a 24% CR rate [59]. With a median follow-up of 29.5 months, the median TTP and PFS times were 28.5 months and the 2-year OS rate was 90.5%. The main AEs included neutropenia, thrombocytopenia, and thromboembolism. These preliminary results suggest that the MPR regimen may be useful in the nontransplant setting; however, confirmation of the results by the ongoing randomized MM015 trial is needed. In addition, the HOVON and the Nordic Myeloma Study Group are conducting a phase III trial in elderly patients comparing melphalan, prednisone, and thalidomide (MPT) plus maintenance thalidomide with MPR followed by maintenance with lenalidomide, which will further clarify the role of lenalidomide in the nontransplant setting.
A subanalysis of the phase III ECOG trial examined the efficacy of RD versus Rd in patients ≥65 years old. The 1-year survival rate was found to be significantly better for patients receiving Rd than for those receiving RD (94% versus 83%, respectively; p = .004) [8].
Bortezomib
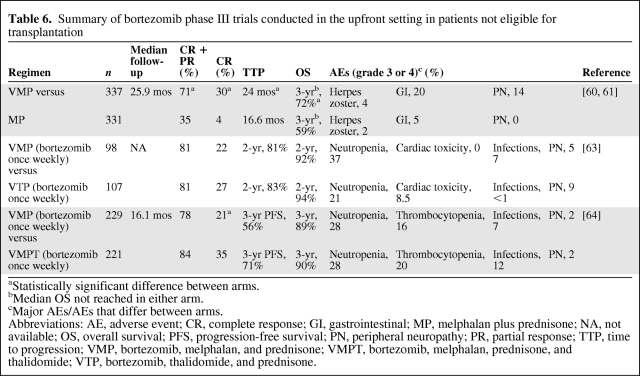
The combination of bortezomib with MP (VMP) was explored in the large phase III Velcade as Initial Standard Therapy in Multiple Myeloma: Assessment with Melphalan and Prednisone (VISTA) trial and was found to be significantly superior to MP alone for all prespecified endpoints, including the CR rate, ORR, TTP, and OS time (Table 6) [60, 61]. VMP was also superior to MP regarding two additional parameters that were included in the comparison of the two arms: time to next therapy (TTNT) and treatment-free interval (TFI). The TTNT was 28.1 months for VMP versus 19.2 months for MP (p < .000001) and the TFI was 16.6 versus 8.4 months (p < .000001). Patient health-related quality of life (HRQoL) data were also collected during the VISTA trial, and an analysis of the association between CR and HRQoL revealed that achievement of a CR resulted in clinically relevant improvements in several aspects of patients' HRQoL [62]. The main differences in grade 3 or 4 AEs between the two treatment arms are detailed in Table 6. Peripheral neuropathy (PN) was more frequent in the VMP arm; however, it was reversible in most patients; 79% of PN events improved (by at least one grade) in a median of 1.9 months and 60% of PN events completely resolved in a median of 5.7 months.
Table 6.
Summary of bortezomib phase III trials conducted in the upfront setting in patients not eligible for transplantation
aStatistically significant difference between arms.
bMedian OS not reached in either arm.
cMajor AEs/AEs that differ between arms.
Abbreviations: AE, adverse event; CR, complete response; GI, gastrointestinal; MP, melphalan plus prednisone; NA, not available; OS, overall survival; PFS, progression-free survival; PN, peripheral neuropathy; PR, partial response; TTP, time to progression; VMP, bortezomib, melphalan, and prednisone; VMPT, bortezomib, melphalan, prednisone, and thalidomide; VTP, bortezomib, thalidomide, and prednisone.
A reduced frequency of administration of bortezomib in combination with MP was investigated in two studies in patients >65 years old. In a trial conducted by the Spanish myeloma group, patients were randomized to receive six cycles of VMP or bortezomib plus thalidomide plus prednisone (VTP) [63]. During cycle 1 of the induction treatment, bortezomib was administered twice weekly, and in subsequent cycles bortezomib was only administered once weekly. The results indicate that efficacy was similar between the two regimens, whereas differences were observed in toxicities (Table 6). Notably, the rate of grade 3 or 4 PN was only 5% with the reduced-dose VMP regimen, and only 12% of patients discontinued treatment.
The Italian myeloma group also investigated a reduced frequency of administration of bortezomib in a trial designed to compare bortezomib, melphalan, prednisone, and thalidomide (VMPT) with VMP in elderly patients [64]. Bortezomib was initially administered twice weekly in a proportion of patients; however, following a protocol amendment, all patients received bortezomib once weekly at 1.3 mg/m2. Efficacy and tolerability results are summarized in Table 6. A comparison of efficacy and toxicity in patients receiving twice-weekly or once-weekly bortezomib in the VMP arm revealed that a shift from twice-weekly to once-weekly bortezomib dosing reduced the rate of CR from 27% to 20%, but that it also substantially reduced the incidence of sensory neuropathy (14% versus 2%) and rate of treatment discontinuation (15% versus 4%).
The results of these two studies appear to suggest that a reduction in bortezomib administration from twice weekly to once weekly leads to a reduction in toxicity of the VMP regimen while retaining significant efficacy, although not at the same level as reported in the original VISTA trial. Longer follow-up is needed to assess the impact on PFS and OS.
Specific Treatment Situations
Renal Impairment
Renal impairment is a serious complication of MM, which affects a major subgroup of patients. Renal impairment requires fast-acting myeloma treatments to reduce tumor burden, with manageable adverse effects that do not further impair the kidneys, or in some cases even allow for recovery of renal function.
In a number of studies, bortezomib was found to be a useful agent in the setting of renal impairment. It has a rapid onset of action and, importantly, its elimination is independent of renal clearance [65], indicating that dose adjustments are not necessary in patients with renal impairment, including those requiring dialysis [66]. In addition, bortezomib may directly act against myeloma kidney disease through its inhibition of nuclear factor κB, the overactivation of which is thought to be a marker of progressive renal disease in humans [67]. Bortezomib may thus reduce inflammation in myeloma kidney disease [68]. In a number of subanalyses, bortezomib efficacy and tolerability were found to be comparable in both young and elderly patients with varying stages of renal impairment and in those with normal renal function [69–71]. Importantly, improvement in renal function has been observed, suggesting that bortezomib may possibly help to normalize renal dysfunction in selected patients. In an ongoing prospective phase II study in patients with acute renal failure, conducted by Ludwig et al. [72], bortezomib in combination with doxorubicin and dexamethasone was found to result in renal responses in 62% and complete renal responses (glomerular filtration rate >60 ml/minute) in 31% of patients.
Thalidomide is also considered a feasible option for the treatment of patients with renal impairment. An analysis of pharmacokinetic data in patients with varying degrees of renal function found that there was no correlation between thalidomide clearance and renal function [73]. In addition, clinical studies have shown that response rates and tolerability with thalidomide are similar in patients with renal failure and in those with normal renal function, both in the relapsed/refractory and front-line settings [74, 75]. In addition, recovery of renal function was observed in the majority of patients whose disease responded to thalidomide treatment [74].
Lenalidomide is primarily excreted by the kidneys and, therefore, careful monitoring of AEs and appropriate dose adjustments in patients with impaired renal function are essential [76–79]. Prospective studies investigating lenalidomide dose adapted to creatinine clearance have been initiated.
Cytogenetic Abnormalities
MM is characterized by various chromosomal changes that carry prognostic information. The deletion of chromosome 17 [del(17)], the translocation of chromosomes 4 and 14 [t(4;14)], the translocation of chromosomes 14 and 16 [t(14;16)], as detected by fluorescence in situ hybridization, the deletion of chromosome 13 [del(13)] by metaphase cytogenetics, and the presence of hypodiploidy are characteristic of high-risk disease [80]. Novel agents may offer the possibility of improving outcomes in patients with these adverse prognostic factors.
Bortezomib appears to be effective in patients with cytogenetic abnormalities, as observed in a number of studies. In the relapsed/refractory setting, the ORR, duration of response, and OS time were not found to be different between patients with and without del(13) [81, 82]. In elderly patients with newly diagnosed disease, the response rate, TTP, and OS time were not negatively affected by the presence of t(4;14), t(14;16), or del(17p) [60]. In the transplant setting, bortezomib induction regimens were also found to remain effective in patients with cytogenetic abnormalities. In the IFM bortezomib plus dexamethasone versus VAD study, the combination of bortezomib plus dexamethasone resulted in a significantly higher ≥VGPR rate than VAD in patients with t(4;14) and/or del(17p) [23]. Similarly, in an Italian trial investigating a bortezomib-based induction regimen, the combination of VTD was significantly superior to TD in patients with t(4;14) and in those with del(17p) in terms of the CR+nCR rate [83].
The addition of thalidomide to the TT2 regimen was found to result in significantly longer survival in patients with cytogenetic abnormalities than in patients who did not receive thalidomide [46]. On the other hand, Cavo et al. [84] showed that TD resulted in a significantly lower probability of response in patients with co-existing del(13) and t(4;14), but not in those with a single abnormality. Moreover, a recent examination of thalidomide maintenance therapy in the MRC Myeloma IX trial found that, in patients with del(17), thalidomide treatment was unfavorable [22, 47].
Lenalidomide has also been investigated in patients with cytogenetic abnormalities. Results from a study by Reece et al. [85] in patients with relapsed/refractory MM indicated that t(4;14) did not influence the response rate, TTP, or OS time, whereas in patients with del(17), the TTP and OS time were significantly shorter. A recent report by the IFM suggested that, in heavily pretreated patients with relapsed or refractory MM, the presence of t(4;14) resulted in a significantly lower response rate and shorter PFS and OS times than in patients without cytogenetic abnormalities [86]. Finally, a report by Kapoor et al. [87] showed that lenalidomide treatment resulted in comparable response rates in patients with newly diagnosed high-risk and standard-risk disease; however, responses were less durable in patients with high-risk disease and the PFS interval was significantly shorter in those patients. However, the OS time was not significantly different between the high-risk and standard-risk groups.
For all novel therapies, there is a lack of prospective data in patients with cytogenetic abnormalities. Currently available results are obtained from reports with small patient numbers and are often derived from subanalyses of trials. Overall, prospective studies with larger patient numbers and longer follow-up are needed to establish the role of novel agents in this setting to enable a risk-adapted approach.
Treatment Decisions for Newly Diagnosed Disease
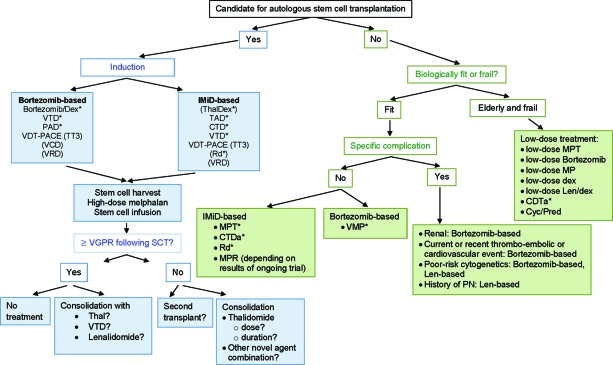
Decision trees for the front-line treatment of MM have been developed, and Figure 1 shows a possible treatment tree based on available data from novel agents. The initial decision in newly diagnosed disease is whether the patient is eligible for transplantation or not. In patients <65 years old and without comorbidities, ASCT after a short induction course (three or four cycles) is considered the standard of care. In recent years, a large number of studies have focused on improving outcomes of transplantation by adding novel agents. In particular, incorporation of novel agents into induction regimens has been studied extensively with a range of different combinations in an effort to improve pre- and post-transplant response rates, especially CR or VGPR rates, because the achievement of a CR is associated with a superior overall outcome. Although long-term survival data are still lacking, the majority of studies involving novel agents have demonstrated superior CR+VGPR rates, compared with VAD postinduction and post-transplant, suggesting that this traditional regimen should no longer be considered standard. Instead, induction regimens should contain at least one novel agent, and ongoing and future studies will establish if three-agent regimens are superior to two-agent combinations. Based on the data from randomized, phase III studies, the following regimens can be recommended over VAD: bortezomib plus dexamethasone, VTD, CTD, PAD, and TAD. TD appears to be similar to VAD in terms of efficacy and may therefore be suboptimal. Other combinations, such as VCD and VRD, are currently undergoing investigation, and data from randomized studies are needed before these regimens can be recommended outside clinical trials. Finally, further data are needed regarding combinations, such as bortezomib plus CTD and Rd.
Figure 1.
MM treatment tree outside clinical trials: front line.
*Indicates data available from a phase III randomized trial.
Abbreviations: CTD, cyclophosphamide, thalidomide, and dexamethasone; CTDa, attenuated cyclophosphamide, thalidomide, and dexamethasone; Cyc, cyclophosphamide; Dex, dexamethasone; IMiD, immunomodulatory drug; Len, lenalidomide; MM, multiple myeloma; MP, melphalan plus prednisone; MPR, melphalan, prednisone, and lenalidomide; MPT, melphalan, prednisone, and thalidomide; PAD, bortezomib, doxorubicin, and dexamethasone; PN, peripheral neuropathy; Pred, prednisone; Rd, lenalidomide plus low-dose dexamethasone; SCT, stem cell transplant; TAD, thalidomide, doxorubicin, and dexamethasone; Thal, thalidomide; TT3, Total Therapy 3; VCD, bortezomib, cyclophosphamide, and dexamethasone; VDT-PACE, bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide; VGPR, very good partial response; VMP, bortezomib, melphalan, and prednisone; VRD, bortezomib, lenalidomide, and dexamethasone; VTD, bortezomib, thalidomide, and dexamethasone.
Treatment decisions need to take into account considerations of patient or disease factors, such as the presence of high-risk disease, comorbidities, and other complications. In cases of renal impairment, bortezomib-based combinations may be the treatment of choice because of accumulating data demonstrating the positive effect of this agent in this setting. In cases of pre-existing clinical PN, a lenalidomide-containing combination may be useful because lenalidomide is not neurotoxic, whereas a history of VTEs may indicate bortezomib use. In patients with high-risk cytogenetic abnormalities, particularly del(17p) and t(4;14), bortezomib has been shown to be effective; however, results from larger trials are needed before recommendations can be given. In addition, more mature data on lenalidomide in this setting are also needed.
Regarding post-transplant therapy, there is currently a lack of strong recommendations, and results from ongoing trials incorporating novel agents are awaited. In cases of the achievement of at least a VGPR following SCT, the options are to not administer any treatment or to consolidate or maintain the response with one of the novel agents, such as thalidomide or a bortezomib-based combination, for which preliminary results appear promising. In cases of a response less than a VGPR following SCT, it is feasible to consider a second transplant or consolidation with thalidomide. However, there are questions regarding the optimal dose or duration of treatment with thalidomide that have to be answered in future studies. Furthermore, certain factors, such as the presence of del(17) may render thalidomide less useful in the setting of maintenance/consolidation. Other novel agent combinations can also be considered in this setting, and a number of studies are currently ongoing that should deliver results in the near future.
In elderly patients and those not eligible for transplantation, results of recent trials indicate that MP should no longer be considered the standard, but that this should be supplemented with novel agents, based on the results of randomized phase III trials. MPT, VMP, and CTDa have been shown to significantly improve outcomes over MP. In addition, the combination of lenalidomide and dexamethasone may be a valuable option for the treatment of elderly patients. A trial comparing MPR with MP was recently closed and results of that study will contribute to defining the role of lenalidomide in this setting.
The group of patients who are not eligible for transplantation is diverse, and it is important to differentiate between those patients who are fit enough to tolerate a full-dose regimen and those patients who are frail and for whom a less intensive treatment approach may be useful to minimize toxicities and optimize treatment duration. Treatment should be selected based on biological age, comorbidities, and overall clinical impression.
The VMP regimen with a reduced frequency of administration of bortezomib may be useful in the frail patient population; however, long-term survival data are needed before firm recommendations can be given. Furthermore, dose-adjusted MPT and low-dose lenalidomide-based combinations are also options in these patients, although there is currently little evidence for lenalidomide in this setting.
Treatment at Relapse
In the majority of cases, MM relapses, even if long periods of remission can be achieved. Prior to the arrival of novel agents, the outcome for patients with relapsed/refractory disease was usually disappointing; however, since novel agents have become available, the outlook for patients has improved steadily, as demonstrated in a recent analysis by Kumar et al. [2].
Since the first report of the activity of thalidomide in the treatment of MM [88], a large number of studies have investigated this agent further in the setting of relapsed/refractory disease. Single-agent activity is limited, and the agent is typically used in combination with dexamethasone and/or chemotherapy [89]. The major toxicities in relapsed disease mirror those in the front-line setting and include PN, deep vein thrombosis, sedation, and gastrointestinal AEs. Although the agent is widely used in the treatment of relapsed/refractory MM, it is not approved for this indication.
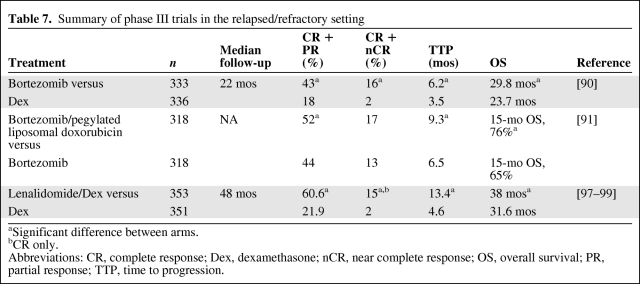
Bortezomib monotherapy was investigated in the phase III Assessment of Proteasome Inhibition for Extending Remissions (APEX) trial and was found to be significantly superior to high-dose dexamethasone in terms of the ORR, CR+nCR rate, TTP, and OS time, despite >62% of patients in the dexamethasone arm crossing over to receive bortezomib [90] (Table 7). In another phase III trial, the addition of pegylated liposomal doxorubicin (PegLD) (Doxil®; Ortho Biotech Products, L.P., Bridgewater, NJ) to bortezomib was found to lead to a significantly longer TTP and OS time versus bortezomib alone [91]. A large number of studies have examined bortezomib in combination with steroids, conventional chemotherapy, or other novel agents and have demonstrated superior efficacy resulting from additive or synergistic effects [92–95]. Major toxicities with bortezomib in the relapsed setting include thrombocytopenia, neutropenia, PN, and gastrointestinal AEs. In the relapsed setting, bortezomib is indicated in the European Union for the treatment of MM at first relapse as a single agent, but clinically, it is almost universally used in combination.
Table 7.
Summary of phase III trials in the relapsed/refractory setting
aSignificant difference between arms.
bCR only.
Abbreviations: CR, complete response; Dex, dexamethasone; nCR, near complete response; OS, overall survival; PR, partial response; TTP, time to progression.
Retreatment with bortezomib was examined in the prospective phase II Retreatment after Initial Response to Velcade® (RETRIEVE) study, in which patients whose disease had responded to previous bortezomib treatment and relapsed after ≥6 months could be retreated with bortezomib (with or without dexamethasone) [96]. Preliminary results of that study indicate that responses were achieved in approximately two thirds of patients and that retreatment is well tolerated with no evidence of cumulative toxicity. In addition, an analysis of response to treatment at relapse in the phase III VISTA trial demonstrated that retreatment with bortezomib appears feasible and that patients can also be effectively treated with immunomodulatory drugs (IMiDs) following front-line bortezomib [61].
Lenalidomide is licensed for the treatment of relapsed/refractory disease in combination with dexamethasone, based on two phase III studies (the MM009 and MM010 studies) that demonstrated a significantly greater ORR, CR rate, TTP, and OS time for the combination versus dexamethasone alone, despite 47.6% of patients in the dexamethasone group crossing over to receive lenalidomide plus dexamethasone (Table 7) [97–99]. The main toxicities reported with the combination of lenalidomide plus dexamethasone include neutropenia, thrombocytopenia, VTE, and infection [97, 98]. An analysis of the effects of prior thalidomide exposure on response, TTP, and OS with lenalidomide plus dexamethasone revealed that the combination remained significantly superior to dexamethasone alone regardless of prior thalidomide [100]. In patients who were refractory to thalidomide, treatment with lenalidomide plus dexamethasone was associated with a lower CR rate and ORR and shorter TTP and PFS time than in those with thalidomide-sensitive disease.
Lenalidomide is also being investigated in combination with other agents, such as doxorubicin, cyclophosphamide, and bortezomib and also in combination with thalidomide. In general, response rates with these combination regimens are superior to those seen with lenalidomide plus dexamethasone alone. For example, the combination of lenalidomide, doxorubicin, and dexamethasone, which was investigated in a phase I/II study in 69 patients, resulted in an ORR of 73% in the overall population, including a 14.5% CR rate and 43% VGPR rate [101]. The median TTP was 10.4 months and the 1-year survival probability was 88%. Longer follow-up is needed to assess the effects on OS in this and other combination studies.
SCT at Relapse
The use of ASCT at relapse has been investigated in a number of studies [102–107] and is considered a useful option in selected patients [107]. Its success appears to be influenced by the efficacy of the previous transplant, the number of prior therapies, as well as the time between the initial and the second transplant step.
Furthermore, allogeneic stem cell transplantation (allo-SCT) may also be feasible in the relapsed setting, and this approach was investigated in a number of recent studies [108–110]. It has been suggested that allo-SCT may be valuable in patients with high-risk disease, but that it currently remains an investigational approach [111].
Treatment Decisions at Relapse
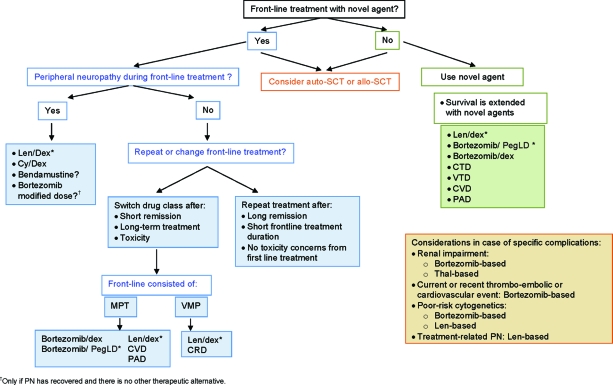
Figure 2 shows a possible treatment decision tree for the treatment of MM at relapse, with a focus on incorporating novel agents into the treatment. It is notable that, although novel agents are widely incorporated into treatment at relapse, data supporting their use does not always stem from randomized studies. Nevertheless, their use is associated with better outcomes [2], and at relapse after front-line treatment with one of the “older” agents, administration of a novel agent should be considered.
Figure 2.
MM treatment tree outside clinical trials: relapse.
*Indicates data available from a phase III randomized trial.
Abbreviations: allo-SCT, allogeneic stem cell transplantation; auto-SCT, autologous stem cell transplantation; CRD, cyclophosphamide, lenalidomide, and dexamethasone; CTD, cyclophosphamide, thalidomide, and dexamethasone; CVD, cyclophosphamide, bortezomib, and dexamethasone; Cyc, cyclophosphamide; Dex, dexamethasone; Len, lenalidomide; MPT, melphalan, prednisone, and thalidomide; PAD, bortezomib, doxorubicin, and dexamethasone; PegLD, pegylated liposomal doxorubicin; PN, peripheral neuropathy; Thal, thalidomide; VMP, bortezomib, melphalan, and prednisone; VTD, bortezomib, thalidomide, and dexamethasone.
Increasingly, novel agents are being incorporated into front-line treatments, which will significantly influence the choices available at relapse and may also influence the efficacy of the different treatments.
A decision to be made at relapse is whether to repeat the initial front-line treatment or switch to a therapy different from that used previously. The decision will be influenced by the duration of remission to initial therapy as well as by the presence of or risk for toxicities. If a long remission was obtained (≥12 months) following a distinct short course of treatment and AEs were acceptable, then rechallenge with the front-line regimen may be possible. On the other hand, if only a short remission (≤6 months) was obtained and the duration of the initial therapy was long, then it may be advisable to switch to a different treatment. For example, transplant at relapse is feasible if a long remission was achieved with the initial transplant. Increasingly, novel agents are being incorporated into front-line therapies, and treatment at relapse has to consider the appropriate treatment sequence. If the relapse happened after long-term thalidomide treatment, then it would be advisable to change treatment and consider a bortezomib-based regimen with the potential addition of alkylating agents. If the disease recurred after front-line treatment with bortezomib, a switch to an IMiD-containing regimen may be indicated. Alternatively, retreatment with bortezomib may be considered if a good response to the initial bortezomib therapy was obtained and if the treatment-free interval was long.
The presence of toxicities will influence the choice of treatment at relapse and may necessitate a change from the front-line treatment. For example, if PN is present from front-line treatment, a switch to a non-neurotoxic agent, such as lenalidomide, may be necessary. The combination of lenalidomide and dexamethasone can be considered a reasonable treatment in thalidomide-refractory patients if PN is present. A history of or high risk for thromboembolic events may indicate a switch from an IMiD-based regimen to a bortezomib combination and the use of low molecular weight heparin (LMWH) thrombophylaxis to avoid further complications.
Specific disease characteristics also influence treatment decisions at relapse, for example, in cases of an aggressive relapse and the presence of poor-risk cytogenetics, treatment with bortezomib or lenalidomide may be indicated. In the presence of renal impairment, a bortezomib-containing regimen may be useful.
In choosing the optimal treatment at relapse, the decision should be individualized based on patient-specific characteristics such as age, presence of comorbidities, type of previous therapy, quality and duration of response, tolerance of therapy, and time without treatment. In addition, decisions have to be made regarding the sequential use of different agents versus combination treatment with simultaneous administration of several agents. Novel agents that have been shown to offer better outcomes than traditional regimens should, whenever feasible, be incorporated into the treatment strategy.
Managing AEs Associated with Novel Agent Use
Thromboembolic events are one of the most significant side effects associated with thalidomide or lenalidomide when these agents are used in combination with steroids or chemotherapy. The risk for developing thromboembolic events appears to be greater when erythropoiesis-stimulating agents (ESAs) are added to IMiDs [112].
Thromboprophylaxis consists of aspirin, LMWH, or warfarin given at either the full dose or a fixed low dose. In a phase III study, fixed low-dose warfarin was not inferior to LMWH or aspirin in patients with a low risk for developing thromboembolic events [113]. On the other hand, LMWH may be recommended in patients at higher risk for developing this complication and in those receiving concomitant high-dose dexamethasone or doxorubicin [114]. Studies are needed to define the optimal agent in the different settings. Prior history or a risk for developing thromboembolic events will require the use of thromboprophylaxis in combination with the chosen IMiD. Alternatively, bortezomib may be useful in this setting because this agent is not associated with a higher risk for thromboembolic events [115], even with the use of ESAs [116].
Both bortezomib and thalidomide can lead to PN, which can be debilitating in some patients. It has been observed that patients who do not develop PN during the first four to six cycles of bortezomib treatment are unlikely to develop this complication during further bortezomib treatment cycles. Notably, bortezomib-associated PN is reversible in the majority of patients. In the APEX trial, grade ≥2 PN resolved or improved in 64% of patients, whereas in the VISTA trial improvement by at least one grade was observed in 79% of patients in a median of 1.9 months [61, 117]. Complete resolution was seen in 60% of patients within a median of 5.7 months. Close monitoring of patients and dose reduction at the first sign of a worsening of the tingling sensation are important. It may be useful to employ a specific questionnaire to help patients recognize the symptoms and to detect the early signs of PN [118].
The risk for developing PN while receiving thalidomide increases with prolonged administration [89]. Dose reduction or discontinuation of treatment is necessary to manage the complication [89]. Lenalidomide is not associated with PN and it may therefore be useful for the treatment of patients with pre-existing PN.
Bortezomib therapy can be associated with reactivation of varicella zoster virus (herpes zoster) [61, 119]; however, the use of antiviral prophylaxis has been shown to successfully prevent this AE [120], and the routine use of antiviral prophylaxis in patients receiving a bortezomib-containing regimen should therefore be considered [119].
Neutropenia is frequently observed with lenalidomide treatment and may necessitate dose reduction, discontinuation of treatment, or administration of growth factors [59, 79, 121, 122]. Bortezomib treatment can lead to thrombocytopenia, which may require dose reduction or temporary treatment discontinuation. However, bortezomib-induced thrombocytopenia is cyclical and platelets typically recover during the rest period of a treatment cycle, so that intervention may not be necessary [66, 115].
MM is characterized by bone disease, and bisphosphonates play an important role in the management of skeletal events. Expert recommendations regarding the use of bisphosphonates have been formulated and were recently published [123]. In patients suffering from lytic bone disease, the use of bisphosphonates is recommended. Treatment should be administered for 2 years and proactive management is needed to avoid renal impairment and osteonecrosis of the jaw.
Clinical Gaps and Ongoing Research
A number of questions surrounding the use of novel agents in the treatment of MM remain. For example, long-term follow-up of the front-line trials is needed so that treatment decisions can be made based on robust survival data. Long-term data will provide answers to questions such as the optimal induction treatment and the appropriate sequencing of agents. Furthermore, new classes of antimyeloma agents, such as heat shock protein-90 and histone deacetylase inhibitors, have shown promising results in preclinical studies and are currently undergoing investigation in the clinical setting. The combination of these newer agents with the agents discussed herein appears attractive and may offer better outcomes.
Studies are needed to further explore the role of transplantation and of consolidation and maintenance therapy. Regarding risk-adapted treatment approaches, large trials or systematic reviews of the available data are needed to define the role of the different agents in the setting of high-risk disease and provide treatment recommendations according to risk factors, such as cytogenetic abnormalities. In addition, further advances in techniques, such as gene-expression profiling and single-nucleotide polymorphism analysis, are eagerly anticipated to further individualize treatment approaches for patients. Moreover, there may be a role for minimal residual disease detection in deciding on the intensity of induction therapy and on the duration of maintenance therapy. Finally, assessment of quality of life has so far been given insufficient attention in MM studies and future studies will help to close this gap.
Acknowledgments
The meeting of the authors and the contribution of Pia Sondergeld were supported by an educational grant from Ortho Biotech, a division of Janssen-Cilag Europe.
A position paper of the European Myeloma Network (EMN).
Author Contributions
Conception/Design: Heinz Ludwig, Meral Beksac, Joan Bladé, Mario Boccadoro, Jamie Cavenagh, Michele Cavo, Meletios Dimopoulos, Johannes Drach, Hermann Einsele, Thierry Facon, Hartmut Goldschmidt, Jean-Luc Harousseau, Urs Hess, Nicolas Ketterer, Martin Kropff, Larisa Mendeleeva, Gareth Morgan, Antonio Palumbo, Torben Plesner, Jesús San Miguel, Ofer Shpilberg, Pieter Sonneveld, Sonja Zweegman
Collection and/or assembly of data: Heinz Ludwig, Meral Beksac, Joan Bladé, Mario Boccadoro, Jamie Cavenagh, Michele Cavo, Meletios Dimopoulos, Johannes Drach, Hermann Einsele, Thierry Facon, Hartmut Goldschmidt, Jean-Luc Harousseau, Urs Hess, Nicolas Ketterer, Martin Kropff, Larisa Mendeleeva, Gareth Morgan, Antonio Palumbo, Torben Plesner, Jesús San Miguel, Ofer Shpilberg, Pieter Sonneveld, Sonja Zweegman
Data analysis and interpretation: Heinz Ludwig, Meral Beksac, Joan Bladé, Mario Boccadoro, Jamie Cavenagh, Michele Cavo, Meletios Dimopoulos, Johannes Drach, Hermann Einsele, Thierry Facon, Hartmut Goldschmidt, Jean-Luc Harousseau, Urs Hess, Nicolas Ketterer, Martin Kropff, Larisa Mendeleeva, Gareth Morgan, Antonio Palumbo, Torben Plesner, Jesús San Miguel, Ofer Shpilberg, Pieter Sonneveld, Sonja Zweegman
Manuscript writing: Heinz Ludwig, Meral Beksac, Joan Bladé, Mario Boccadoro, Jamie Cavenagh, Michele Cavo, Meletios Dimopoulos, Johannes Drach, Hermann Einsele, Thierry Facon, Hartmut Goldschmidt, Jean-Luc Harousseau, Urs Hess, Nicolas Ketterer, Martin Kropff, Larisa Mendeleeva, Gareth Morgan, Antonio Palumbo, Torben Plesner, Jesús San Miguel, Ofer Shpilberg, Pia Sondergeld, Pieter Sonneveld, Sonja Zweegman
Final approval of manuscript: Heinz Ludwig, Meral Beksac, Joan Bladé, Mario Boccadoro, Jamie Cavenagh, Michele Cavo, Meletios Dimopoulos, Johannes Drach, Hermann Einsele, Thierry Facon, Hartmut Goldschmidt, Jean-Luc Harousseau, Urs Hess, Nicolas Ketterer, Martin Kropff, Larisa Mendeleeva, Gareth Morgan, Antonio Palumbo, Torben Plesner, Jesús San Miguel, Ofer Shpilberg, Pia Sondergeld, Pieter Sonneveld, Sonja Zweegman
References
- 1.Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111:2521–2526. doi: 10.1182/blood-2007-08-104984. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Velde HJ, Liu X, Chen G, et al. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;92:1399–1406. doi: 10.3324/haematol.11534. [DOI] [PubMed] [Google Scholar]
- 4.Harousseau JL, Attal M, Avet-Loiseau H. The role of complete response in multiple myeloma. Blood. 2009;114:3139–3146. doi: 10.1182/blood-2009-03-201053. [DOI] [PubMed] [Google Scholar]
- 5.Lahuerta JJ, Mateos MV, Martínez-López J, et al. Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: Sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol. 2008;26:5775–5782. doi: 10.1200/JCO.2008.17.9721. [DOI] [PubMed] [Google Scholar]
- 6.Barlogie B, Anaissie E, Haessler J, et al. Complete remission sustained 3 years from treatment initiation is a powerful surrogate for extended survival in multiple myeloma. Cancer. 2008;113:355–359. doi: 10.1002/cncr.23546. [DOI] [PubMed] [Google Scholar]
- 7.Ludwig H, Hajek R, Tóthová E, et al. Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood. 2009;113:3435–3442. doi: 10.1182/blood-2008-07-169565. [DOI] [PubMed] [Google Scholar]
- 8.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2009 Oct 21; doi: 10.1016/S1470-2045(09)70284-0. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pineda-Roman M, Bolejack V, Arzoumanian V, et al. Complete response in myeloma extends survival without, but not with history of prior monoclonal gammopathy of undetermined significance or smouldering disease. Br J Haematol. 2007;136:393–399. doi: 10.1111/j.1365-2141.2006.06441.x. [DOI] [PubMed] [Google Scholar]
- 10.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 11.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 12.Palumbo A, Triolo S, Argentino C, et al. Dose-intensive melphalan with stem cell support (MEL100) is superior to standard treatment in elderly myeloma patients. Blood. 1999;94:1248–1253. [PubMed] [Google Scholar]
- 13.Alexanian R, Barlogie B, Tucker S. VAD-based regimens as primary treatment for multiple myeloma. Am J Hematol. 1990;33:86–89. doi: 10.1002/ajh.2830330203. [DOI] [PubMed] [Google Scholar]
- 14.Reece DE. An update of the management of multiple myeloma: The changing landscape. Hematology Am Soc Hematol Educ Program. 2005:353–359. doi: 10.1182/asheducation-2005.1.353. [DOI] [PubMed] [Google Scholar]
- 15.Cavo M, Zamagni E, Tosi P, et al. Superiority of thalidomide and dexamethasone over vincristine-doxorubicin-dexamethasone (VAD) as primary therapy in preparation for autologous transplantation for multiple myeloma. Blood. 2005;106:35–39. doi: 10.1182/blood-2005-02-0522. [DOI] [PubMed] [Google Scholar]
- 16.Rajkumar SV, Rosiñol L, Hussein M, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008;26:2171–2177. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavo M, Di Raimondo F, Zamagni E, et al. Short-term thalidomide incorporated into double autologous stem-cell transplantation improves outcomes in comparison with double autotransplantation for multiple myeloma. J Clin Oncol. 2009;27:5001–5007. doi: 10.1200/JCO.2009.22.7389. [DOI] [PubMed] [Google Scholar]
- 18.Lokhorst HM, Schmidt-Wolf I, Sonneveld P, et al. Thalidomide in induction treatment increases the very good partial response rate before and after high-dose therapy in previously untreated multiple myeloma. Haematologica. 2008;93:124–127. doi: 10.3324/haematol.11644. [DOI] [PubMed] [Google Scholar]
- 19.Lokhorst H, van der Holt B, Zweegman S, et al. HOVON-50 final analysis of thalidomide combined with adriamycine, dexamethasone (AD) and high dose melphalan (HDM) [abstract 46] Clin Lymphoma Myeloma. 2009;9(1 suppl):S9. [Google Scholar]
- 20.Macro M, Divine M, Uzunhan Y, et al. Dexamethasone+thalidomide (dex/thal) compared to VAD as a pre-transplant treatment in newly diagnosed multiple myeloma (MM): A randomized trial [abstract 57] Blood. 2006;108:22a. [Google Scholar]
- 21.Morgan GJ, Davies FE, Owen RG, et al. Thalidomide combinations improve response rates; results from the MRC IX study [abstract 3593] Blood. 2007;110 [Google Scholar]
- 22.Owen RG, Child JA, Jackson GH, et al. MRC Myeloma IX: Preliminary results from the intensive pathway study [abstract 546] Clin Lymphoma Myeloma. 2009;9(1 suppl):79. [Google Scholar]
- 23.Harousseau JL, Mathiot C, Attal M, et al. VELCADE/dexamethasone (Vel/D) versus VAD as induction treatment prior to autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma (MM): Updated results of the IFM 2005/01 trial. Presented at the 2008 meeting of the American Society of Hematology; December 7, 2008; San Francisco, California. [Google Scholar]
- 24.Cavo M, Tacchetti P, Patriarca F, et al. Superior complete response rate and progression-free survival after autologous transplantation with up-front Velcade-thalidomide-dexamethasone compared with thalidomide-dexamethasone in newly diagnosed multiple myeloma [abstract 158] Blood. 2008;112:65. [Google Scholar]
- 25.Rosinol L, Cibeira MT, Martinez J, et al. Thalidomide/dexamethasone (TD) vs. bortezomib(Velcade®)/thalidomide/dexamethasone (VTD) vs. VBMCP/VBAD/Velcade® as induction regimens prior autologous stem cell transplantation (ASCT) in younger patients with multiple myeloma (MM): First results of a prospective phase III PETHEMA/Gem trial [abstract 654] Blood. 2008;112:244. [Google Scholar]
- 26.Barlogie B, Anaissie EJ, Shaughnessy JD, et al. Ninety percent sustained complete response (CR) rate projected 4 years after onset of CR in gene expression profiling (GEP)-defined low-risk multiple myeloma (MM) treated with Total Therapy 3 (TT3): Basis for GEP-risk-adapted TT4 and TT5 [abstract 162] Blood. 2008b;112:66. [Google Scholar]
- 27.Sonneveld P, van der Holt B, Schmidt-Wolf IGH, et al. First analysis of HOVON-65/GMMG-HD4 randomized phase III trial comparing bortezomib, adriamycine, dexamethasone (PAD) vs VAD as induction treatment prior to high dose melphalan (HDM) in patients with newly diagnosed multiple myeloma (MM) [abstract 473] Haematologica. 2009;94(2 suppl):191. [Google Scholar]
- 28.Davies FE, Wu P, Jenner M, et al. The combination of cyclophosphamide, velcade and dexamethasone induces high response rates with comparable toxicity to velcade alone and velcade plus dexamethasone. Haematologica. 2007;92:1149–1150. doi: 10.3324/haematol.11228. [DOI] [PubMed] [Google Scholar]
- 29.Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: High response rates in a phase II clinical trial. Leukemia. 2009;23:1337–1341. doi: 10.1038/leu.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knop S, Liebisch P, Wandt H, et al. Bortezomib, IV cyclophosphamide, and dexamethasone (VelCD) as induction therapy in newly diagnosed multiple myeloma: Results of an interim analysis of the German DSMM Xia trial [abstract 8516] J Clin Oncol. 2009;27(18 suppl):437s. [Google Scholar]
- 31.Rajkumar SV, Jacobus S, Callander N, et al. Randomized trial of lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone in newly diagnosed myeloma (E4A03), a trial coordinated by the Eastern Cooperative Oncology Group: Analysis of response, survival, and outcome with primary therapy and with stem cell transplantation. Presented at the 2008 meeting of the American Society of Hematology; December 7, 2008; San Francisco, CA. Joint ASH/ASCO symposium. [Google Scholar]
- 32.Richardson PG, Lonial S, Jakubowiak A, et al. Lenalidomide, bortezomib, and dexamethasone in patients with newly diagnosed multiple myeloma: Encouraging efficacy in high risk groups with updated results of a phase I/II study [abstract 92] Blood. 2008;112:41. [Google Scholar]
- 33.Kumar S, Hayman S, Buadi F, et al. Phase II trial of lenalidomide (Revlimid™) with cyclophosphamide and dexamethasone (RCd) for newly diagnosed myeloma [abstract 91] Blood. 2008;112:40. [Google Scholar]
- 34.Kumar S, Flinn IW, Noga SJ, et al. Safety and efficacy of novel combination therapy with bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in newly diagnosed multiple myeloma: Initial results from the phase I/II multi-center EVOLUTION study [abstract 93] Blood. 2008;112:41. [Google Scholar]
- 35.Breitkreutz I, Lokhorst HM, Raab MS, et al. Thalidomide in newly diagnosed multiple myeloma: Influence of thalidomide treatment on peripheral blood stem cell collection yield. Leukemia. 2007;21:1294–1299. doi: 10.1038/sj.leu.2404661. [DOI] [PubMed] [Google Scholar]
- 36.Kumar S, Giralt S, Stadtmauer EA, et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009;114:1729–1735. doi: 10.1182/blood-2009-04-205013. [DOI] [PubMed] [Google Scholar]
- 37.Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–2042. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 38.Paripati H, Stewart AK, Cabou S, et al. Compromised stem cell mobilization following induction therapy with lenalidomide in myeloma. Leukemia. 2008;22:1282–1284. doi: 10.1038/sj.leu.2405100. [DOI] [PubMed] [Google Scholar]
- 39.Mark T, Stern J, Furst JR, et al. Stem cell mobilization with cyclophosphamide overcomes the suppressive effect of lenalidomide therapy on stem cell collection in multiple myeloma. Biol Blood Marrow Transplant. 2008;14:795–798. doi: 10.1016/j.bbmt.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oakervee H, Popat R, Cavenagh JD. Use of bortezomib as induction therapy prior to stem cell transplantation in frontline treatment of multiple myeloma: Impact on stem cell harvesting and engraftment. Leuk Lymphoma. 2007;48:1910–1921. doi: 10.1080/10428190701540991. [DOI] [PubMed] [Google Scholar]
- 41.Durie BG, Kyle RA, Belch A, et al. Myeloma management guidelines: A consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J. 2003;4:379–398. [PubMed] [Google Scholar]
- 42.Barosi G, Boccadoro M, Cavo M, et al. Management of multiple myeloma and related-disorders: Guidelines from the Italian Society of Hematology (SIE), Italian Society of Experimental Hematology (SIES) and Italian Group for Bone Marrow Transplantation (GITMO) Haematologica. 2004;89:717–741. [PubMed] [Google Scholar]
- 43.Attal M, Harousseau JL, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 44.Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009;27:1788–1793. doi: 10.1200/JCO.2008.18.8573. [DOI] [PubMed] [Google Scholar]
- 45.Barlogie B, Tricot G, Anaissie E, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354:1021–1030. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- 46.Barlogie B, Pineda-Roman M, van Rhee F, et al. Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities. Blood. 2008;112:3115–3121. doi: 10.1182/blood-2008-03-145235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgan GJ, Jackson GH, Davies FE, et al. Maintenance thalidomide may improve progression free but not overall survival; results from the Myeloma IX maintenance randomisation [abstract 656] Blood. 2008;112:245. [Google Scholar]
- 48.Owen RG, Morgan GJ, Jackson H, et al. MRC Myeloma IX: Preliminary results from the non-intensive study [abstract 547] Clin Lymphoma Myeloma. 2009;9(1 suppl):79. [Google Scholar]
- 49.Ludwig H, Zdenek A, Tothova E, et al. Thalidomide/interferon versus interferon maintenance after thal/dex versus MP [abstract 384] Haematologica. 2009;94(2 suppl):153. [Google Scholar]
- 50.Cavo M, Pantani L, Tacchetti P, et al. Thalidomide maintenance in multiple myeloma: Certainties and controversies. J Clin Oncol. 2009;27:e186–e187. doi: 10.1200/JCO.2009.24.0150. [DOI] [PubMed] [Google Scholar]
- 51.Palumbo A, Cavallo F, Pagliano G, et al. Post transplant consolidation with bortezomib, thalidomide and dexamethasone induces a clinically significant shrinkage of residual tumor burden measured by real time PCR [abstract 1071] Haematologica. 2009;94(2 suppl):431. [Google Scholar]
- 52.Benevolo G, Larocca A, Pregno P, et al. Use of maintenance therapy with bortezomib and dexamethasone (VD) in advanced multiple myeloma [abstract 398] Haematologica. 2009;94(2 suppl):160. [Google Scholar]
- 53.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): A randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 54.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: Randomised controlled trial. Lancet. 2006;367:825–831. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]
- 55.Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: Updated results of a randomized, controlled trial. Blood. 2008;112:3107–3114. doi: 10.1182/blood-2008-04-149427. [DOI] [PubMed] [Google Scholar]
- 56.Gulbrandsen N, Waage A, Gimsing P, et al. A randomised placebo controlled study with melphalan/prednisone versus melphalan/prednisone/thalidomide: Quality of life and toxicity [abstract 209] Haematologica. 2008;93(1 suppl):84. [Google Scholar]
- 57.Wijermans PW, Zweegman S, van Marwijk Kooy M, et al. MP versus MPT in elderly myeloma patients: The final outcome of the HOVON-49 study [abstract 116] Clin Lymphoma Myeloma. 2009;9(1 suppl):S14. [Google Scholar]
- 58.Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 Trial. J Clin Oncol. 2009;27:3664–3670. doi: 10.1200/JCO.2008.21.0948. [DOI] [PubMed] [Google Scholar]
- 59.Palumbo A, Falco P, Falcone A, et al. Melphalan, prednisone, and lenalidomide for newly diagnosed myeloma: Kinetics of neutropenia and thrombocytopenia and time-to-event results. Clin Lymphoma Myeloma. 2009;9:145–150. doi: 10.3816/CLM.2009.n.035. [DOI] [PubMed] [Google Scholar]
- 60.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 61.San Miguel J, Schlag R, Khuageva N, et al. Updated follow-up and results of subsequent therapy in the phase III VISTA trial: Bortezomib plus melphalan–prednisone versus melphalan–prednisone in newly diagnosed multiple myeloma [abstract 650] Blood. 2008;112:242. [Google Scholar]
- 62.Dhawan R, Meunier J, Regnault R, et al. Impact of complete response on health-related quality of life in newly diagnosed multiple myeloma patients: Results from the VISTA trial [abstract 352] Clin Lymphoma Myeloma. 2009;9(1 suppl):53. [Google Scholar]
- 63.Mateos MV, Oriol A, Martinez J, et al. Bortezomib (Velcade)/melphalan/prednisone (VMP) versus Velcade/thalidomide/prednisonse (VTP) in elderly untreated multiple myeloma (MM) patients [abstract 471] Haematologica. 2009;94(2 suppl):190. [Google Scholar]
- 64.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib, melphalan, prednisone and thalidomide (VMPT) versus bortezomib, melphalan and prednisone (VMP) in elderly newly diagnosed myeloma patients: A prospective, randomized phase III study [abstract 472] Haematologica. 2009;94(2 suppl):190. [Google Scholar]
- 65.Mulkerin D, Remick S, Takimoto C, et al. Safety, tolerability, and pharmacology of bortezomib in cancer patients with renal failure requiring dialysis: Results from a prospective phase 1 study [abstract 3477] Blood. 2007;110:1018a. [Google Scholar]
- 66.European Medicines Agency. Bortezomib EU Prescribing Information. [accessed September 10, 2009]. Available at http://www.emea.europa.eu/humandocs/Humans/EPAR/velcade/velcade.htm.
- 67.Mezzano SA, Barría M, Droguett MA, et al. Tubular NF-κB and AP-1 activation in human proteinuric renal disease. Kidney Int. 2001;60:1366–1377. doi: 10.1046/j.1523-1755.2001.00941.x. [DOI] [PubMed] [Google Scholar]
- 68.Ludwig H, Drach J, Graf H, et al. Reversal of acute renal failure by bortezomib-based chemotherapy in patients with multiple myeloma. Haematologica. 2007;92:1411–1414. doi: 10.3324/haematol.11463. [DOI] [PubMed] [Google Scholar]
- 69.Jagannath S, Barlogie B, Berenson JR, et al. Bortezomib in recurrent and/or refractory multiple myeloma. Initial clinical experience in patients with impaired renal function. Cancer. 2005;103:1195–1200. doi: 10.1002/cncr.20888. [DOI] [PubMed] [Google Scholar]
- 70.San-Miguel JF, Richardson PG, Sonneveld P, et al. Efficacy and safety of bortezomib in patients with renal impairment: Results from the APEX phase 3 study. Leukemia. 2008;22:842–849. doi: 10.1038/sj.leu.2405087. [DOI] [PubMed] [Google Scholar]
- 71.Dimopoulos MA, Richardson PG, Schlag R, et al. VMP (bortezomib, melphalan, and prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: Cohort analysis of the phase III VISTA study. J Clin Oncol. 2009;27:6086–6093. doi: 10.1200/JCO.2009.22.2232. [DOI] [PubMed] [Google Scholar]
- 72.Ludwig H, Adam Z, Greil R, et al. Reversal of acute renal impairment by bortezomib-doxorubicin-dexamethasone (BDD) in multiple myeloma (MM). Results from a phase II study [abstract 385] Haematologica. 2009;94(2 suppl):154. [Google Scholar]
- 73.Eriksson T, Höglund P, Turesson I, et al. Pharmacokinetics of thalidomide in patients with impaired renal function and while on and off dialysis. J Pharm Pharmacol. 2003;55:1701–1706. doi: 10.1211/0022357022241. [DOI] [PubMed] [Google Scholar]
- 74.Tosi P, Zamagni E, Cellini C, et al. Thalidomide alone or in combination with dexamethasone in patients with advanced, relapsed or refractory multiple myeloma and renal failure. Eur J Haematol. 2004;73:98–103. doi: 10.1111/j.1600-0609.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- 75.Tosi P, Tacchetti P, Zamagni E, et al. Thalidomide/dexamethasone induction therapy in newly diagnosed myeloma patients with renal failure [abstract 174] Clin Lymphoma Myeloma. 2009;9(1 suppl):25. [Google Scholar]
- 76.Chen N, Lau H, Kong L, et al. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol. 2007;47:1466–1475. doi: 10.1177/0091270007309563. [DOI] [PubMed] [Google Scholar]
- 77.Niesvizky R, Naib T, Christos PJ, et al. Lenalidomide-induced myelosuppression is associated with renal dysfunction: Adverse events evaluation of treatment-naïve patients undergoing front-line lenalidomide and dexamethasone therapy. Br J Haematol. 2007;138:640–643. doi: 10.1111/j.1365-2141.2007.06698.x. [DOI] [PubMed] [Google Scholar]
- 78.Weber DM, Spencer A, Wang M, et al. The efficacy and safety of lenalidomide plus dexamethasone in relapsed or refractory multiple myeloma patients with impaired renal function [abstract 8542] J Clin Oncol. 2008;26(15 suppl):464s. [Google Scholar]
- 79.European Medicines Agency. Revlimid EU prescribing information. [accessed September 10, 2009]. Available at http://www.emea.europa.eu/humandocs/Humans/EPAR/revlimid/revlimid.htm.
- 80.Stewart AK, Bergsagel PL, Greipp PR, et al. A practical guide to defining high-risk myeloma for clinical trials, patient counseling and choice of therapy. Leukemia. 2007;21:529–534. doi: 10.1038/sj.leu.2404516. [DOI] [PubMed] [Google Scholar]
- 81.Jagannath S, Richardson PG, Sonneveld P, et al. Bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials. Leukemia. 2007;21:151–157. doi: 10.1038/sj.leu.2404442. [DOI] [PubMed] [Google Scholar]
- 82.Sagaster V, Ludwig H, Kaufmann H, et al. Bortezomib in relapsed multiple myeloma: Response rates and duration of response are independent of a chromosome 13q-deletion. Leukemia. 2007;21:164–168. doi: 10.1038/sj.leu.2404459. [DOI] [PubMed] [Google Scholar]
- 83.Cavo M, Testoni N, Terragna C, et al. Superior rate of complete response with up-front Velcade-thalidomide-dexamethasone versus thalidomide-dexamethasone in newly diagnosed multiple myeloma is not affected by adverse prognostic factors, including high-risk cytogenetic abnormalities [abstract 1662] Blood. 2008;112:586. [Google Scholar]
- 84.Cavo M, Testoni N, Terragna C, et al. Up-front thalidomide-dexamethasone (thal) and double autologous transplantation (double TX) for multiple myeloma: Comparison with double TX without added thalidomide and prognostic implications of chromosome 13 deletion and translocation t(4;14) [abstract 3081] Blood. 2006;108:878a. [Google Scholar]
- 85.Reece D, Song KW, Fu T, et al. Influence of cytogenetics in patients with relapsed or refractory multiple myeloma treated with lenalidomide plus dexamethasone: Adverse effect of deletion 17p13. Blood. 2009;114:522–525. doi: 10.1182/blood-2008-12-193458. [DOI] [PubMed] [Google Scholar]
- 86.Avet-Loiseau H, Soulier J, Fermand JP, et al. Impact of chromosomal abnormalities del(13), t(4;14), and del(17p) and prior treatment on outcomes in patients with relapsed or refractory multiple myeloma treated with lenalidomide [abstract 3685] Blood. 2008;112:1262. [Google Scholar]
- 87.Kapoor P, Kumar S, Fonseca R, et al. Impact of risk stratification on outcome among patients with multiple myeloma receiving initial therapy with lenalidomide and dexamethasone. Blood. 2009;114:518–521. doi: 10.1182/blood-2009-01-202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 89.Palumbo A, Facon T, Sonneveld P, et al. Thalidomide for treatment of multiple myeloma: 10 years later. Blood. 2008;111:3968–3977. doi: 10.1182/blood-2007-10-117457. [DOI] [PubMed] [Google Scholar]
- 90.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: Final time-to-event results of the APEX trial. Blood. 2007;110:3557–3560. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 91.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: Combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 92.Kropff M, Bisping G, Schuck E, et al. Bortezomib in combination with intermediate-dose dexamethasone and continuous low-dose oral cyclophosphamide for relapsed multiple myeloma. Br J Haematol. 2007;138:330–337. doi: 10.1111/j.1365-2141.2007.06656.x. [DOI] [PubMed] [Google Scholar]
- 93.Palumbo A, Gay F, Bringhen S, et al. Bortezomib, doxorubicin and dexamethasone in advanced multiple myeloma. Ann Oncol. 2008;19:1160–1165. doi: 10.1093/annonc/mdn018. [DOI] [PubMed] [Google Scholar]
- 94.Mikhael JR, Belch AR, Prince HM, et al. High response rate to bortezomib with or without dexamethasone in patients with relapsed or refractory multiple myeloma: Results of a global phase 3b expanded access program. Br J Haematol. 2009;144:169–175. doi: 10.1111/j.1365-2141.2008.07409.x. [DOI] [PubMed] [Google Scholar]
- 95.Anderson KC, Jagannath S, Jakubowiak A, et al. Lenalidomide, bortezomib, and dexamethasone in relapsed/refractory multiple myeloma (MM): Encouraging outcomes and tolerability in a phase II study [abstract 8536] J Clin Oncol. 2009;27(18 suppl):442s. [Google Scholar]
- 96.Petrucci MT, Blau IW, Corradini P, et al. Efficacy and safety of re-treatment with bortezomib (Velcade®) in patients with multiple myeloma: Results from a prospective international phase II trial [abstract 3690] Blood. 2008;112:1264. [Google Scholar]
- 97.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 98.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 99.Dimopoulos MA, Chen C, Spencer A, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23:2147–2152. doi: 10.1038/leu.2009.147. [DOI] [PubMed] [Google Scholar]
- 100.Wang M, Dimopoulos MA, Chen C, et al. Lenalidomide plus dexamethasone is more effective than dexamethasone alone in patients with relapsed or refractory multiple myeloma regardless of prior thalidomide exposure. Blood. 2008;112:4445–4451. doi: 10.1182/blood-2008-02-141614. [DOI] [PubMed] [Google Scholar]
- 101.Knop S, Gerecke C, Liebisch P, et al. Lenalidomide, adriamycin, and dexamethasone (RAD) in patients with relapsed and refractory multiple myeloma: A report from the German Myeloma Study Group DSMM (Deutsche Studiengruppe Multiples Myelom) Blood. 2009;113:4137–4143. doi: 10.1182/blood-2008-10-184135. [DOI] [PubMed] [Google Scholar]
- 102.Mehta J, Tricot G, Jagannath S, et al. Salvage autologous or allogeneic transplantation for multiple myeloma refractory to or relapsing after a first-line autograft? Bone Marrow Transplant. 1998;21:887–892. doi: 10.1038/sj.bmt.1701208. [DOI] [PubMed] [Google Scholar]
- 103.Elice F, Raimondi R, Tosetto A, et al. Prolonged overall survival with second on-demand autologous transplant in multiple myeloma. Am J Hematol. 2006;81:426–431. doi: 10.1002/ajh.20641. [DOI] [PubMed] [Google Scholar]
- 104.Mikhael J, Samiee S, Stewart AK, et al. Second autologous stem cell transplantation as salvage therapy in patients with relapsed multiple myeloma: Improved outcomes in patients with longer disease free interval after first autologous stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(1 suppl.):117. [Google Scholar]
- 105.Simpson L, Verma R, Kumar S, et al. Outcome after second stem cell transplantation for relapsed multiple myeloma [abstract 8118] J Clin Oncol. 2007;25(18 suppl):470s. [Google Scholar]
- 106.Olin RL, Vogl DT, Porter DL, et al. Second auto-SCT is safe and effective salvage therapy for relapsed multiple myeloma. Bone Marrow Transplant. 2009;43:417–422. doi: 10.1038/bmt.2008.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alvares CL, Davies FE, Horton C, et al. The role of second autografts in the management of myeloma at first relapse. Haematologica. 2006;91:141–142. [PubMed] [Google Scholar]
- 108.de Lavallade H, El-Cheikh J, Faucher C, et al. Reduced-intensity conditioning allogeneic SCT as salvage treatment for relapsed multiple myeloma. Bone Marrow Transplant. 2008;41:953–960. doi: 10.1038/bmt.2008.22. [DOI] [PubMed] [Google Scholar]
- 109.Minnema MC, van Dorp S, van de Donk N, et al. Non-myeloablative allo-SCT for relapsed myeloma: A single-center experience [abstract 288] Clin Lymphoma Myeloma. 2009;9(1 suppl):42. [Google Scholar]
- 110.Elliott B, Osman K, Mandeli J, et al. Nonmyeloablative conditioning and allogeneic transplantation for multiple myeloma [abstract 621] Clin Lymphoma Myeloma. 2009;9(1 suppl):87. [Google Scholar]
- 111.San-Miguel J, Harousseau JL, Joshua D, et al. Individualizing treatment of patients with myeloma in the era of novel agents. J Clin Oncol. 2008;26:2761–2766. doi: 10.1200/JCO.2007.15.2546. [DOI] [PubMed] [Google Scholar]
- 112.Niesvizky R, Spencer A, Wang M, et al. Increased risk of thrombosis with lenalidomide in combination with dexamethasone and erythropoietin [abstract 7506] J Clin Oncol. 2006;24(18 suppl):423s. [Google Scholar]
- 113.Cavo M, Palumbo A, Bringhen S, et al. A phase III study of enoxaparin versus low-dose warfarin versus aspirin as thromboprophylaxis for patients with newly diagnosed multiple myeloma treated up-front with thalidomide-containing regimens [abstract 3017] Blood. 2008;112:1038. [Google Scholar]
- 114.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–423. doi: 10.1038/sj.leu.2405062. [DOI] [PubMed] [Google Scholar]
- 115.Lonial S, Richardson PG, San Miguel J, et al. Characterisation of haematological profiles and low risk of thromboembolic events with bortezomib in patients with relapsed multiple myeloma. Br J Haematol. 2008;143:222–229. doi: 10.1111/j.1365-2141.2008.07321.x. [DOI] [PubMed] [Google Scholar]
- 116.Richardson P, Schlag R, Khuageva NK, et al. Erythropoiesis-stimulating agents do not adversely affect long-term outcomes nor increase the risk of thromboembolic events in multiple myeloma patients treated in the phase III VISTA trial [abstract 1741] Blood. 2008;112:614. [Google Scholar]
- 117.Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: Impact of a dose-modification guideline. Br J Haematol. 2009;144:895–903. doi: 10.1111/j.1365-2141.2008.07573.x. [DOI] [PubMed] [Google Scholar]
- 118.Colson K, Doss DS, Swift R, et al. Expanding role of bortezomib in multiple myeloma: Nursing implications. Cancer Nurs. 2008;31:239–249. doi: 10.1097/01.NCC.0000305733.80592.8e. [DOI] [PubMed] [Google Scholar]
- 119.Chanan-Khan A, Sonneveld P, Schuster MW, et al. Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol. 2008;26:4784–4790. doi: 10.1200/JCO.2007.14.9641. [DOI] [PubMed] [Google Scholar]
- 120.Vickrey E, Allen S, Mehta J, et al. Acyclovir to prevent reactivation of varicella zoster virus (herpes zoster) in multiple myeloma patients receiving bortezomib therapy. Cancer. 2009;115:229–232. doi: 10.1002/cncr.24006. [DOI] [PubMed] [Google Scholar]
- 121.Mateos MV, García-Sanz R, Colado E, et al. Should prophylactic granulocyte-colony stimulating factor be used in multiple myeloma patients developing neutropenia under lenalidomide-based therapy? Br J Haematol. 2008;140:324–326. doi: 10.1111/j.1365-2141.2007.06946.x. [DOI] [PubMed] [Google Scholar]
- 122.Richardson P, Jagannath S, Hussein M, et al. Safety and efficacy of single-agent lenalidomide in patients with relapsed and refractory multiple myeloma. Blood. 2009;114:772–778. doi: 10.1182/blood-2008-12-196238. [DOI] [PubMed] [Google Scholar]
- 123.Terpos E, Sezer O, Croucher PI, et al. The use of bisphosphonates in multiple myeloma: Recommendations of an expert panel on behalf of the European Myeloma Network. Ann Oncol. 2009;20:1303–1317. doi: 10.1093/annonc/mdn796. [DOI] [PubMed] [Google Scholar]