The article reports on a case of ectopic adrenocorticotropic hormone production with esthesioneuroblastoma with a review of the literature and management.
Keywords: Esthesioneuroblastoma, Paraneoplastic disease, Ectopic ACTH, Skull-based tumors
Abstract
The case of a patient with recurrent esthesioneuroblastoma complicated by ectopic adrenocorticotropic hormone production is presented, including the workup and management of this uncommon complication of an uncommon disease.
Case Presentation
In 1992, a 55-year-old white man presented with epistaxis and was diagnosed with esthesioneuroblastoma. He underwent a craniofacial resection with postoperative radiation therapy to his tumor site. In 1996, he relapsed in the cervical lymph nodes and underwent a bilateral cervical lymph node dissection. In total, eight positive lymph nodes were removed. He was then treated with chemotherapy with etoposide and cisplatin for four cycles. Although radiation therapy to the neck was recommended, the patient refused. In 1998, he relapsed again in the left submandibular area, and two involved lymph nodes were resected. He then agreed to and received radiation therapy to both sides of the neck. In 2002, he had evidence of further disease progression in the posterior nasopharynx and a left preauricular node. In 2004, he was treated with etoposide and carboplatin for four cycles with a minor response. Following that, he was observed without further therapy, given the indolent nature of his disease and minimal symptoms. He did, however, experience focal seizures, occasional epistaxis, hyponatremia, hypothyroidism, and an episode of pneumococcal meningitis.
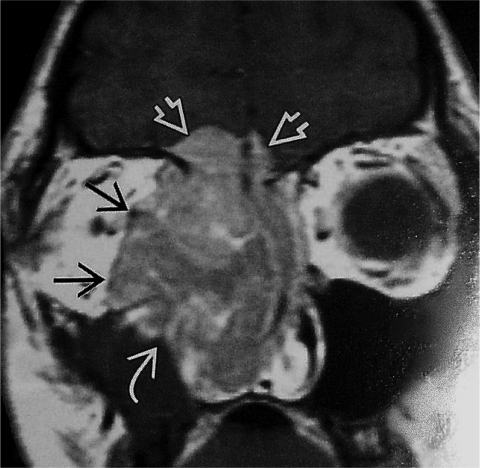
In August 2007, he developed fatigue, confusion, severe hypertension, and proximal muscle weakness. Laboratory studies revealed a serum glucose level of 228 mg/dl (normal, 70–99 mg/dl), serum potassium of 2.9 mmol/l (normal, 3.5–5.1 mmol/l), pH of 7.570 (normal, 7.350–7.450), and HCO3 of 45.8 mEq/l (normal, 23.0–27.0 mEq/l). His serum cortisol was 88 μg/ml (normal, 5–25 μg/ml). His serum adrenocorticotropic hormone (ACTH) was 455 pg/ml (normal, 7–50 pg/ml) and his 24-hour urine free cortisol concentration was 15,261.4 μg/24 hours (normal, 20–90 μg/24 hours). An octreotide scan revealed uptake in the region of the left temporal bone and skull base, corresponding to the masses identified on a magnetic resonance imaging (MRI) scan (Fig. 1). There was no evidence of distant metastatic deposits.
Figure 1.
Coronal MRI image demonstrating an esthesioneuroblastoma with extension to brain, nasal cavity, and orbit.
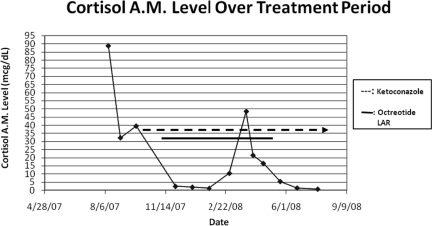
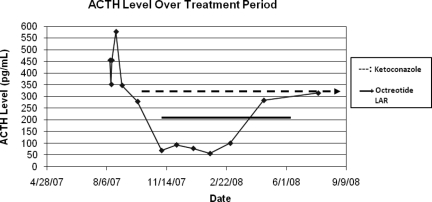
He was diagnosed with ectopic ACTH syndrome and started on ketoconazole, 400 mg three times a day, along with hydrocortisone replacement. In September 2007, octreotide (Sandostatin® LAR®; Novartis AG, Basel, Switzerland) was added. With treatment, the patient's cortisol concentration dropped (Fig. 2). His serum ACTH concentration was reduced, but it remained elevated and above the range of normal (Fig. 3). In April 2008, octreotide was discontinued.
Figure 2.
Graph of serum cortisol level over treatment period.
Figure 3.
Graph of ACTH level over treatment period.
From October through December 2007, the patient received a trial of oral temozolomide for three cycles. However, a follow-up MRI scan showed no response, so this was discontinued. In July 2008, he developed dysphasia with a progressive intracranial tumor. He underwent craniotomy with a partial resection. Postoperatively, his speech and mental status improved. Currently, the patient's cortisol, glucose, blood pressure, potassium, and serum bicarbonate levels have remained normal as he continues on ketoconazale, 400 mg three times daily, with maintenance hydrocortisone.
Pathology
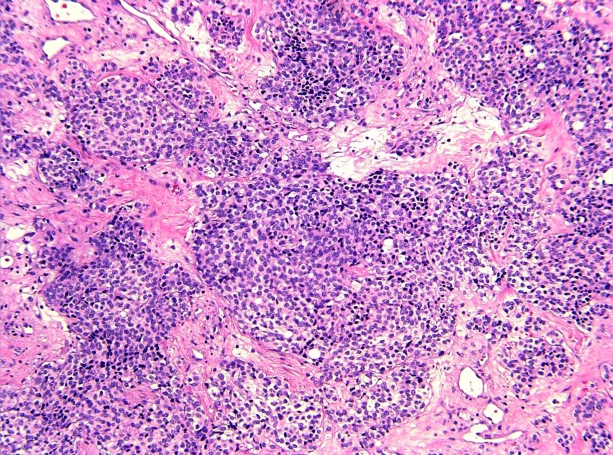
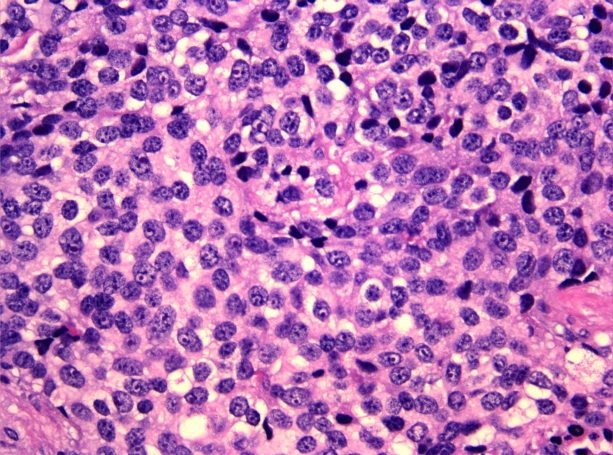
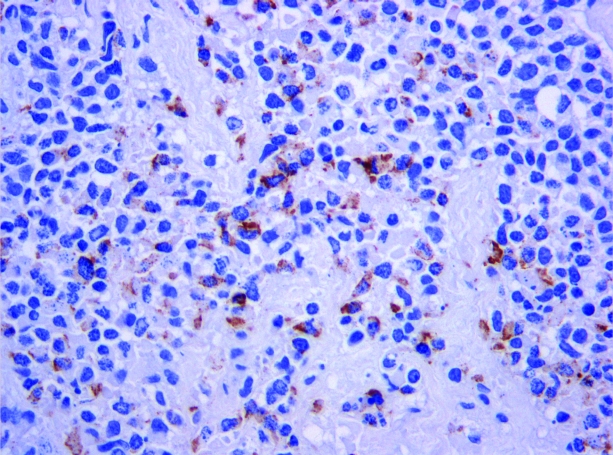
Pathology from the most recent procedure in July 2008 confirmed recurrent esthesioneuroblastoma, also known as olfactory neuroblastoma (ON). The tumor was removed in multiple fragments. Histologically, the tumor demonstrates islands and nests of round to oval cells with scant cytoplasm. The nuclei are small, monomorphic, and have a fine chromatin pattern (Figs. 4 and 5). Immunohistochemistry demonstrated expression of synaptophysin, chromogranin, and neuron-specific enolase; stains for S100, AE1/AE3, and Cam 5.2 were negative. Immunostaining for ACTH was focally positive (Fig. 6). The morphologic and immunohistochemical observations support the diagnosis of ON with ectopic ACTH production [1].
Figure 4.
Medium-power view with islands and nests of tumor cells (×200, hematoxylin and eosin).
Figure 5.
High-power view of tumor cells (×400, hematoxylin and eosin).
Figure 6.
ACTH expression in tumor cells (×200, ACTH immunohistochemistry).
Hyams et al. [2] proposed a histologic grading system based on the degree of lobular architecture preservation, presence of fibrillary matrix, nuclear pleomorphism, mitotic activity, necrosis, rosette and gland formation, and calcification. This four-tiered system is often simplified into low grade (Hyams grades I and II) and high grade (Hyams grades III and IV) [3]. The correlation between tumor grade and prognosis is somewhat controversial, but the majority of ONs are of low grade, as in the present case.
Immunohistochemistry typically demonstrates ON to be positive for chromogranin, synaptophysin, and neuron-specific enolase, and negative for cytokeratin and epithelial membrane antigen in the majority of cases, as in the present case. The histogenesis of ON is controversial, and definitive precursor lesions have not been identified, although these tumors are assumed to originate from immature olfactory neurons of neuroectodermal origin [4].
ON is distinct from classic neuroblastoma clinically and genetically. In contrast to ON, neuroblastoma is primarily a tumor of children, being the most common extracranial solid malignant tumor diagnosed during the first 2 years of life. It presents most commonly as an intra-abdominal mass [5]. Genetically, ON lacks the typical cytogenetic abnormalities seen in neuroblastoma, such as deletion of the short arm of chromosome 1 (1p), MYCN amplification, and expression of neurotrophin receptor TrkA [6].
Discussion
Esthesioneuroblastoma (ON) is an uncommon malignant neoplasm of the nasal vault, believed to develop from olfactory neuroepithelium. The cancer exhibits a wide spectrum of behavior, ranging from indolent progression, with patients surviving >20 years, to rapid widespread metastatic disease, with survival durations of only a few months. Five-year survival rates are reported to be in the range of 45%–62% [7–10]. The Kadish staging system divides patients into: A, limited to the nasal cavity; B, lesions involving the nasal cavity and paranasal sinuses; and C, extension beyond the nasal cavity and paranasal sinuses. Morita et al. [8] proposed a modification of the Kadish system, with a stage D for patients with regional nodal or distant metastases. Jethanamest et al. [9] recently reviewed 311 cases available in the Surveillance, Epidemiology, and End Results data base for 1973–2002. The overall 5- and 10-year survival rates were 62.1% and 45.6%, respectively. Their review confirmed that the modified Kadish staging system was prognostic for survival. Age, nodal status, and treatment were also prognostic indicators.
For early-stage disease, resection is considered the treatment of choice. Radiation therapy has been used in conjunction with surgery, either pre- or postoperatively in some centers, and as definitive treatment for patients with unresectable disease. In some centers, chemotherapy, alone or in combination with radiation, has been used in the neoadjuvant setting to downstage tumors prior to surgery [7–14].
Surgical Approach to Skull-Base Tumors
Esthesioneuroblastoma is a relatively rare diagnosis, even among sinonasal carcinoma, representing 3%–10% of sinonasal malignancies [14]. Unlike many other sinonasal cancers, there are no known causative factors. Patients may present with seemingly benign and common symptoms such as nasal obstruction, epistaxis, sinusitis, and headaches. Cranial neuropathies may develop. There is often a delay in diagnosis; the time frame from when a patient first presents with symptoms to diagnosis is often between 6 months and 1 year.
The differential diagnosis includes a wide range of pathologies, both benign and malignant. Once a mass is noted in the sinonasal cavity, the possibilities include entities such as sinonasal polyp, mucocele, inverting papilloma, granuloma, angiofibroma, meningocele, and myelomeningocele. Other possible malignancies include sinonasal undifferentiated carcinoma, squamous cell carcinoma/nasopharyngeal carcinoma, adenocarcinoma, sarcoma, mucosal melanoma, lymphoma, and metastasis. The diagnosis of esthesioneuroblastoma can generally be established via transnasal endoscopic biopsy with appropriate immunohistochemical staining.
Staging plays an important role in treatment, because survival in most studies is linked to stage [3]. Although there are several staging systems, they generally include the same characteristics to define stages. There is no true standard of care for treatment, because no controlled trials have demonstrated superior outcomes. However, multimodality therapy is generally recommended for all but the smallest of tumors [15, 16]. Surgery remains the mainstay of treatment for this malignancy. However, negative margin surgery is sometimes impossible because of the structures involved in the removal of these tumors, including the intracranial carotid, the cavernous sinus, and the orbit.
Over the last several decades, the surgical management of these tumors has undergone significant change. Cranial base surgery, developed in the 1950s, is a rapidly changing field. There has been a significant evolution over this time, leading to a continuing decrease in morbidity and mortality. Esthesioneuroblastomas that used to be treated via an open craniotomy with facial incisions are now treated almost entirely endoscopically through the nose. The indication for these minimally invasive surgeries continues to grow. As our technology continues to improve, and our ability to close the defects that separate the intracranial compartment from the sinonasal cavity grows, we are able to remove larger tumors through the endonasal route. Historically, the large communications from the intracranial compartment to the sinonasal cavity were repaired using skin grafts and periosteal flaps [17]. The rate of complications was quite high. Now, separating the intracranial cavity from the rest of the sinonasal cavity can be accomplished through the use of pericranial flaps and vascularized free flaps. Novel procedures are also being employed to close defects created endoscopically, including pedicled nasoseptal flaps [18]. Additionally, technology such as intraoperative navigation allows surgeons to pinpoint and avoid critical structures, as well as confidently remove parts of the tumor that would otherwise be missed.
Chemotherapy
Local recurrence of esthesioneuroblastoma occurs with a rate of 20%–40% whereas 25%–50% of patients develop metastatic disease [6–15]. Despite this, the data regarding the benefits of chemotherapy are limited for both the adjuvant and metastatic settings, given the rarity of this tumor. What data exist, however, indicate that esthesioneuroblastoma can be chemotherapy responsive. Platinum-based regimens, sometimes with etoposide, have been frequently used, although other regimens have been used as well [11–13, 19–21].
Kim et al. [11] reported the use of neoadjuvant etoposide, ifosfamide, and cisplatin, with an 82% response rate in 11 patients. Loy et al. [12] updated the University of Virginia experience reviewing 50 patients treated in 1976–2004. Their approach included preoperative radiotherapy followed by surgery for Kadish A/B stage disease, whereas patients with Kadish stage C disease received preoperative chemotherapy and radiotherapy followed by resection. Porter et al. [13] reviewed 76 patients treated at the Mayo Clinic in 1976–2003. They concluded that adjuvant chemotherapy may lead to longer disease-free and overall survival times in patients with high-grade, stage C disease, by comparing results in only six patients who did and six patients who did not receive adjuvant chemotherapy. Chamberlain reported that four of six patients with Kadish C disease responded to a combination of carboplatin, lomustine, and vincristine [19]. Kiyota et al. [20] reported on 12 cases of ON treated with a nonplatinum regimen containing irinotecan and docetaxel, of whom three responded.
Given our patient's progressive disease and two prior treatments with etoposide and a platinum with minimal, if any, response, we treated our patient with three cycles of temozolomide. Temozolomide, usually used to treat glioblastoma multiforme and melanoma, was used in a 2004 case report for a patient with intracranial metastatic esthesioneuroblastoma who achieved stable disease with this agent for >23 months [21]. When our patient was treated with temozolomide, he showed no indication of response and his cancer continued to progress.
Ectopic ACTH Syndrome
Ectopic ACTH syndrome, one cause of Cushing's syndrome, has been rarely reported with esthesioneuroblastoma. Eight cases have previously been described [22–28].
Ectopic ACTH production, stimulating cortisol secretion and producing Cushing's syndrome, is most commonly a result of small cell lung carcinomas, bronchial carcinoids, and islet cell tumors [29, 30]. Diagnosing ectopic ACTH syndrome (ACTH production by nonpituitary ACTH-secreting tumors) requires distinction from Cushing's disease (ACTH secretion from corticotroph adenomas) because both cause ACTH-dependent Cushing's syndrome (elevated cortisol and nonsuppressed ACTH concentration). Myopathy and hypokalemic alkalosis, as seen in our case, tend to be prominent presenting features with ectopic ACTH syndrome. Our patient also developed hypertension, hyperglycemia, and edema as part of his syndrome. Weight loss, rather than the weight gain with Cushingoid features typically seen with Cushing's disease, may also be a sign of ectopic ACTH associated with advanced malignancies. Likewise, marked elevations in serum cortisol and ACTH are more often seen with ectopic ACTH syndrome. Patients with ectopic ACTH secretion usually fail to suppress with high-dose dexamethasone, although the sensitivity of this test is <100%. Inferior petrosal sinus sampling has been regarded as the test that best detects ectopic ACTH secretion [30].
Although in the case presented here, the presence and location of the tumor was evident prior to the diagnosis of ectopic ACTH syndrome, in some cases determining the location of the ACTH-secreting tumor is challenging, often because of its small size and the limited ability of present imaging techniques to detect the tumor. The octreotide scan, which revealed uptake corresponding to the esthesioneuroblastoma, confirmed suspicions that this patient's ectopic ACTH syndrome was caused by the tumor. Up to 80% of ectopic ACTH-producing tumors have somatostatin receptors by scintigraphy [29–31]. In a recent review of 90 patients with ectopic ACTH syndrome, it was noted that the standard 6 mCi octreotide scan had a sensitivity of 49% and, as in our case, did not detect any new lesions not already noted by computed tomography or MRI [30].
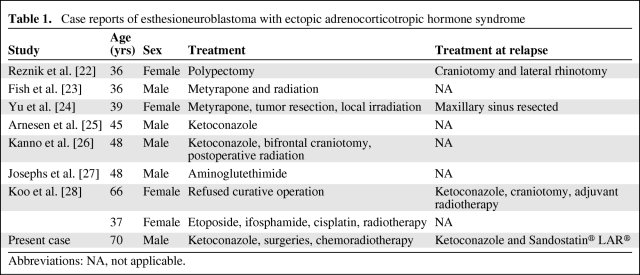
Our patient was treated with ketoconazole, which successfully normalized his cortisol concentration. Of the eight other case reports of ACTH-secreting esthesioneuroblastoma, four patients received ketoconazole with successful control of cortisol secretion. Although none of the patients in the case reports used octreotide to lower ACTH concentration, in the literature the drug has been shown to effectively reduce ACTH secretion by nonpituitary tumors through the cell-surface receptor somatostatin [32]. Apparently, however, the therapeutic response to octreotide cannot be predicted by the results of an octreotide scan [33]. In treating ectopic ACTH syndrome, the optimal therapy is total resection of the ACTH-secreting tumor. In four of the eight previous case reports of patients who underwent surgical excision of their ectopic ACTH-secreting esthesioneuroblastomas, all experienced disappearance of symptoms along with decreased ACTH and cortisol concentrations (Table 1). Postoperative radiation therapy was often used to minimize the risk for local recurrence. Hypercortisolism should be treated to minimize the risks for immunosuppression, thromboembolism, hypokalemia, and diabetes. Adrenal enzyme inhibitors, such as aminoglutethimide, ketoconazole, etomidate, and metyrapone, are commonly used alone or in combination with control hypercortisolism. Other treatment options include glucocorticoid antagonists such as mifepristone and adrenalectomy, either surgical (bilateral) or medical using mitotane [29, 30, 32, 33].
Table 1.
Case reports of esthesioneuroblastoma with ectopic adrenocorticotropic hormone syndrome
Abbreviations: NA, not applicable.
Conclusion
Esthesioneuroblastoma is a rare disease, likely best approached with primarily surgical resection, when possible. Newer, less invasive endoscopic surgical techniques are being used with increasing frequency. Adjuvant radiotherapy, and sometimes chemotherapy, is frequently given either before or after surgery. However, because of the rarity of the tumor, there are no randomized clinical trials to definitively support specific recommendations.
Ectopic ACTH production is an uncommon manifestation of this uncommon disease. We report here a ninth case of ectopic ACTH production with esthesioneuroblastoma with a review of the literature and management.
Author Contributions
Conception/Design: David M. Mintzer
Administrative support: David M. Mintzer, Sarah Zheng
Provision of study material or patients: David M. Mintzer, Sarah Zheng, Michiko Nagamine, Jason Newman
Collection and/or assembly of data: David M. Mintzer, Sarah Zheng, Michiko Nagamine, Jason Newman
Data analysis and interpretation: David M. Mintzer, Maria Benito
Manuscript writing: David M. Mintzer, Sarah Zheng, Michiko Nagamine, Jason Newman, Maria Benito
Final approval of manuscript: David M. Mintzer
References
- 1.Finkelstein SD, Hirose T, VandenBerg SR. Olfactory neuroblastoma. In: Kleihues P, Cavenee WK, editors. Pathology and Genetics: Tumours of the Nervous System. Lyon, France: World Health Organization, IARC; 2000. pp. 150–152. [Google Scholar]
- 2.Hyams V, Batsakis J, Michaels L. Atlas of tumor pathology. Armed Forces Institute of Pathology. 1988;25:240–248. [Google Scholar]
- 3.Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: A meta-analysis and review. Lancet Oncol. 2001;2:683–690. doi: 10.1016/S1470-2045(01)00558-7. [DOI] [PubMed] [Google Scholar]
- 4.Carney ME, O'Reilly RC, Sholevar B, et al. Expression of the human Achaete-scute 1 gene in olfactory neuroblastoma (esthesioneuroblastoma) J Neurooncol. 1995;26:35–43. doi: 10.1007/BF01054767. [DOI] [PubMed] [Google Scholar]
- 5.Schwab M, Shimada H, Joshi V, et al. Neuroblastic tumours of adrenal gland and sympathetic nervous system. In: Kleihues P, Cavenee WK, editors. Pathology and Genetics of Tumours of the Nervous System. Lyon, France: World Health Organization, IARC; 2000. p. 153. [Google Scholar]
- 6.Sorensen PH, Wu JK, Berean KW, et al. Olfactory neuroblastoma is a peripheral primitive neuroectodermal tumor related to Ewing sarcoma. Proc Natl Acad Sci U S A. 1996;93:1038–1043. doi: 10.1073/pnas.93.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sciñska A, Korkosz A, Sienkiewicz-Jarosz H, et al. [Olfactory neuroblastoma (esthesioneuroblastoma): Etiopathogenesis, diagnosis, and treatment] Otolaryngol Pol. 2006;60:849–858. In Polish. [PubMed] [Google Scholar]
- 8.Morita A, Ebersold MJ, Olsen KD, et al. Esthesioneuroblastoma: Prognosis and management. Neurosurgery. 1993;32:706–714. doi: 10.1227/00006123-199305000-00002. discussion 714–715. [DOI] [PubMed] [Google Scholar]
- 9.Jethanamest D, Morris LG, Sikora AG, et al. Esthesioneuroblastoma: A population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg. 2007;133:276–280. doi: 10.1001/archotol.133.3.276. [DOI] [PubMed] [Google Scholar]
- 10.McElroy EA, Jr, Buckner JC, Lewis JE. Chemotherapy for advanced esthesioneuroblastoma: The Mayo Clinic experience. Neurosurgery. 1998;42:1023–1027. doi: 10.1097/00006123-199805000-00040. discussion 1027–1028. [DOI] [PubMed] [Google Scholar]
- 11.Kim DW, Jo YH, Kim JH, et al. Neoadjuvant etoposide, ifosfamide, and cisplatin for the treatment of olfactory neuroblastoma. Cancer. 2004;101:2257–2260. doi: 10.1002/cncr.20648. [DOI] [PubMed] [Google Scholar]
- 12.Loy AH, Reibel JF, Read PW, et al. Esthesioneuroblastoma: Continued follow-up of a single institution's experience. Arch Otolaryngol Head Neck Surg. 2006;132:134–138. doi: 10.1001/archotol.132.2.134. [DOI] [PubMed] [Google Scholar]
- 13.Porter AB, Bernold DM, Giannini C, et al. Retrospective review of adjuvant chemotherapy for esthesioneuroblastoma. J Neurooncol. 2008;90:201–204. doi: 10.1007/s11060-008-9645-y. [DOI] [PubMed] [Google Scholar]
- 14.Broich G, Pagliari A, Ottaviani F. Esthesioneuroblastoma: A general review of the cases published since the discovery of the tumour in 1924. Anticancer Res. 1997;7:2683–2706. [PubMed] [Google Scholar]
- 15.Bachar G, Goldstein DP, Shah M, et al. Esthesioneuroblastoma: The Princess Margaret Hospital experience. Head Neck. 2008;30:1607–1614. doi: 10.1002/hed.20920. [DOI] [PubMed] [Google Scholar]
- 16.Dias FL, Sa GM, Lima RA, et al. Patterns of failure and outcome in esthesioneuroblastoma. Arch Otolaryngol Head Neck Surg. 2003;129:1186–1192. doi: 10.1001/archotol.129.11.1186. [DOI] [PubMed] [Google Scholar]
- 17.Shah JP, Galicich JH. Craniofacial resection for malignant tumors of ethmoid and anterior skull base. Arch Otolaryngol. 1977;103:514–517. doi: 10.1001/archotol.1977.00780260044002. [DOI] [PubMed] [Google Scholar]
- 18.Kassam AB, Thomas A, Carrau RL, et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery. 2008;63(suppl 1):ONS44–ONS52. doi: 10.1227/01.neu.0000297074.13423.f5. discussion ONS52–ONS53. [DOI] [PubMed] [Google Scholar]
- 19.Chamberlain MC. Treatment of intracranial metastatic esthesioneuroblastoma. J Clin Oncol. 2002;20:357–358. doi: 10.1200/JCO.2002.20.1.357. [DOI] [PubMed] [Google Scholar]
- 20.Kiyota N, Tahara M, Fujii S, et al. Nonplatinum-based chemotherapy with irinotecan plus docetaxel for advanced or metastatic olfactory neuroblastoma: A retrospective analysis of 12 cases. Cancer. 2008;112:885–891. doi: 10.1002/cncr.23246. [DOI] [PubMed] [Google Scholar]
- 21.Wick W, Wick A, Kk̈er W, et al. Intracranial metastatic esthesioneuroblastoma responsive to temozolomide. J Neurooncol. 2004;70:73–75. doi: 10.1023/b:neon.0000040826.30636.4a. [DOI] [PubMed] [Google Scholar]
- 22.Reznik M, Melon J, Lambricht M, et al. [Neuroendocrine tumor of the nasal cavity (esthesioneuroblastoma) Apropos of a case with paraneoplastic Cushing's syndrome.] Ann Pathol. 1987;7:137–142. In French. [PubMed] [Google Scholar]
- 23.Fish S, Harish S, Tapino E, et al. Ectopic ACTH syndrome caused by olfactory neuroblastoma. Resid Staff Physician. 2005;51:30–33. [Google Scholar]
- 24.Yu J, Koch CA, Patsalides A, et al. Ectopic Cushing's syndrome caused by an esthesioneuroblastoma. Endocr Pract. 2004;10:119–124. doi: 10.4158/EP.10.2.119. [DOI] [PubMed] [Google Scholar]
- 25.Arnesen MA, Scheithauer BW, Freeman S, et al. Cushing's syndrome secondary to olfactory neuroblastoma. Ultrastruct Pathol. 1994;18:61–68. doi: 10.3109/01913129409016275. [DOI] [PubMed] [Google Scholar]
- 26.Kanno K, Morokuma Y, Tateno T, et al. Olfactory neuroblastoma causing ectopic ACTH syndrome. Endocr J. 2005;52:675–681. doi: 10.1507/endocrj.52.675. [DOI] [PubMed] [Google Scholar]
- 27.Josephs L, Jones L, Marenette L, et al. Cushing's syndrome: An unusual presentation of olfactory neuroblastoma. Skull Base. 2008;18:73–76. doi: 10.1055/s-2007-994291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koo BK, An JH, Jeon KH, et al. Two cases of ectopic adrenocorticotropic hormone syndrome with olfactory neuroblastoma and literature review. Endocr J. 2008;55:469–475. doi: 10.1507/endocrj.k07e-005. [DOI] [PubMed] [Google Scholar]
- 29.Isidori AM, Kaltsas GA, Pozza C, et al. The ectopic adrenocorticotropin syndrome: Clinical features, diagnosis, management and long-term follow-up. J Clin Endocrinol Metab. 2006;91:371–377. doi: 10.1210/jc.2005-1542. [DOI] [PubMed] [Google Scholar]
- 30.Ilias I, Torpy DJ, Pacak K, et al. Cushing's syndrome due to ectopic corticotrophin secretion: Twenty years' experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90:4955–4962. doi: 10.1210/jc.2004-2527. [DOI] [PubMed] [Google Scholar]
- 31.Tabarin A, Valli N, Chanson P, et al. Usefulness of somatostatin receptor scintigraphy in patients with occult ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1999;84:1193–1202. doi: 10.1210/jcem.84.4.5583. [DOI] [PubMed] [Google Scholar]
- 32.von Werder K, Muller OA, Stalla GK. Somatostatin analogs in ectopic corticotropin production. Metabolism. 1996;45(suppl 1):129–131. doi: 10.1016/s0026-0495(96)90107-9. [DOI] [PubMed] [Google Scholar]
- 33.Uwaifo GI, Koch CA, Hishberg B, et al. Is there a therapeutic role for octreotide in patients with ectopic Cushing's syndrome? J Endocrinol Invest. 2003;26:710–717. doi: 10.1007/BF03347351. [DOI] [PubMed] [Google Scholar]
Additional Reading
- 1.Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: A meta-analysis and review. Lancet Oncol. 2001;2:683–690. doi: 10.1016/S1470-2045(01)00558-7. [DOI] [PubMed] [Google Scholar]
- 2.Jethanamest D, Morris LG, Sikora AG, et al. Esthesioneuroblastoma: A population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg. 2007;133:276–280. doi: 10.1001/archotol.133.3.276. [DOI] [PubMed] [Google Scholar]
- 3.Ilias I, Torpy DJ, Pacak K, et al. Cushing's syndrome due to ectopic corticotrophin secretion: twenty years' experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90:4955–4962. doi: 10.1210/jc.2004-2527. [DOI] [PubMed] [Google Scholar]