This review summarizes the current knowledge on parathyroid carcinoma and describes new information on parathyroid carcinoma gene expression and operative management using intraoperative parathyroid hormone monitoring.
Keywords: Parathyroid carcinoma, Intraoperative PTH, Gene expression
Learning Objectives
After completing this course, the reader will be able to:
Discuss the association of parathyroid cancers with severe hypercalcemia and markedly elevated parathyroid hormone levels.
Describe the effect of the risk factors, clinical presentation, and gene expression on diagnosis of parathyroid cancers.
Analyze the probability of cure for parathyroid cancers with treatment by aggressive surgery with en bloc resection and lymph node dissection.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Parathyroid carcinoma is an indolent but ultimately life-threatening malignancy. Due to the lack of definitive diagnostic markers and overlapping clinical features of benign primary hyperparathyroidism (PHPT), this disease is often misdiagnosed as parathyroid adenoma. Therefore, a high index of suspicion preoperatively and early intraoperative recognition with en bloc surgical resection are crucial for favorable outcome. Owing to the rarity of the disease, little is known about the molecular pathogenesis of parathyroid carcinoma. Here, we review the literature to present current understanding of the disease and provide new information on gene expression and use of intraoperative parathyroid hormone (PTH) monitoring in the surgical management of this rare malignancy. Specifically, using microarray transcriptome analysis of an unequivocal case of parathyroid carcinoma and a biopsy from the same patient's normal parathyroid gland, we identify APP, CDH1, KCNJ16, and UCHL1 as differentially expressed genes in parathyroid carcinoma. Further, using case records from four cases of unequivocal parathyroid carcinoma, we compared intraoperative PTH kinetics of these patients to 475 patients with benign PHPT, and show that intraoperative PTH monitoring is accurate in predicting postoperative normocalcemia in initial en bloc operations for parathyroid carcinoma.
Introduction
Parathyroid carcinoma (PCA) is a rare malignant neoplasm derived from the parenchymal cells of the parathyroid glands. Patients with PCA typically have severe primary hyperparathyroidism (PHPT) with marked hypercalcemia (>14 mg/dl) and profoundly elevated (three to ten times normal) parathyroid hormone (PTH) levels [1]. These patients are more likely to have metabolic bone and renal sequelae than are those with benign parathyroid adenomas. They also may have local symptoms resulting from tumor growth. The mainstay of treatment for PCA is complete en bloc resection. Although gross appearance and/or histologic features of the parathyroid tumor may suggest the diagnosis of PCA, presently there is no histopathologic marker diagnostic for PCA. A definitive diagnosis of PCA currently relies upon demonstration of local invasion, lymph node metastasis, or distant metastasis. The rarity of the disease has contributed, at least in part, to the paucity of clinical and basic science knowledge of PCA [2]. This review summarizes the current knowledge of PCA and describes new information on PCA gene expression and operative management using intraoperative PTH monitoring.
Epidemiology and Risk Factors
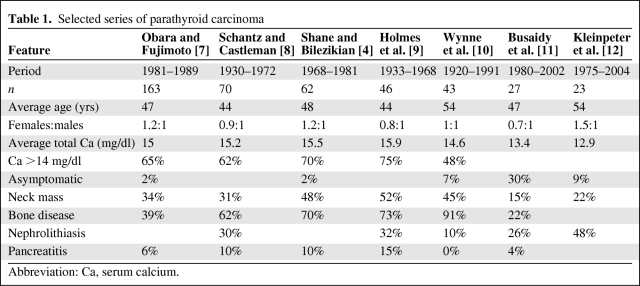
The estimated incidence of PCA is 5.73 per 10 million persons [3]. The incidence of carcinoma does not appear to differ among races. However, among patients with PHPT in the U.S., PCA occurs in 1% of cases. In two series from Japan and Italy, the published incidence was as high as 5% of all cases of PHPT [4–6]. It is unclear whether the variation in the reported incidence rate among countries/regions reflects true ethnic differences in disease susceptibility or varying diagnostic criteria. In multiple retrospective studies of PCA, the female-to-male ratio has been consistently observed as 1:1 (Table 1). This finding is in contrast to benign parathyroid tumors, in which a female-to-male ratio of 3–4:1 is reported [1]. The average age at presentation for patients with PCA is 48 years [4, 7–12], approximately 10 years earlier than for patients with parathyroid adenomas. PCA may rarely occur in children, in either a sporadic or familial fashion [13, 14].
Table 1.
Selected series of parathyroid carcinoma
Abbreviation: Ca, serum calcium.
Risk factors for PCA are largely unknown. However, membership in a kindred with either familial PCA or hyperparathyroidism-jaw tumor syndrome (HPT-JT) is a clear risk factor. HPT-JT is an autosomal dominant syndrome primarily characterized by hyperparathyroidism resulting from multigland parathyroid neoplasia with a high predisposition for parathyroid cancer development. Approximately 15% of patients with HPT-JT develop PCA. Limited data from case reports have also described a history of head and neck irradiation as a possible risk factor [15, 16]. PCA has been reported in several patients with secondary hyperparathyroidism (SHPT), suggesting that chronic stimulation of the parathyroid glands can result in malignant transformation of hyperplastic parathyroid cells [17, 18]. Asymmetric nodular parathyroid growth in SHPT supports the notion of clonal expansion of cells within regions of polyclonal parathyroid hyperplasia [19].
Natural History
Usually, PCA arises in the normal parathyroid gland locations within the central neck. Rarely, PCA develops in ectopic glands in mediastinal locations [20]. In the majority of cases, PCA arises in a single gland, but multiple carcinomas have been reported [21]. The clinical course of PCA is not readily predictable [22, 23]. PCA is often quite indolent, with recurrence occurring up to 15–20 years after the initial diagnosis [4]. PCA may infiltrate locally and/or metastasize distantly, with a specific predilection toward the thyroid and the lungs, respectively. Other organs can be involved with metastases, including the liver, bone, adrenals, and pancreas [22, 24]. The median survival time following first recurrence is 28 months [5]. The lethality of the disease is usually a result of the high level of serum calcium rather than local tumor growth.
Pathogenesis
As with benign parathyroid tumors, the pathogenesis of PCA is incompletely understood. Initial data by Cryns et al. [25–29] showed somatic loss of DNA at the Rb locus in PCA with decreased immunohistochemical staining of Rb protein in a subset of cases. Shattuck and colleagues detected no Rb mutations in six carcinoma specimens (from four patients) with documented 13q loss [30]. Allelic loss of p53 and abnormal p53 protein expression were also described by Cryns et al. [31] in a small number of PCA cases. Although PCA is not a feature of multiple endocrine neoplasia type 1, Haven et al. [32] identified a missense and two frameshift menin mutations in three of 23 sporadic PCA cases, suggesting that menin may be involved in the development or progression of PCA. Cyclin D1 is highly expressed in PCA. In a study by Vasef et al. [33], 91% (10 of 11) of the carcinoma specimens were positive for cyclin D1 overexpression, compared with 39% (11 of 28) of the adenoma specimens and 61% (11 of 18) of the hyperplasia cases.
It is possible that cyclin D1 overexpression in PCA is a reflection of increased proliferation as opposed to a direct pathogenetic contributor, as has been described in the PRAD1 translocation observed in some parathyroid adenomas. Alternatively, cyclin D1 overexpression may be a result of loss of parafibromin expression, because parafibromin has been shown to negatively regulate cyclin D1 expression [34].
Parafibromin (CDC73), a tumor suppressor encoded by the HRPT2 gene on chromosome 1q25, has been identified to play a central role in the development of familial PCA, sporadic nonfamilial PCA, and HPT-JT [30, 35, 36]. Inactivating germline mutations in HRPT2 have been proposed to contribute to the development of HPT-JT and sporadic PCA [36, 37]. Shattuck et al. [37] identified HRPT2 mutations (somatic and/or germline) in 10 of 15 patients with sporadic PCA. The mechanism by which loss of parafibromin leads to PCA is not fully elucidated. Overexpression of wild-type parafibromin in human HEK-293 cells inhibits proliferation via a reduction in cyclin D1 expression [34]. Parafibromin also can inhibit the c-myc proto-oncogene via the PAF1 transcriptional regulatory complex [38].
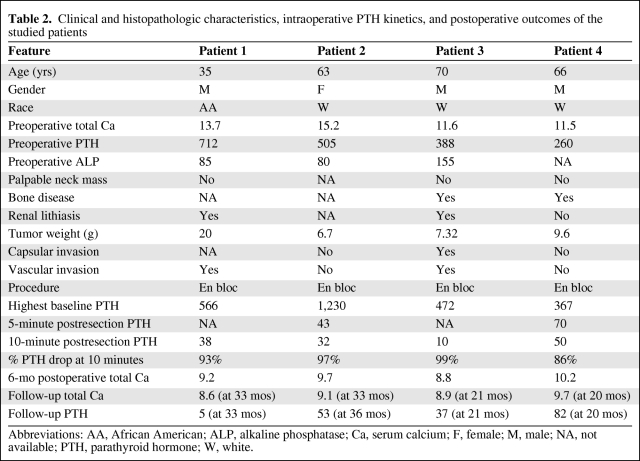
Microarray analysis of tumor transcriptomes is a powerful technology that is capable of assessing the expression levels of thousands of genes simultaneously in one sample. It has been used to explore the molecular mechanisms and the genetic etiologies of PHPT and SHPT [39, 40]. Forsberg et al. [41] used microarray analysis to compare gene expression in parathyroid adenomas and normal parathyroid glands. However, very limited studies have used microarray technology to analyze gene expression in PCA. Haven and colleagues investigated the expression profiles of 53 parathyroid tumors, including two PCA specimens. They identified three genes as potential markers for PCA, including histone 1 family 2, amyloid β precursor protein (APP), and E-cadherin [42]. Recently, we arrayed a PCA and a normal parathyroid gland (both from patient 1, Table 2) to examine gene expression in PCA.
Table 2.
Clinical and histopathologic characteristics, intraoperative PTH kinetics, and postoperative outcomes of the studied patients
Abbreviations: AA, African American; ALP, alkaline phosphatase; Ca, serum calcium; F, female; M, male; NA, not available; PTH, parathyroid hormone; W, white.
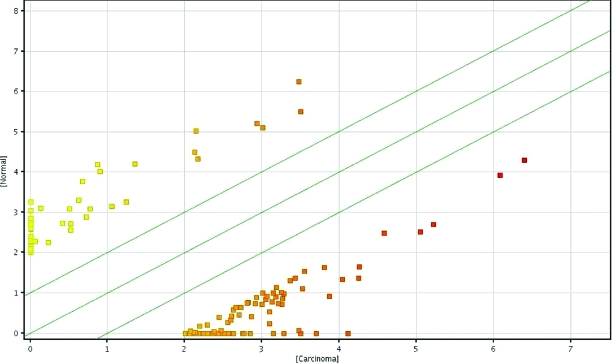
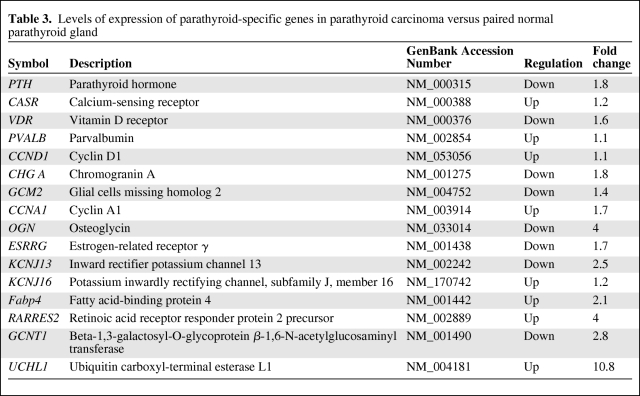
Fold change analyses were used to identify differentially expressed genes. In total, 1,561 probes were found to be differentially expressed (≥ twofold) between the patient's carcinoma sample and the normal parathyroid gland. Upon raising the fold change cutoff to four, 129 probes were identified to be differentially expressed in the carcinoma versus the normal sample. Of the 129 differentially expressed probes, 87 showed greater expression and 42 genes showed lower expression in the carcinoma sample (Fig. 1). Parathyroid-specific genes were compared between the carcinoma and the normal parathyroid (Table 3).
Figure 1.
Scatter plot depicting differentially expressed genes in parathyroid carcinoma versus paired normal parathyroid at fourfold change cutoff.
Table 3.
Levels of expression of parathyroid-specific genes in parathyroid carcinoma versus paired normal parathyroid gland
Interestingly, the PTH gene was highly expressed in the carcinoma sample (18th highest expressed), but the expression level was lower than in the normal gland. The calcium-sensing receptor (CASR) gene was upregulated (1.2-fold) and the vitamin D receptor gene was downregulated (1.6-fold) in the carcinoma sample. Cyclin D1 expression was low in both specimens. Notably, cyclin A1 (CCNA1), potassium inwardly rectifying channel, subfamily J, member 16 (KCNJ16), and ubiquitin carboxyl-terminal esterase L1 (UCHL1) were upregulated in the carcinoma sample compared with the normal parathyroid sample. UCHL1 encodes the protein gene product (PGP)9.5 protein, which has been found to be associated with loss of nuclear expression of parafibromin, a characteristic of PCA [43]. APP and E-cadherin (CDH1) were overexpressed in the carcinoma sample, consistent with findings reported by Haven et al. [42]. Genes underexpressed in the carcinoma sample compared with the normal gland included chromogranin A (CHG A), glial cells missing homolog 2 (GCM2), osteoglycin (OGN), and estrogen-receptor related gamma (ESRRG). Each of these downregulated genes (CHG A, GCM2, OGN, and ESRRG) is highly expressed in sporadic parathyroid adenoma (unpublished data). This unique gene expression data give an initial insight into potential diagnostic markers and deregulated gene expression in PCA; however, larger studies are needed to fully characterize PCA gene expression.
Clinical Presentation
Most patients with PCA present with severe hyperparathyroidism. The most common symptoms of PCA include thirst, polydipsia, anorexia, severe weakness and fatigability, constipation, renal colic, bone pain, and vague abdominal pain. The overlap in symptoms between PCA and benign HPT makes it very challenging to differentiate between parathyroid adenoma and carcinoma on clinical grounds. Unfortunately, PCA most often is misdiagnosed as benign PHPT [44].
In a series of 163 patients with PCA, a palpable neck mass was found in 34% of the patients [7]. This finding is very uncommon in primary HPT resulting from parathyroid adenoma and its presence along with a very high PTH level can be use as a differentiating feature of PCA [4, 10, 22]. In addition, unilateral vocal cord paralysis with hypercalcemia in a patient without a previous neck operation is very suspicious for PCA. Rarely, cases of nonfunctioning PCA have been reported to present with locally advanced neck masses [45, 46]. Table 1 summarizes the demographic and clinical characteristics of selected published series of PCA patients.
Diagnostic Evaluation
Laboratory Testing
PCA is characterized by very high levels of PTH, which ultimately result in severe hypercalcemia. The hypercalcemia is usually very profound, with serum calcium concentration >14 mg/dl [1, 4, 47]. Obara and Fujimoto reported that 65% of 133 patients with carcinoma had a serum calcium level >14 mg/dl, with a range of 10- 24 mg/dl [7]. This is in contrast to the hypercalcemia found in benign PHPT, which is usually within 1 mg/dl above the normal range. In addition, high alkaline phosphatase, hypophosphatemia, and hyperchloremic metabolic acidosis have also been observed in PCA patients [48, 49].
Imaging Studies
Ultrasonography is useful to localize parathyroid tumors, and may help to differentiate PCA from parathyroid adenoma. In a study of 16 patients with PCA and 61 patients with adenoma, Hara et al. [50] found that large size, inhomogeneous appearance, and irregular borders were ultrasonographic features of PCA. In contrast, benign adenomas were found to be smaller, homogeneous, and hypoechoic with smooth borders. Moreover, 94% of the 16 patients with PCA had a depth width (DW) ratio ≥1, compared with only 5% of the 61 patients with adenoma [50]. These imaging characteristics, though nonspecific, should alert the clinician to the possibility of PCA when evaluating patients with PHPT.
Technetium-99 (99Tc)-sestamibi scanning has been used successfully for preoperative localization of PCA [51]. However, it is an unreliable tool for differentiating between adenoma and carcinoma [52]. 99Tc-sestamibi is also a useful diagnostic tool to detect metastatic PCA [53].
Both parathyroid scintigraphy and computed tomography (CT) are useful for preoperative planning in patients with recurrent PCA. In a series of eight patients with recurrent PCA, Clark et al. [54] studied parathyroid scintigraphy and CT in detecting disease recurrences. 99Tc-sestamibi scintigraphy correctly identified 67% of the recurrences, compared with 53% for CT. Discordant results were noted in 78% of the cases, and the authors recommended both studies for preoperative planning.
Biopsy
Generally, biopsy (including fine-needle aspiration cytology [FNAC]) of PCA is unnecessary and should be avoided in resectable cases because disruption of the tumor capsule increases the possibility of tumor implantation [55, 56]. Biopsy of metastatic sites is reasonable to confirm recurrence and plan potential resection.
Treatment of Primary Disease
Surgery
Surgery is the only cure for PCA. Extensive surgical resection is the treatment of choice in patients with PCA limited to the neck. The recommended initial procedure is en bloc removal of the parathyroid cancer including ipsilateral thyroid lobectomy, isthmectomy, tracheal skeletonization, and excision of any adherent muscle [9]. Simple parathyroidectomy or subtotal resection is not recommended in cases of suspected PCA to avoid cancer spillage and eventual disease recurrence (parathyromatosis) [8]. En bloc resection has a survival advantage over simple parathyroidectomy. In a study by Koea and Shaw [57], patients treated with en bloc resection had an overall survival rate of 89% at a mean follow-up of 69 months, compared with 53% at a mean follow-up of 62 months for patients treated with simple parathyroidectomy. In addition, en bloc resection is associated with lower recurrence rates than parathyroidectomy alone. Obara and Fujimoto [7] reported that 70% of the patients who were recurrence free for >1 year had undergone en bloc resection as their initial procedure. Shortell et al. [58] also demonstrated the superiority of initial aggressive treatment over simple parathyroidectomy. Only one of the eight patients who underwent aggressive surgical treatment in that series developed recurrence. In contrast, all of the three patients who had simple parathyroidectomy developed recurrence. In a retrospective study of 13 patients, Wiseman et al. [59] found that all five patients who underwent simple local excision developed recurrent disease within 1–6 years, although they achieved normocalcemia in the immediate postoperative period. In contrast, only two of the five patients who underwent extensive en bloc resection developed recurrence [59].
Despite the apparent survival advantage of en bloc resection, a greater number of patients undergo simple parathyroidectomy as their initial procedure. Data from the Surveillance Epidemiology and End Results registry (1988–2003) show that only 12.5% (28 of 224) of PCA patients in the database underwent en bloc resection, as opposed to 78.6% (176 of 224) who underwent parathyroidectomy alone [3]. In another study of 23 patients, only 39% underwent initial en bloc resection [12]. This trend may be a reflection of the diagnostic challenges prior to initial surgery and it emphasizes the need for intraoperative recognition of PCA. Along with en bloc resection, bilateral neck exploration and identification of all four parathyroid glands is indicated, because PCA may coexist with multiple gland disease [60–62].
In order to identify patients at risk for PCA and appropriately employ en bloc resection, the diagnosis should be suspected based on the clinical presentation, with early recognition based on gross appearance during surgery. Macroscopically, surgeons should consider the possibility of PCA when certain gross features are noted during neck exploration. These include firm texture, thick grey or white capsule, and large tumor size [8, 47]. Adherence of the diseased gland to surrounding structures (trachea, esophagus, carotid sheath, and/or recurrent laryngeal nerve) is suggestive of PCA, but not definitive [47]. Adherence to adjacent structures was reported in 23% of 163 of cases of PCA published by Obara and Fujimoto [7], with the thyroid found to be the most commonly involved organ. This is in contrast to benign PHPT conditions, which are easily dissectible. Nevertheless, adherence to the surrounding tissues has been found, by some, in benign PHPT [63].
During neck exploration, effort must be made not to violate or rupture the capsule, which often leads to tumor spillage and eventual disease recurrence. Unfortunately, there is a high incidence of iatrogenic spillage that often leads to multiple operations and unfavorable outcomes [64], and it usually happens during the dissection of a presumed adenoma.
There has been disagreement concerning the management of an involved recurrent laryngeal nerve (RLN). Some authors call for the removal of a functioning involved nerve whereas others recommend dissection of the cancer off an involved nerve [1, 63, 65, 66]. Given the propensity for local recurrence with tumor violation during resection and the absence of effective adjuvant therapy for microscopic or gross residual disease, we prefer resection of the involved RLN if the tumor cannot be dissected from the nerve completely. This dissection is greatly facilitated in our experience with the recurrent laryngeal nerve monitor (Neurovision, Inc., Ventura, CA).
The role of cervical lymphadenectomy for PCA is controversial, with most reports supporting lymph node dissection in cases in which they are grossly enlarged, there is extensive local invasion, or local recurrence is present [2, 5, 7]. Holmes et al. [9] reported an incidence of 32% for cervical lymph node metastases in their study of 46 patients and advocated complete removal of the lymph nodes in the tracheoesophageal groove and ipsilateral radical neck dissection. Others have detected an extremely low rate (3%) of lymph node metastases and argued against lymphadenectomy [2, 5]. We routinely perform ipsilateral level VI lymphadenectomy in cases of suspected or known PCA.
PCA patients are at risk to develop hungry bone syndrome in the postoperative period because of the deposition of calcium and phosphate in the bones. An abnormally elevated preoperative alkaline phosphatase level can identify patients at risk for this syndrome. The symptoms may become severe and protracted, necessitating large doses of calcium [67, 68] and calcitriol.
Intraoperative PTH Monitoring
Intraoperative measurements of intact PTH have proven utility in limiting operation time and predicting cure rate in PHPT resulting from benign adenoma and hyperplasia [69, 70]. In cases of PHPT, appropriate decay ≥50% in the intraoperative PTH (ioPTH) level after resection of the offending gland accurately predicts postoperative normocalcemia.
Despite the multiplicity of publications supporting the use of ioPTH monitoring in PHPT and SHPT, very little information has been published on the utility of ioPTH monitoring in PCA. Solórzano et al. [71] described the ioPTH kinetics of their eight PCA patients. All of their patients treated with initial en bloc resections (five of five) achieved an ioPTH drop >50% from baseline and this predicted at least 6 months of postoperative normocalcemia. However, ioPTH was less accurate in predicting 6 months of postoperative normocalcemia in reoperative cases.
From a prospective database of 480 consecutive patients with PHPT treated surgically at Duke University Medical Center between 2000 and 2009, we identified four patients with unequivocal PCA. The diagnosis of PCA was made by histopathologic demonstration of local invasion and/or lymph node metastasis. Clinical characteristics of these patients were similar to those in reported series of PCA patients (Table 2).
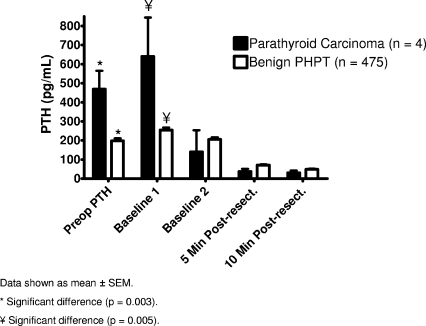
Our standard operative approach to PCA is a four-gland exploration and en bloc resection of carcinoma with confirmation of adequate resection of hyperfunctioning glands by ioPTH monitoring. ioPTH is measured using a rapid two-site chemiluminescent automated assay (Immulite Turbo PTH Assay; Diagnostic Products Corporation, Los Angeles, CA), as previously published [72]. All operations were initial en bloc resections and 100% (four of four) had normocalcemia in the 6-month postoperative period. The ioPTH results of the four studied patients are shown in Table 2. All patients achieved >50% reduction in ioPTH, with an average PTH drop of 94%. The preoperative PTH and the ioPTH baseline 1 were significantly (p < .05) higher in the carcinoma patients (n = 4) than in the benign PHPT patients (n = 475) (466 and 640 versus 197 and 255 pg/ml, respectively) (Fig. 2). However, the percent PTH drop (from the highest baseline) at 10 minutes postresection was not different between the carcinoma and the benign PHPT patients (94 ± 6 versus 82 ± 16; p = .2). None of the patients had recurred after a follow-up interval of 20–36 months.
Figure 2.
Pre- and intraoperative parathyroid hormone (PTH) kinetics in benign and malignant primary hyperparathyroidism (PHPT).
Abbreviation: SEM, standard error of the mean.
Based on these limited data, ioPTH monitoring is accurate in predicting postoperative normocalcemia in initial en bloc resected patients, and we advocate its use in the surgical treatment of PCA.
Pathologic Diagnosis
Because it is difficult to establish the diagnosis of PCA by virtue of the clinical profile, it can also be challenging to confirm the diagnosis by histopathologic examination. The diagnosis of PCA relies upon findings of microscopic infiltrative growth pattern and/or the presence of metastasis. In a large series of 92 patients with PCA, only 45% of the patients had these two criteria [5]; this necessitates the need for more sensitive but accurate diagnostic criteria. To date, the most commonly accepted diagnostic criteria were proposed by Schantz and Castleman [8], and include the presence of fibrous trabeculae, mitotic figures, and capsular and blood vessel invasions. Despite the widespread acceptance of these criteria, some authors have debated their practical applicability, because these features are not found consistently [23], and each of these features can occasionally be seen in parathyroid adenomas, with the exception of capsular and vascular invasion [73]. As suggested by others, when these criteria are not met or when there are equivocal findings, we advocate close follow-up with calcium, PTH, and ultrasound monitoring for disease recurrence [24, 63, 74].
Immunohistochemistry
Given the high incidence of HRPT2 mutations (70%) in sporadic PCA cases and their rarity in benign PHPT, it has been suggested that HRPT2 mutations can be used to diagnose PCA [43, 75]. Mutations in HRPT2 result in lack of nuclear staining for the HRPT2 protein, parafibromin, which may be used as a surrogate for HRPT2 mutations. Indeed, emerging data suggest that, in the appropriate clinical and pathological context, complete absence of parafibromin staining in a parathyroid tumor is diagnostic of PCA or HPT-JT tumor [43, 75]. Expression of PGP9.5 correlates well with loss of nuclear expression of parafibromin in PCA, and PGP9.5 immunohistochemistry was recently introduced to assist in diagnosing PCA. PGP9.5 is a protein encoded by the UCHL1 gene family, which was found to be highly expressed in PCA/HPT-JT–related tumors as well as several other cancers [42, 43]. Howell et al. [43] demonstrated that PGP9.5 staining has superior sensitivity and specificity to the lack of parafibromin staining. In addition, positive parafibromin staining does not exclude HRPT2 mutation because some tumors with HRPT2 missense mutations may show weak parafibromin expression [75], which may explain the relatively poor sensitivity of parafibromin staining. Given the slightly superior sensitivity of PGP9.5 staining and its ability to detect the missense HRPT2 mutation (L64P), it has been advocated to use PGP9.5 staining in addition to parafibromin staining to screen for HRPT2 mutation [43] and PCA.
Treatment of Persistent/Recurrent Disease
Surgery
The neck is the most common site for local recurrence of PCA. For isolated cervical recurrence of PCA, en bloc resection of the tumor and involved structures as well as regional lymphadenectomy is recommended. The lungs are the most common site of distant metastasis; Obara et al. [76] concluded that aggressive excision of pulmonary metastases is associated with favorable outcomes and long-term survival. Resection of PCA metastases may also improve PTH levels and provide effective palliation of hypercalcemia.
When the diagnosis of PCA is made postoperatively, thorough evaluation of the metabolic indices, pathologic results, and operative reports is warranted to determine the adequacy of the initial resection. In our experience, PCA is diagnosed intraoperatively and adequate resection is undertaken. In cases in which PCA is recognized postoperatively, we base reoperative surgery on postoperative PTH levels. Incomplete resection can cause disease recurrence, and 8% of these recurrences occur after en bloc resection, versus 51% after simple parathyroidectomy [57].
Calcimimetics
Surgical resection is the most effective method of controlling hypercalcemia resulting from PCA. Nonetheless, in some patients, hypercalcemia may be refractory to surgery, and these patients require other treatment options [77].
Recently, the U.S. Food and Drug Administration approved cinacalcet HCl (cinacalcet) to control symptomatic hypercalcemia in PCA patients. Calcimimetics are therapeutic agents that suppress the secretion of PTH by increasing the sensitivity of the calcium-sensing receptor.
Given that hypercalcemia is the main cause of morbidity and mortality in PCA patients, reducing calcium levels with calcimimetics can conceivably improve clinical outcome. In a multicountry, open-label, single-arm, dose titration study of 29 patients with PCA, cinacalcet effectively reduced the level of calcium in 18 of the 29 patients. In the responders, serum calcium fell at least 1 mg/dl (from 15 ± 0.5 to 11 ± 0.3 mg/dl) [78]. Adverse effects are mild and include nausea and vomiting, but they can lead to the discontinuation of treatment in some patients. In addition to cinacalcet, others have tried another calcimimetic, R-568, in a patient with refractory hypercalcemia. The calcium level dropped in a dose-dependent manner with symptomatic improvement [79].
Radiotherapy
PCA is not a radiosensitive neoplasm [9]. In most patients studied, radiotherapy failed to slow tumor growth and reduce hormonal secretion [80]. However, there are a few reports of success using palliative radiation as adjuvant therapy. In a large series of 27 patients with a minimum follow-up of 2 years, six patients received adjuvant radiotherapy after initial surgery. Only one of the six patients who received radiotherapy developed a relapse, compared with 10 of the 20 patients who did not receive adjuvant radiotherapy [11].
Chemotherapy
Cytotoxic chemotherapy is an ineffective approach to treating PCA. A combination of doxorubicin, cyclophosphamide, and 5-fluorouracil did not achieve success in two patients with unresectable metastatic carcinoma [60]. Others have used dacarbazine, cyclophosphamide, 5-fluorouracil, and vincristine, but these agents failed to correct hypercalcemia [76]. However, there have been very rare reports of success. Calandra and colleagues reported a patient with recurrent disease in whom dacarbazine produced a marked drop in serum calcium [81].
Prognosis
Recurrence of PCA occurs about 30%–50% of the time, even after extensive surgical resection [5, 8]. The average time between initial surgery and first recurrence is 3 years, and once it occurs cure is rare [1]. Recurrence within 2 years carries a poor prognosis [8]. The overall 5-year survival rate is 45%–85%. According to several studies, the 10-year survival rate is in the range of 50%–70% [2, 82].
There are neither reliable prognostic parameters nor a tumor–node-metastasis staging system for PCA. However, one group of investigators found several factors, including age, type of initial surgery, and histopathology, as the most predictive of mortality in their series of 95 patients [5]. Others have used nuclear DNA content as a prognostic indicator, and demonstrated its correlation with aggressive disease [23]. Moreover, aneuploidy has been associated with poor prognosis [83, 84]. In addition, increased mitotic activity in unequivocal PCA is an indicator of poor prognosis [85]. Interestingly, Rubin and others reported and demonstrated a prognostic and diagnostic value for the malignant hyperglycosylated isoform of urinary human chorionic gonadotropin [86].
Follow-Up
Long-term follow-up is recommended, because recurrence can happen up to 15 years after surgery [22]. More specifically, close follow-up is required during the first 2 years from initial surgery because of the higher rate of recurrence during this period. If postoperative hypercalcemia is found, disease recurrence should be suspected and thoroughly investigated with extensive localization studies to assess the adequacy of the initial surgery. Recurrent disease in the neck and isolated distant metastases can be resected with palliative intent to alleviate symptoms of PTH excess [7]. Unresectable disease is best treated with calcimimetics.
Summary and Conclusions
PCA is a rare but life-threatening tumor that represents 1% of PHPT cases. The clinical manifestations are usually a reflection of the severe PTH-associated hypercalcemia rather than a tumor mass effect. Frequently, PCA presents as a severe form of PHPT, and it can be very challenging to establish the diagnosis by virtue of clinical presentation alone. Severe hypercalcemia (>14 mg/dl) with high PTH levels in a male patient with palpable neck mass is concerning for PCA. Ultrasonographic signs of PCA include heterogeneous parathyroid mass with irregular contours and DW >1. FNAC should be avoided in cases suspicious for PCA, because there have been reports of carcinoma implantation resulting from FNAC. Intraoperative recognition of PCA is critical to avoid tumor spillage and to allow en bloc resection of the tumor and involved structures. At surgery, a large, white, hard mass rather than the soft consistency of parathyroid adenoma may be indicative of carcinoma. Aggressive en bloc resection along with ipsilateral thyroid lobectomy and ipsilateral paratracheal lymphadenectomy is the best initial treatment. Great care should be taken not to violate the capsule during surgical dissection, because seeding of the cancer has been reported. During surgery for benign PHPT, if a suspicious cancerous gland is found, it should not be biopsied in vivo, because this may lead to cancer spillage and eventual disease recurrence. Intraoperative PTH monitoring is useful in guiding the operation and predicting normocalcemia 6 months postoperatively. The histologic diagnosis of PCA can be challenging; however, the presence of capsular and/or vascular invasions can be definitive. Parafibromin and PGP9.5 staining are of diagnostic value in PCA. Close follow-up is highly recommended, especially during the first 2-year window from operation. Metastasectomy is of palliative benefit in those with recurrent disease. Disease recurrence within 2 years is indicative of poor prognosis. The overall 5-year survival rate is 45%–85% and the 10-year survival rate is in the range of 50%–70%.
Author Contributions
Conception/Design: Mohamed Abdelgadir Adam, John Olson
Financial support: John Olson
Provision of study material or patients: John Olson
Collection and/or assembly of data: John Olson
Data analysis and interpretation: Mohamed Abdelgadir Adam, Brian Untch, John Olson
Manuscript writing: Mohamed Abdelgadir Adam, Brian Untch, John Olson
Final approval of manuscript: Mohamed Abdelgadir Adam, Brian Untch, John Olson
References
- 1.Shane E. Clinical review 122: Parathyroid carcinoma. J Clin Endocrinol Metab. 2001;86:485–493. doi: 10.1210/jcem.86.2.7207. [DOI] [PubMed] [Google Scholar]
- 2.Hundahl SA, Fleming ID, Fremgen AM, et al. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985–1995: A National Cancer Data Base Report. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1999;86:538–544. doi: 10.1002/(sici)1097-0142(19990801)86:3<538::aid-cncr25>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 3.Lee PK, Jarosek SL, Virnig BA, et al. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer. 2007;109:1736–1741. doi: 10.1002/cncr.22599. [DOI] [PubMed] [Google Scholar]
- 4.Shane E, Bilezikian JP. Parathyroid carcinoma: A review of 62 patients. Endocr Rev. 1982;3:218–226. doi: 10.1210/edrv-3-2-218. [DOI] [PubMed] [Google Scholar]
- 5.Sandelin K, Auer G, Bondeson L, et al. Prognostic factors in parathyroid cancer: A review of 95 cases. World J Surg. 1992;16:724–731. doi: 10.1007/BF02067369. [DOI] [PubMed] [Google Scholar]
- 6.Favia G, Lumachi F, Polistina F, et al. Parathyroid carcinoma: Sixteen new cases and suggestions for correct management. World J Surg. 1998;22:1225–1230. doi: 10.1007/s002689900549. [DOI] [PubMed] [Google Scholar]
- 7.Obara T, Fujimoto Y. Diagnosis and treatment of patients with parathyroid carcinoma: An update and review. World J Surg. 1991;15:738–744. doi: 10.1007/BF01665308. [DOI] [PubMed] [Google Scholar]
- 8.Schantz A, Castleman B. Parathyroid carcinoma. A study of 70 cases. Cancer. 1973;31:600–605. doi: 10.1002/1097-0142(197303)31:3<600::aid-cncr2820310316>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Holmes EC, Morton DL, Ketcham AS. Parathyroid carcinoma: A collective review. Ann Surg. 1969;169:631–640. doi: 10.1097/00000658-196904000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wynne AG, van Heerden J, Carney JA, et al. Parathyroid carcinoma: Clinical and pathologic features in 43 patients. Medicine (Baltimore) 1992;71:197–205. [PubMed] [Google Scholar]
- 11.Busaidy NL, Jimenez C, Habra MA, et al. Parathyroid carcinoma: A 22-year experience. Head Neck. 2004;26:716–726. doi: 10.1002/hed.20049. [DOI] [PubMed] [Google Scholar]
- 12.Kleinpeter KP, Lovato JF, Clark PB, et al. Is parathyroid carcinoma indeed a lethal disease? Ann Surg Oncol. 2005;12:260–266. doi: 10.1245/ASO.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 13.Hamill J, Maoate K, Beasley SW, et al. Familial parathyroid carcinoma in a child. J Paediatr Child Health. 2002;38:314–317. doi: 10.1046/j.1440-1754.2002.00802.x. [DOI] [PubMed] [Google Scholar]
- 14.Righi A, Dimosthenous K, Mize J. Mediastinal parathyroid carcinoma with tumor implants in a child: A unique occurrence. Int J Surg Pathol. 2008;16:458–460. doi: 10.1177/1066896908315821. [DOI] [PubMed] [Google Scholar]
- 15.Mashburn MA, Chonkich GD, Chase DR, et al. Parathyroid carcinoma: Two new cases—diagnosis, therapy, and treatment. Laryngoscope. 1987;97:215–218. doi: 10.1288/00005537-198702000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Ireland JP, Fleming SJ, Levison DA, et al. Parathyroid carcinoma associated with chronic renal failure and previous radiotherapy to the neck. J Clin Pathol. 1985;38:1114–1118. doi: 10.1136/jcp.38.10.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miki H, Sumitomo M, Inoue H, et al. Parathyroid carcinoma in patients with chronic renal failure on maintenance hemodialysis. Surgery. 1996;120:897–901. doi: 10.1016/s0039-6060(96)80101-2. [DOI] [PubMed] [Google Scholar]
- 18.Tominaga Y, Numano M, Uchida K, et al. Lung metastasis from parathyroid carcinoma causing recurrent renal hyperparathyroidism in a hemodialysis patient: Report of a case. Surg Today. 1995;25:984–986. doi: 10.1007/BF00312388. [DOI] [PubMed] [Google Scholar]
- 19.Tominaga Y, Takagi H. Molecular genetics of hyperparathyroid disease. Curr Opin Nephrol Hypertens. 1996;5:336–341. doi: 10.1097/00041552-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Kelly MD, Sheridan BF, Farnsworth AE, et al. Parathyroid carcinoma in a mediastinal sixth parathyroid gland. Aust N Z J Surg. 1994;64:446–449. doi: 10.1111/j.1445-2197.1994.tb02251.x. [DOI] [PubMed] [Google Scholar]
- 21.Kameyama K, Takami H. Double parathyroid carcinoma. Endocr J. 2003;50:477–479. doi: 10.1507/endocrj.50.477. [DOI] [PubMed] [Google Scholar]
- 22.Wang CA, Gaz RD. Natural history of parathyroid carcinoma. Diagnosis, treatment, and results. Am J Surg. 1985;149:522–527. doi: 10.1016/s0002-9610(85)80050-7. [DOI] [PubMed] [Google Scholar]
- 23.Sandelin K, Tullgren O, Farnebo LO. Clinical course of metastatic parathyroid cancer. World J Surg. 1994;18:594–598. doi: 10.1007/BF00353773. discussion 599. [DOI] [PubMed] [Google Scholar]
- 24.Sandelin K, Thompson NW, Bondeson L. Metastatic parathyroid carcinoma: Dilemmas in management. Surgery. 1991;110:978–986. discussion 986–988. [PubMed] [Google Scholar]
- 25.Cryns VL, Thor A, Xu HJ, et al. Loss of the retinoblastoma tumor-suppressor gene in parathyroid carcinoma. N Engl J Med. 1994;330:757–761. doi: 10.1056/NEJM199403173301105. [DOI] [PubMed] [Google Scholar]
- 26.Dotzenrath C, Teh BT, Farnebo F, et al. Allelic loss of the retinoblastoma tumor suppressor gene: A marker for aggressive parathyroid tumors? J Clin Endocrinol Metab. 1996;81:3194–3196. doi: 10.1210/jcem.81.9.8784068. [DOI] [PubMed] [Google Scholar]
- 27.Pearce SH, Trump D, Wooding C, et al. Loss of heterozygosity studies at the retinoblastoma and breast cancer susceptibility (BRCA2) loci in pituitary, parathyroid, pancreatic and carcinoid tumours. Clin Endocrinol (Oxf) 1996;45:195–200. doi: 10.1046/j.1365-2265.1996.d01-1561.x. [DOI] [PubMed] [Google Scholar]
- 28.Subramaniam P, Wilkinson S, Shepherd JJ. Inactivation of retinoblastoma gene in malignant parathyroid growths: A candidate genetic trigger? Aust N Z J Surg. 1995;65:714–716. doi: 10.1111/j.1445-2197.1995.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 29.Farnebo F, Auer G, Farnebo LO, et al. Evaluation of retinoblastoma and Ki-67 immunostaining as diagnostic markers of benign and malignant parathyroid disease. World J Surg. 1999;23:68–74. doi: 10.1007/s002689900567. [DOI] [PubMed] [Google Scholar]
- 30.Shattuck TM, Kim TS, Costa J, et al. Mutational analyses of RB and BRCA2 as candidate tumour suppressor genes in parathyroid carcinoma. Clin Endocrinol (Oxf) 2003;59:180–189. doi: 10.1046/j.1365-2265.2003.01814.x. [DOI] [PubMed] [Google Scholar]
- 31.Cryns VL, Rubio MP, Thor AD, et al. p53 abnormalities in human parathyroid carcinoma. J Clin Endocrinol Metab. 1994;78:1320–1324. doi: 10.1210/jcem.78.6.8200932. [DOI] [PubMed] [Google Scholar]
- 32.Haven CJ, van Puijenbroek M, Tan MH, et al. Identification of MEN1 and HRPT2 somatic mutations in paraffin-embedded (sporadic) parathyroid carcinomas. Clin Endocrinol (Oxf) 2007;67:370–376. doi: 10.1111/j.1365-2265.2007.02894.x. [DOI] [PubMed] [Google Scholar]
- 33.Vasef MA, Brynes RK, Sturm M, et al. Expression of cyclin D1 in parathyroid carcinomas, adenomas, and hyperplasias: A paraffin immunohistochemical study. Mod Pathol. 1999;12:412–416. [PubMed] [Google Scholar]
- 34.Woodard GE, Lin L, Zhang JH, et al. Parafibromin, product of the hyperparathyroidism-jaw tumor syndrome gene HRPT2, regulates cyclin D1/PRAD1 expression. Oncogene. 2005;24:1272–1276. doi: 10.1038/sj.onc.1208274. [DOI] [PubMed] [Google Scholar]
- 35.Howell VM, Haven CJ, Kahnoski K, et al. HRPT2 mutations are associated with malignancy in sporadic parathyroid tumours. J Med Genet. 2003;40:657–663. doi: 10.1136/jmg.40.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpten JD, Robbins CM, Villablanca A, et al. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet. 2002;32:676–680. doi: 10.1038/ng1048. [DOI] [PubMed] [Google Scholar]
- 37.Shattuck TM, Välimäki S, Obara T, et al. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med. 2003;349:1722–1729. doi: 10.1056/NEJMoa031237. [DOI] [PubMed] [Google Scholar]
- 38.Lin L, Zhang JH, Panicker LM, et al. The parafibromin tumor suppressor protein inhibits cell proliferation by repression of the c-myc proto-oncogene. Proc Natl Acad Sci U S A. 2008;105:17420–17425. doi: 10.1073/pnas.0710725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakajima K, Umino K, Azuma Y, et al. Stimulating parathyroid cell proliferation and PTH release with phosphate in organ cultures obtained from patients with primary and secondary hyperparathyroidism for a prolonged period. J Bone Miner Metab. 2009;27:224–233. doi: 10.1007/s00774-008-0032-8. [DOI] [PubMed] [Google Scholar]
- 40.Velázquez-Fernández D, Laurell C, Saqui-Salces M, et al. Differential RNA expression profile by cDNA microarray in sporadic primary hyperparathyroidism (pHPT): Primary parathyroid hyperplasia versus adenoma. World J Surg. 2006;30:705–713. doi: 10.1007/s00268-005-0708-3. [DOI] [PubMed] [Google Scholar]
- 41.Forsberg L, Bjorck E, Hashemi J, et al. Distinction in gene expression profiles demonstrated in parathyroid adenomas by high-density oligoarray technology. Eur J Endocrinol. 2005;152:459–470. doi: 10.1530/eje.1.01864. [DOI] [PubMed] [Google Scholar]
- 42.Haven CJ, Howell VM, Eilers PH, et al. Gene expression of parathyroid tumors: Molecular subclassification and identification of the potential malignant phenotype. Cancer Res. 2004;64:7405–7411. doi: 10.1158/0008-5472.CAN-04-2063. [DOI] [PubMed] [Google Scholar]
- 43.Howell VM, Gill A, Clarkson A, et al. Accuracy of combined protein gene product 9.5 and parafibromin markers for immunohistochemical diagnosis of parathyroid carcinoma. J Clin Endocrinol Metab. 2009;94:434–441. doi: 10.1210/jc.2008-1740. [DOI] [PubMed] [Google Scholar]
- 44.Schoretsanitis G, Daskalakis M, Melissas J, et al. Parathyroid carcinoma: Clinical presentation and management. Am J Otolaryngol. 2009;30:277–280. doi: 10.1016/j.amjoto.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Klink BK, Karulf RE, Maimon WN, et al. Nonfunctioning parathyroid carcinoma. Am Surg. 1991;57:463–467. [PubMed] [Google Scholar]
- 46.Aldinger KA, Hickey RC, Ibanez ML, et al. Parathyroid carcinoma: A clinical study of seven cases of functioning and two cases of nonfunctioning parathyroid cancer. Cancer. 1982;49:388–397. doi: 10.1002/1097-0142(19820115)49:2<388::aid-cncr2820490230>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 47.Fujimoto Y, Obara T. How to recognize and treat parathyroid carcinoma. Surg Clin North Am. 1987;67:343–357. doi: 10.1016/s0039-6109(16)44188-5. [DOI] [PubMed] [Google Scholar]
- 48.Silverberg SJ, Shane E, Jacobs TP, et al. Nephrolithiasis and bone involvement in primary hyperparathyroidism. Am J Med. 1990;89:327–334. doi: 10.1016/0002-9343(90)90346-f. [DOI] [PubMed] [Google Scholar]
- 49.Laks MS, Kahn SE, Favus MJ, et al. Case report: Clinical pathological correlations in a case of primary parathyroid carcinoma. Ann Clin Lab Sci. 1984;14:458–463. [PubMed] [Google Scholar]
- 50.Hara H, Igarashi A, Yano Y, et al. Ultrasonographic features of parathyroid carcinoma. Endocr J. 2001;48:213–217. doi: 10.1507/endocrj.48.213. [DOI] [PubMed] [Google Scholar]
- 51.Singhal T, Jacobs M, Mantil JC. Tc-99m pertechnetate/sestamibi subtraction scan in a case of parathyroid carcinoma. Clin Nucl Med. 2008;33:196–197. doi: 10.1097/RLU.0b013e318162dd4d. [DOI] [PubMed] [Google Scholar]
- 52.Aigner RM, Fueger GF, Lax S. A case of parathyroid carcinoma visualized on Tc-99m-sestamibi scintigraphy. Nuklearmedizin. 1997;36:256–258. [PubMed] [Google Scholar]
- 53.Al-Sobhi S, Ashari LH, Ingemansson S. Detection of metastatic parathyroid carcinoma with Tc-99m sestamibi imaging. Clin Nucl Med. 1999;24:21–23. doi: 10.1097/00003072-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Clark P, Wooldridge T, Kleinpeter K, et al. Providing optimal preoperative localization for recurrent parathyroid carcinoma: A combined parathyroid scintigraphy and computed tomography approach. Clin Nucl Med. 2004;29:681–684. doi: 10.1097/00003072-200411000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal G, Dhingra S, Mishra SK, et al. Implantation of parathyroid carcinoma along fine needle aspiration track. Langenbecks Arch Surg. 2006;391:623–626. doi: 10.1007/s00423-006-0095-8. [DOI] [PubMed] [Google Scholar]
- 56.Spinelli C, Bonadio AG, Berti P, et al. Cutaneous spreading of parathyroid carcinoma after fine needle aspiration cytology. J Endocrinol Invest. 2000;23:255–257. doi: 10.1007/BF03343718. [DOI] [PubMed] [Google Scholar]
- 57.Koea JB, Shaw JH. Parathyroid cancer: Biology and management. Surg Oncol. 1999;8:155–165. doi: 10.1016/s0960-7404(99)00037-7. [DOI] [PubMed] [Google Scholar]
- 58.Shortell CK, Andrus CH, Phillips CE, Jr, et al. Carcinoma of the parathyroid gland: A 30-year experience. Surgery. 1991;110:704–708. [PubMed] [Google Scholar]
- 59.Wiseman SM, Rigual NR, Hicks WL, Jr, et al. Parathyroid carcinoma: A multicenter review of clinicopathologic features and treatment outcomes. Ear Nose Throat J. 2004;83:491–494. [PubMed] [Google Scholar]
- 60.Anderson BJ, Samaan NA, Vassilopoulou-Sellin R, et al. Parathyroid carcinoma: Features and difficulties in diagnosis and management. Surgery. 1983;94:906–915. [PubMed] [Google Scholar]
- 61.Berland Y, Olmer M, Lebreuil G, et al. Parathyroid carcinoma, adenoma and hyperplasia in a case of chronic renal insufficiency on dialysis. Clin Nephrol. 1982;18:154–158. [PubMed] [Google Scholar]
- 62.Boyle NH, Ogg CS, Hartley RB, et al. Parathyroid carcinoma secondary to prolonged hyperplasia in chronic renal failure and in coeliac disease. Eur J Surg Oncol. 1999;25:100–103. [PubMed] [Google Scholar]
- 63.Levin KE, Galante M, Clark OH. Parathyroid carcinoma versus parathyroid adenoma in patients with profound hypercalcemia. Surgery. 1987;101:649–660. [PubMed] [Google Scholar]
- 64.Rattner DW, Marrone GC, Kasdon E, et al. Recurrent hyperparathyroidism due to implantation of parathyroid tissue. Am J Surg. 1985;149:745–748. doi: 10.1016/s0002-9610(85)80178-1. [DOI] [PubMed] [Google Scholar]
- 65.Clayman GL, Gonzalez HE, El-Naggar A, et al. Parathyroid carcinoma: Evaluation and interdisciplinary management. Cancer. 2004;100:900–905. doi: 10.1002/cncr.20089. [DOI] [PubMed] [Google Scholar]
- 66.Vetto JT, Brennan MF, Woodruf J, et al. Parathyroid carcinoma: Diagnosis and clinical history. Surgery. 1993;114:882–892. [PubMed] [Google Scholar]
- 67.Rathi MS, Ajjan R, Orme SM. A case of parathyroid carcinoma with severe hungry bone syndrome and review of literature. Exp Clin Endocrinol Diabetes. 2008;116:487–490. doi: 10.1055/s-2007-992155. [DOI] [PubMed] [Google Scholar]
- 68.Chandran M, Deftos LJ, Stuenkel CA, et al. Thymic parathyroid carcinoma and postoperative hungry bone syndrome. Endocr Pract. 2003;9:152–156. doi: 10.4158/EP.9.2.152. [DOI] [PubMed] [Google Scholar]
- 69.Irvin GL, 3rd, Solorzano CC, Carneiro DM. Quick intraoperative parathyroid hormone assay: Surgical adjunct to allow limited parathyroidectomy, improve success rate, and predict outcome. World J Surg. 2004;28:1287–1292. doi: 10.1007/s00268-004-7708-6. [DOI] [PubMed] [Google Scholar]
- 70.Clary BM, Garner SC, Leight GS., Jr Intraoperative parathyroid hormone monitoring during parathyroidectomy for secondary hyperparathyroidism. Surgery. 1997;122:1034–1038. doi: 10.1016/s0039-6060(97)90206-3. discussion 1038–1039. [DOI] [PubMed] [Google Scholar]
- 71.Solórzano CC, Carneiro-Pla DM, Lew JI, et al. Intra-operative parathyroid hormone monitoring in patients with parathyroid cancer. Ann Surg Oncol. 2007;14:3216–3222. doi: 10.1245/s10434-007-9590-6. [DOI] [PubMed] [Google Scholar]
- 72.Untch BR, Barfield ME, Dar M, et al. Impact of 25-hydroxyvitamin D deficiency on perioperative parathyroid hormone kinetics and results in patients with primary hyperparathyroidism. Surgery. 2007;142:1022–1026. doi: 10.1016/j.surg.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 73.Kameyama K, Takami H. Proposal for the histological classification of parathyroid carcinoma. Endocr Pathol. 2005;16:49–52. doi: 10.1385/ep:16:1:049. [DOI] [PubMed] [Google Scholar]
- 74.Fujimoto Y, Obara T, Ito Y, et al. Surgical treatment of ten cases of parathyroid carcinoma: Importance of an initial en bloc tumor resection. World J Surg. 1984;8:392–400. doi: 10.1007/BF01655086. [DOI] [PubMed] [Google Scholar]
- 75.Gill AJ, Clarkson A, Gimm O, et al. Loss of nuclear expression of parafibromin distinguishes parathyroid carcinomas and hyperparathyroidism-jaw tumor (HPT-JT) syndrome-related adenomas from sporadic parathyroid adenomas and hyperplasias. Am J Surg Pathol. 2006;30:1140–1149. doi: 10.1097/01.pas.0000209827.39477.4f. [DOI] [PubMed] [Google Scholar]
- 76.Obara T, Okamoto T, Ito Y, et al. Surgical and medical management of patients with pulmonary metastasis from parathyroid carcinoma. Surgery. 1993;114:1040–1048. discussion 1048–1049. [PubMed] [Google Scholar]
- 77.Hoelting T, Weber T, Werner J, et al. Surgical treatment of parathyroid carcinoma (review) Oncol Rep. 2001;8:931–934. doi: 10.3892/or.8.4.931. [DOI] [PubMed] [Google Scholar]
- 78.Silverberg SJ, Rubin MR, Faiman C, et al. Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocrinol Metab. 2007;92:3803–3808. doi: 10.1210/jc.2007-0585. [DOI] [PubMed] [Google Scholar]
- 79.Collins MT, Skarulis MC, Bilezikian JP, et al. Treatment of hypercalcemia secondary to parathyroid carcinoma with a novel calcimimetic agent. J Clin Endocrinol Metab. 1998;83:1083–1088. doi: 10.1210/jcem.83.4.4726. [DOI] [PubMed] [Google Scholar]
- 80.Cohn K, Silverman M, Corrado J, et al. Parathyroid carcinoma: The Lahey Clinic experience. Surgery. 1985;98:1095–1100. [PubMed] [Google Scholar]
- 81.Calandra DB, Chejfec G, Foy BK, et al. Parathyroid carcinoma: Biochemical and pathologic response to DTIC. Surgery. 1984;96:1132–1137. [PubMed] [Google Scholar]
- 82.Yip L, Seethala RR, Nikiforova MN, et al. Loss of heterozygosity of selected tumor suppressor genes in parathyroid carcinoma. Surgery. 2008;144:949–955. doi: 10.1016/j.surg.2008.08.030. discussion 954–955. [DOI] [PubMed] [Google Scholar]
- 83.Obara T, Fujimoto Y, Kanaji Y, et al. Flow cytometric DNA analysis of parathyroid tumors. Implication of aneuploidy for pathologic and biologic classification. Cancer. 1990;66:1555–1562. doi: 10.1002/1097-0142(19901001)66:7<1555::aid-cncr2820660721>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 84.Obara T, Fujimoto Y, Hirayama A, et al. Flow cytometric DNA analysis of parathyroid tumors with special reference to its diagnostic and prognostic value in parathyroid carcinoma. Cancer. 1990;65:1789–1793. doi: 10.1002/1097-0142(19900415)65:8<1789::aid-cncr2820650820>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 85.Bondeson L, Sandelin K, Grimelius L. Histopathological variables and DNA cytometry in parathyroid carcinoma. Am J Surg Pathol. 1993;17:820–829. doi: 10.1097/00000478-199308000-00007. [DOI] [PubMed] [Google Scholar]
- 86.Rubin MR, Bilezikian JP, Birken S, et al. Human chorionic gonadotropin measurements in parathyroid carcinoma. Eur J Endocrinol. 2008;159:469–474. doi: 10.1530/EJE-08-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]