The article investigates the effects of MBL2 genotypes on irinotecan-induced febrile neutropenia in patients with solid tumors. Patients with high MBL2 promoter genotypes and haplotypes seemed more at risk for developing febrile neutropenia.
Keywords: Irinotecan, MBL, Polymorphisms, Genotypes, Toxicity, Febrile neutropenia
Abstract
Objective.
Mannose-binding lectin (MBL) is important in the innate immune response. MBL2 gene polymorphisms affect MBL expression, and genotypes yielding low MBL levels have been associated with an elevated risk for infections in hematological cancer patients undergoing chemotherapy. However, these reported associations are inconsistent, and data on patients with solid tumors are lacking. Here, we investigated the effects of MBL2 genotypes on irinotecan-induced febrile neutropenia in patients with solid tumors.
Patients and Methods.
Irinotecan-treated patients were genotyped for the MBL2 gene. Two promoter (−550 H/L and −221 X/Y) and three exon polymorphisms (52 A/D, 54 A/B, and 57 A/C) were determined, together with known risk factors for irinotecan-induced toxicity. Neutropenia and febrile neutropenia were recorded during the first course.
Results.
Of the 133 patients, 28% experienced severe neutropenia and 10% experienced febrile neutropenia. No associations were found between exon polymorphisms and febrile neutropenia. However, patients with the H/H promoter genotype, associated with high MBL levels, experienced significantly more febrile neutropenia than patients with the H/L and L/L genotypes (20% versus 13% versus 5%). Moreover, patients with the HYA haplotype encountered significantly more febrile neutropenia than patients without this high MBL-producing haplotype (16% versus 4%). In the subgroup with wild-type exon polymorphisms (A/A), patients with the high MBL promoter phenotype had the highest incidence of febrile neutropenia, regardless of known risk factors.
Conclusion.
Patients with high MBL2 promoter genotypes and haplotypes seem more at risk for developing febrile neutropenia. If confirmed, these preliminary findings may contribute to more individualized approaches of irinotecan treatment.
Introduction
Mannose-binding lectin (MBL) is produced in the liver and plays an important role in the innate immune system [1–3]. As a member of the collectin family, MBL possesses a carbohydrate recognition domain, which recognizes and binds microbial surface carbohydrates [4]. A wide range of Gram-positive and Gram-negative bacteria, viruses, fungi, and protozoa can be bound by MBL, mediating opsonophagocytosis directly and indirectly by activation of the lectin complement pathway [1, 5]. Especially when the adaptive immune system is immature or compromised, MBL becomes very important. In these instances, the innate immune response forms the principal defense against infection, thereby theoretically rendering MBL deficiency a serious risk factor for infection [1]. This seems particularly relevant when a patient is under immunosuppressive therapy or is receiving a bone marrow transplant and/or chemotherapy for hematological malignancies [3, 6–11]. However, research on the effects of MBL on hematological malignancies has yielded conflicting results [7, 12–16].
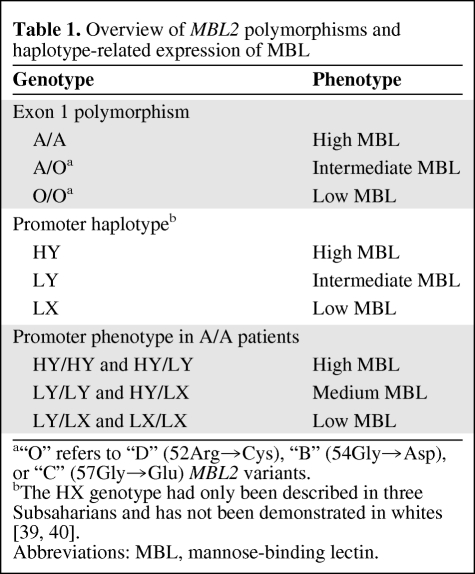
Five different polymorphisms in the MBL2 gene, located on chromosome 10, are related to serum MBL protein levels [17–20]. Two promoter polymorphisms, −550 H/L (rs11003125) and −221 X/Y (rs7096206), together form three haplotypes, which result in high (HY), intermediate (LY), and low (LX) MBL levels [18, 21] (Table 1). The remaining three polymorphisms, also referred to as exon polymorphisms, are located on the first exon of the MBL2 gene—codon 52 (Arg→Cys, rs5030737; also known as the MBL2-D variant allele), codon 54 (Gly→Asp, rs1800450; also known as the MBL2-B variant allele), and codon 57 (Gly→Glu, rs1800451; also known as the MBL2-C variant allele). Homozygous wild-type patients for each of these exon polymorphisms are referred to as A/A and have high circulating MBL levels [22]. Patients carrying one variant allele have intermediate MBL levels and are referred to as A/O, in which the “O” stands for one of the MBL2 variants (D, B, or C). Patients carrying two variant alleles, whether two equal or two different variants, are referred to as O/O and have low functional MBL levels [22]. The occurrence of these variants is rather frequent, with approximately 40% of whites bearing at least one variant exon polymorphism resulting in reduced serum MBL levels [21, 23, 24].
Table 1.
Overview of MBL2 polymorphisms and haplotype-related expression of MBL
Irinotecan, a prodrug of the topoisomerase I inhibitor SN-38, is widely used in patients with colorectal cancer. It is typically known for its unpredictable toxicities, mainly consisting of (febrile) neutropenia and late-onset diarrhea. The occurrence of these side effects may lead to dose reductions or even treatment discontinuation, thereby attenuating the antitumor activity of irinotecan [25, 26]. Several inherited and environmental factors affect the occurrence of these adverse effects. These include polymorphisms in genes encoding drug-metabolizing enzymes and transport proteins, lifestyle factors such as smoking habits, renal function, and comedication [27–31]. However, despite the elucidation of these risk factors, there is an obvious need to get more insight into factors rendering patients at risk for developing irinotecan-induced toxicities, in particular, febrile neutropenia.
Therefore, together with the facts that data on the association of MBL2 genotypes with the occurrence of febrile neutropenia in hematological cancer patients are inconsistent and data on such an association in solid cancer patients are lacking, we explored the effect of MBL2 polymorphisms on febrile neutropenia in irinotecan-treated patients.
Patients and Methods
Patients and Treatment
Patients who were treated with single-agent irinotecan once every 3 weeks over 90 minutes at a dose of 350 mg/m2, its 600 mg flat-fixed (irrespective of BSA) dose equivalent [32, 33], or at a dose that was based on cytochrome P450 (CYP)3A4 phenotyping [34] were studied during their first treatment course. Premedication consisted of dexamethasone and granisetron. In cases of acute cholinergic syndrome, atropine was administered s.c. Delayed-type diarrhea was treated with loperamide and, if necessary, with antibiotics, at the discretion of the treating physician. Febrile neutropenia was treated with the broad-spectrum antibiotic imipenem.
All patients had participated in four prospective trials involving pharmacokinetic and pharmacodynamic analyses [34–37]. Those trials were approved by the institutional medical ethical boards of the participating medical centers and were performed in accordance with the Declaration of Helsinki. All patients gave written informed consent for participation in those studies and for additional pharmacogenetic analyses. The most important inclusion criteria of these studies were: a histologically or cytologically confirmed diagnosis of any solid tumor that was believed to be sensitive to irinotecan treatment; age >18 years; a World Health Organization performance status score <2; and adequate hematological, renal, and hepatic function. Additionally, the time between the last anticancer treatment and the start of irinotecan treatment had to be >4 weeks. Furthermore, the use of any known CYP3A and/or P-glycoprotein inhibitor or inducer during the whole study period (starting 2 weeks before the first irinotecan administration) was not allowed. Patients with unresolved bowel obstruction or chronic colic disease were excluded from the study.
In one of the four studies, some of the patients were prophylactically treated with neomycin [35]. Because this aminoglycoside antibiotic is poorly absorbed [38], and in this study no effects of neomycin prophylaxis on the systemic clearance of SN-38 and the incidence of serious neutropenia and leukopenia were noted [35], no effect of neomycin cotreatment on febrile neutropenia was anticipated.
Toxicities
Before the start of irinotecan treatment, a complete medical history, physical examination, and hematological and chemical blood analyses were performed. During the 3-week follow-up period after the first administration of irinotecan, patients were seen weekly at the outpatient clinic for follow-up, which included a physical examination and routine hematological, renal, and hepatic laboratory analyses. When patients were admitted to the hospital because of severe toxicities, laboratory tests were performed more frequently (at least thrice a week). Toxicities were graded according to the Common Terminology Criteria for Adverse Events, version 3.0, of the National Cancer Institute. Febrile neutropenia was defined as a neutrophil count ≤1.0 × 109/ml in the presence of fever. Fever was defined as a temperature >38.5°C on a single measurement or a temperature >38.0°C on two separate occasions [9, 13].
Genotyping
DNA was obtained from whole blood as described previously [37], and samples were genotyped for polymorphisms in the MBL2 gene. Five common polymorphisms of the MBL2 gene—two in the promoter region (−550 H/L and −221 X/Y) and three in the first exon (52 A/D, 54 A/B, and 57 A/C—were analyzed by allele-specific amplification reactions, according to Mullighan et al. [39]. The polymerase chain reaction (PCR) amplification was performed in a 25-μl reaction volume, containing 15 ng genomic DNA, PCR Buffer II (Perkin Elmer, Waltham, MA), 1.5 mM MgCl2, 0.2 mM of each of the deoxynucleotide triphosphates (Roche, Indianapolis, IN), 0.5 U Amplitaq Gold (Perkin Elmer), and 20 pmol of each of the primers, as described elsewhere [39]. PCR products were analyzed on a 1% agarose gel with ethidium bromide, and results were archived using a Gel Doc GD 2000 system (Bio-Rad, Hercules, CA).
Construction of MBL2 Haplotypes and Phenotypes
The two promoter polymorphisms, −550 H/L and −221 X/Y, were combined to form three promoter haplotypes: HY, LY, and LX [12], which are related to high, intermediate, and low levels of MBL, respectively (Table 1) [18, 21]. The HX haplotype probably does not occur in whites because of linkage disequilibrium [39, 40]. Combination of these haplotypes in patients having the wild-type exon genotype (A/A) yielded three MBL2 promoter phenotypes: high (HY/HY and HY/LY), medium (HY/LX and LY/LY), and low (LY/LX and LX/LX).
Additional Analyses
Pharmacokinetic analyses and parameters of irinotecan and the active metabolite SN-38, as well as the presence of UGT1A1*28 and UGT1A1*93 (−3156G>A) alleles, which are known to influence irinotecan pharmacokinetics and toxicities by decreasing glucuronidation [30, 41], were determined as described previously [37].
Statistical Analyses
All exploratory analyses were performed anonymously and without awareness of clinical outcome to exclude any form of potential bias. Differences in the incidence of febrile neutropenia among exon polymorphisms (A/A, A/O, O/O), promoter polymorphisms (−550 H/H, H/L, L/L and −221 X/X, X/Y, Y/Y), and promoter phenotypes (high, medium, low) were analyzed using χ2 tests for trends.
Associations between the toxicities and the presence or absence of the HYA and LXA haplotypes were analyzed using χ2 tests. In addition, odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Differences in patient characteristics among the MBL phenotype groups and the promoter polymorphism and haplotype groups were analyzed using Kruskal-Wallis tests in cases of continuous variables and χ2 tests (for trends) in cases of nominal variables.
Statistical calculations were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL). p-values < .05 were considered significant.
Results
Patient Characteristics
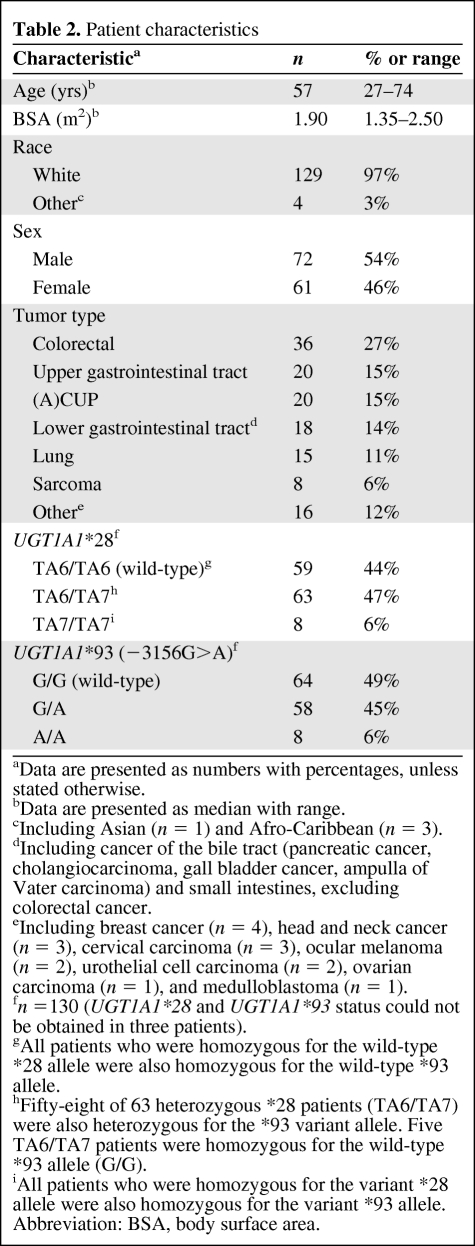
One hundred thirty-three patients were successfully genotyped for MBL2 and were included in the analyses. Thirteen patients were excluded from the analyses because there was no blood available for genotyping (n = 7), MBL genotype could not be obtained (n = 4), or no toxicity data were available (n = 2). The characteristics of these patients are shown in Table 2. Seventy-two patients (54%) were male and 61 patients (46%) were female. Ninety-seven percent of the patients were white. The most common tumor types were colorectal cancer (27%), upper gastrointestinal cancer (15%), and (adeno)carcinoma of unknown primary (15%). Nineteen patients (15%) had been prophylactically treated with neomycin, as mentioned before [35].
Table 2.
Patient characteristics
aData are presented as numbers with percentages, unless stated otherwise.
bData are presented as median with range.
cIncluding Asian (n = 1) and Afro-Caribbean (n = 3).
dIncluding cancer of the bile tract (pancreatic cancer, cholangiocarcinoma, gall bladder cancer, ampulla of Vater carcinoma) and small intestines, excluding colorectal cancer.
eIncluding breast cancer (n = 4), head and neck cancer (n = 3), cervical carcinoma (n = 3), ocular melanoma (n = 2), urothelial cell carcinoma (n = 2), ovarian carcinoma (n = 1), and medulloblastoma (n = 1).
fn =130 (UGT1A1*28 and UGT1A1*93 status could not be obtained in three patients).
gAll patients who were homozygous for the wild-type *28 allele were also homozygous for the wild-type *93 allele.
hFifty-eight of 63 heterozygous *28 patients (TA6/TA7) were also heterozygous for the *93 variant allele. Five TA6/TA7 patients were homozygous for the wild-type *93 allele (G/G).
iAll patients who were homozygous for the variant *28 allele were also homozygous for the variant *93 allele.
Abbreviation: BSA, body surface area.
Toxicities
Twenty-nine patients (22%) experienced fever during their first irinotecan course. Twenty patients (15%) had grade 3 neutropenia and 17 patients (13%) experienced grade 4 neutropenia. Febrile neutropenia was seen in 13 patients (10%) and lasted a median of 1.5 days (range, 1–4 days). In 11 patients with febrile neutropenia (85%), there were neither positive blood cultures nor any clinical signs of infection other than fever. In the only two positive blood cultures that were found, one showed Escherichia coli and the other showed Pseudomonas aeruginosa. No effect of cotreatment with neomycin on the incidence of febrile neutropenia was seen in this study. Of the 19 patients who received neomycin, three developed febrile neutropenia (16%), whereas in patients who were not treated with neomycin, nine of 102 (8%) developed febrile neutropenia (p = .285).
MBL2 Polymorphism Frequencies
In four patients (2.9%), MBL2 genotyping was not successful, and therefore those patients were excluded from the analyses. In line with recent publications [8, 14], 50 patients (38%) carried at least one exon 1 mutation. The allele frequency of the wild-type allele (A) was 78.0%, whereas the rest of the alleles were variably present, with allele frequencies of 5%, 15%, and 2% for the D, B, and C variants, respectively. The frequencies of the −550H and −550L alleles in the promoter were 32% and 68%, respectively, and the frequencies of the −221X and −221Y promoter alleles were 22% and 78%, respectively.
Combining the promoter and exon 1 polymorphisms, six haplotypes were found based on the genotyping results and available literature [18, 39]. The most frequent haplotypes were LYA, HYA, and LXA, with haplotype frequencies of 20%–30%, whereas LYC and HYD were found sporadically, with haplotype frequencies <5%. The haplotype frequency of LYB was 15%. These frequencies were in accordance with previously reported frequencies in whites [8, 14, 24, 42, 43].
MBL2 Exon Polymorphisms in Relation to Irinotecan-Induced Febrile Neutropenia
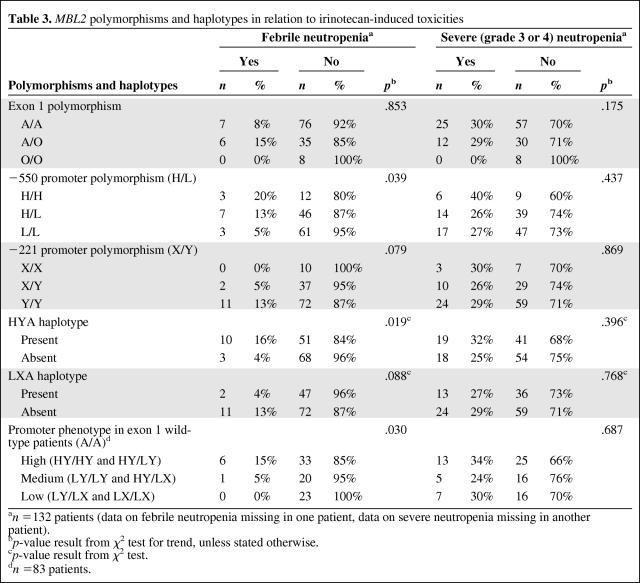
Febrile neutropenia was not significantly associated with the presence of a certain exon polymorphism (Table 3). The incidences of febrile neutropenia were 8% (seven of 83 patients) in patients with the A/A genotype, 15% (six of 41 patients) in patients with the A/O genotype, and 0% (none of eight patients) in the O/O patients (p = .853). In the subgroup of patients with severe (grade 3 or 4) neutropenia, no relation was found between the incidence of fever and the presence of a certain exon polymorphism.
Table 3.
MBL2 polymorphisms and haplotypes in relation to irinotecan-induced toxicities
an =132 patients (data on febrile neutropenia missing in one patient, data on severe neutropenia missing in another patient).
bp-value result from χ2 test for trend, unless stated otherwise.
cp-value result from χ2 test.
dn =83 patients.
MBL2 Promoter Polymorphisms in Relation to Irinotecan-Induced Febrile Neutropenia
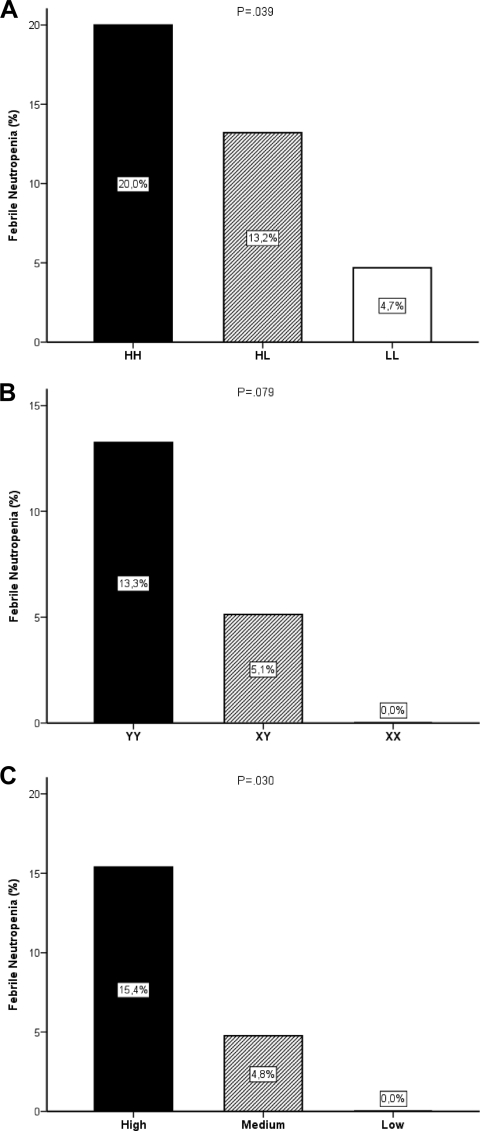
As illustrated in Figure 1A and Table 3, patients with the H/H genotype had a higher incidence of febrile neutropenia than patients with the H/L and L/L genotype (three of 15 patients [20%] versus seven of 53 patients [13%] versus three of 64 patients [5%]; p = .039). A higher incidence of febrile neutropenia was also observed in patients carrying the Y/Y genotype than in those carrying the X/Y and X/X genotypes (11 of 83 patients [13%] versus two of 39 patients [5%] versus none of 10 patients [0%]) (Fig. 1B), although this finding did not reach statistical significance (p = .079). In both analyses, the differences in the incidence of febrile neutropenia could not be explained by a different incidence of severe (grade 3 or 4) neutropenia in the groups (p > .437).
Figure 1.
Bar graphs showing the effects of the MBL2-promoter −550H/L polymorphism (A), MBL2-promoter −221X/Y polymorphism (B), and MBL2-promoter phenotypes (high, HY/HY and HY/LY; medium, LY/LY and HY/LX; and low, LY/LX and LX/LX) in patients with the wild-type exon polymorphism (A/A) (C) on the incidence of febrile neutropenia in cancer patients treated with irinotecan.
Similarly, in patients experiencing severe neutropenia, the risk for febrile neutropenia was likewise dependent on promoter genotype, illustrating the role of MBL in immune-compromised patients. Patients with the H/H and the H/L genotypes had higher incidences of febrile neutropenia than patients with the L/L genotype (50% versus 50% versus 18%; p = .066), and patients carrying the Y/Y genotype had a higher incidence of febrile neutropenia than patients carrying the X/Y and X/X genotypes (48% versus 20% versus 0%; p = .044).
MBL2 Promoter Haplotypes and Phenotypes in Relation to Irinotecan-Induced Febrile Neutropenia
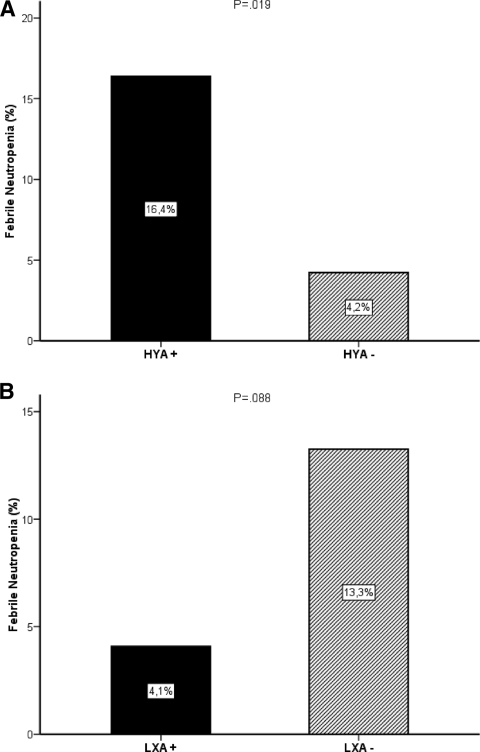
In line with analyses of individual promoter polymorphisms, patients with at least one HYA haplotype had a four times higher incidence of febrile neutropenia than patients who did not have this high MBL level haplotype (16% versus 4%; OR, 4.4; 95% CI, 1.2–17.0; p = .019) (Fig. 2A). In the subgroup of patients with a wild-type exon polymorphism (A/A), patients with the HYA haplotype also had a higher incidence of febrile neutropenia (n = 83; 15% versus 0%; OR, 1.2; 95% CI, 1.0–1.3; p = .018). Importantly, in patients with severe neutropenia (grade 3 or 4), the presence of at least one HYA haplotype resulted in a higher risk for developing fever during the neutropenic episode (n = 36; 53% versus 18%; OR, 5.2; 95% CI, 1.1–24.1; p = .029). This was also the case in the subgroup of patients with the wild-type exon polymorphism who had severe neutropenia (n = 25; 47% versus 0%; OR, 1.9; 95% CI, 1.2–3.0; p = .011).
Figure 2.
Bar graphs showing the negative effects of the presence of at least one MBL2 HYA haplotype (A) and the absence of any MBL2 LXA haplotype (B) on the incidence of febrile neutropenia in cancer patients treated with single-agent irinotecan.
Although not significant, patients without the low MBL-producing haplotype LXA had a trend toward a more than three times higher risk for febrile neutropenia than patients with this haplotype (13% versus 4%; OR, 3.6; 95% CI, 0.8–16.9; p =.088) (Fig. 2B).
In the subgroup of patients with the wild-type exon polymorphism (A/A), patients with the high MBL2 promoter phenotype (HY/HY and HY/LY) had the highest incidence of febrile neutropenia, compared with the medium (LY/LY and HY/LX) and low (LY/LX and LX/LX) MBL2 promoter phenotypes, as illustrated in Figure 1C (15% versus 5% versus 0%; p = .030). This could not be explained by a higher incidence of severe (grade 3 or 4) neutropenia in this group (34% versus 24% versus 30%; p = .687). Likewise, no effect of other potential confounders such as age, exposure to SN-38, or the presence of the UGT1A1*28 or UGT1A1*93 alleles was found (see online supplementary Tables A–C).
Also, in the subgroup of patients with the wild-type exon polymorphism who experienced severe neutropenia, a higher incidence of febrile neutropenia was observed in patients with the high MBL2 promoter phenotypes (46% versus 20% versus 0%; p = .029).
Discussion
This study explored the influence of MBL2 polymorphisms on chemotherapy-induced neutropenic fever in adult cancer patients with solid tumors. Because the innate immune response, with MBL as a key factor, is thought to play a crucial role in the defense against infectious pathogens (in particular, in cases of neutropenia), we initially hypothesized that patients with functional genotypes associated with low MBL production would be more prone to develop neutropenic fever. In contrast, we found that patients with high MBL-producing promoter genotypes, haplotypes, and haplotype combinations of the MBL2 gene suffer more often from febrile neutropenia after irinotecan chemotherapy than patients with low MBL-producing genotypes. Also, in the group of patients who developed grade 3 or 4 neutropenia, the incidence of neutropenic fever was higher in patients with genotypes yielding high MBL levels.
In view of our results, we hypothesize that low MBL levels (as determined by genotype) might have a protective effect against the occurrence of fever in neutropenic patients because of insufficient activation of the complement system. A high frequency of unexplained fever during neutropenic episodes was shown previously [44, 45]. Also, in our study population, almost no infectious causes could be identified in the patients developing febrile neutropenia. Therefore, it could be hypothesized that fever in (most) patients with neutropenia is induced by the neutropenic state as such, rather than by the presence of infectious pathogens. This might explain why patients with high MBL genotypes had a higher incidence of febrile neutropenia in our study, because higher MBL levels might induce the occurrence of an immunological response [46]. Another explanation for our findings is that transient neutropenia is commonly associated with subclinical infections. If true, those patients with genotypes yielding high MBL levels may be more prone to developing fever as a response to neutropenia than patients with genotypes yielding low MBL concentrations. However, these explanations are hypothetical and should be further investigated.
To the best of our knowledge, this is the first study addressing the association of MBL genotypes and neutropenic fever in adult patients with solid malignancies who are treated with chemotherapy. Previous research on the influence of MBL2 polymorphisms and MBL serum levels on the incidence and duration of infectious toxicities has been conducted only in hematological and pediatric cancer patients, and yielded conflicting results. In patients with genotypes producing low MBL levels, a longer duration of febrile neutropenia and a higher risk for major infections were reported in pediatric patients undergoing chemotherapy for (mainly) hematological malignancies and in adults after allogeneic hemopoietic stem cell transplantation [7, 9]. The differences between these and our results may be explained by the, in general, much more myelosuppressive (or even myeloablative) effect of chemotherapy for hematological malignancies than for chemotherapy administered to our population, apart from the fact that hematological malignancies themselves also cause myelosuppression [47], and that the mode of action of MBL is influenced by the depth and duration of myelosuppression.
In line with our findings, Schlapbach et al. [10] found that, not only children with the lowest MBL levels (<100 μg/l), but also children with the highest MBL levels (>1,000 μg/l) had more frequent episodes of febrile neutropenia and were hospitalized longer than children with intermediate MBL levels after chemotherapeutic treatment for solid and hematological cancers. They showed that high MBL levels may be less beneficial for patients, possibly as a result of greater complement activation and the stimulation of proinflammatory signals [46], and that moderate MBL deficiency may be favorable, especially in patients who briefly receive immunosuppressive chemotherapy. This could explain why we only found an effect of promoter polymorphisms, which are known to have a less dramatic effect on MBL levels than exon polymorphisms, on the incidence of febrile neutropenia [18]. Another interesting analysis showed a trend toward more grade 4 neutropenic infections in multiple myeloma patients carrying the high MBL-producing wild-type exon polymorphism [14]. Interestingly, Klostergaard et al. [48] found a significantly higher incidence of Gram-negative blood cultures in high MBL genotypes, which could explain why we found a higher incidence of febrile neutropenia in high MBL genotypes and positive blood cultures of only Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) in our study.
In addition to these studies, several other studies could not reveal an association between MBL2 genotype or serum MBL levels and infectious complications after chemotherapy [12, 13, 16, 49]. The conflicting outcomes of these studies clearly underline that more research is required to unravel the exact relationship among MBL2 genotypes, MBL serum levels, and the occurrence of neutropenic events in cancer patients treated with chemotherapy. We, therefore, feel that the initiation of studies on MBL replacement therapy, as has occurred in patients with multiple myeloma or children with chemotherapy-induced neutropenia, is premature [50].
An important caveat of studies on the relationship between MBL2 gene polymorphisms and neutropenic events, including ours, is the fact that, in ill people, MBL levels are determined not only by genotype but likely also by the disease itself in the context of an acute-phase response [8, 12–13]. Moreover, our study population was relatively small, which resulted in a low absolute number of neutropenic events seen. In addition, several tumor types were involved, which could have influenced outcomes. Because this study was exploratory in nature, no corrections for multiple analyses were performed. Therefore, our findings should be interpreted with caution and should be confirmed in larger (and more uniform) study populations, preferably in combination with MBL serum level measurements, to determine whether the known genotype/phenotype relations in healthy humans are comparable in cancer patients. If confirmed, the finding that patients having polymorphisms in the MBL2 gene, associated with high MBL levels, have an elevated risk for developing irinotecan-induced febrile neutropenia is of importance. As a result, MBL2 genotyping could have a meaningful place in clinical decision making and contribute to more personalized patient management.
Supplementary Material
Acknowledgment
This work was presented in part at the Annual Meeting of the American Society of Clinical Pharmacology and Therapeutics 2009 (# OI-C-3), Washington DC, March 19, 2009.
Author Contributions
Conception/Design: Jessica M. van der Bol, Ron H. Mathijssen, Floris A. de Jong
Provision of study material or patients: Jaap Verweij, Stefan Sleijfer, Ron H.
Mathijssen
Collection and/or assembly of data: Jessica M. van der Bol, Ron H. Mathijssen, Ron van Schaik
Data analysis and interpretation: Jessica M. van der Bol, Stefan Sleijfer, Ron H. Mathijssen, Floris A. de Jong, Ron van Schaik, Alex Sparreboom, Marianne van Fessem, Fleur van de Geijn, Paul van Daele
Manuscript writing: Jessica M. van der Bol, Ron H. Mathijssen, Floris A. de Jong, Fleur van de Geijn
Final approval of manuscript: Jessica M. van der Bol, Jaap Verweij, Stefan Sleijfer, Ron H. Mathijssen, Floris A. de Jong, Ron van Schaik, Alex Sparreboom, Marianne van Fessem, Fleur van de Geijn, Paul van Daele
References
- 1.Eisen DP, Minchinton RM. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin Infect Dis. 2003;37:1496–1505. doi: 10.1086/379324. [DOI] [PubMed] [Google Scholar]
- 2.Ip WK, Takahashi K, Ezekowitz RA, et al. Mannose-binding lectin and innate immunity. Immunol Rev. 2009;230:9–21. doi: 10.1111/j.1600-065X.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- 3.Tsutsumi A, Takahashi R, Sumida T. Mannose binding lectin: Genetics and autoimmune disease. Autoimmun Rev. 2005;4:364–372. doi: 10.1016/j.autrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Holmskov U, Malhotra R, Sim RB, et al. Collectins: Collagenous C-type lectins of the innate immune defense system. Immunol Today. 1994;15:67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 5.Dommett RM, Klein N, Turner MW. Mannose-binding lectin in innate immunity: Past, present and future. Tissue Antigens. 2006;68:193–209. doi: 10.1111/j.1399-0039.2006.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullighan CG, Bardy PG. Mannose-binding lectin and infection following allogeneic hemopoietic stem cell transplantation. Leuk Lymphoma. 2004;45:247–256. doi: 10.1080/1042819031000146983. [DOI] [PubMed] [Google Scholar]
- 7.Mullighan CG, Heatley S, Doherty K, et al. Mannose-binding lectin gene polymorphisms are associated with major infection following allogeneic hemopoietic stem cell transplantation. Blood. 2002;99:3524–3529. doi: 10.1182/blood.v99.10.3524. [DOI] [PubMed] [Google Scholar]
- 8.Mullighan CG, Heatley SL, Danner S, et al. Mannose-binding lectin status is associated with risk of major infection following myeloablative sibling allogeneic hematopoietic stem cell transplantation. Blood. 2008;112:2120–2128. doi: 10.1182/blood-2007-07-100222. [DOI] [PubMed] [Google Scholar]
- 9.Neth O, Hann I, Turner MW, et al. Deficiency of mannose-binding lectin and burden of infection in children with malignancy: A prospective study. Lancet. 2001;358:614–618. doi: 10.1016/S0140-6736(01)05776-2. [DOI] [PubMed] [Google Scholar]
- 10.Schlapbach LJ, Aebi C, Otth M, et al. Serum levels of mannose-binding lectin and the risk of fever in neutropenia pediatric cancer patients. Pediatr Blood Cancer. 2007;49:11–16. doi: 10.1002/pbc.21097. [DOI] [PubMed] [Google Scholar]
- 11.Vekemans M, Robinson J, Georgala A, et al. Low mannose-binding lectin concentration is associated with severe infection in patients with hematological cancer who are undergoing chemotherapy. Clin Infect Dis. 2007;44:1593–1601. doi: 10.1086/518171. [DOI] [PubMed] [Google Scholar]
- 12.Bergmann OJ, Christiansen M, Laursen I, et al. Low levels of mannose-binding lectin do not affect occurrence of severe infections or duration of fever in acute myeloid leukaemia during remission induction therapy. Eur J Haematol. 2003;70:91–97. doi: 10.1034/j.1600-0609.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 13.Kilpatrick DC, McLintock LA, Allan EK, et al. No strong relationship between mannan binding lectin or plasma ficolins and chemotherapy-related infections. Clin Exp Immunol. 2003;134:279–284. doi: 10.1046/j.1365-2249.2003.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mølle I, Steffensen R, Thiel S, et al. Chemotherapy-related infections in patients with multiple myeloma: Associations with mannan-binding lectin genotypes. Eur J Haematol. 2006;77:19–26. doi: 10.1111/j.1600-0609.2006.00669.x. [DOI] [PubMed] [Google Scholar]
- 15.Peterslund NA, Koch C, Jensenius JC, et al. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 2001;358:637–638. doi: 10.1016/S0140-6736(01)05785-3. [DOI] [PubMed] [Google Scholar]
- 16.Rocha V, Franco RF, Porcher R, et al. Host defense and inflammatory gene polymorphisms are associated with outcomes after HLA-identical sibling bone marrow transplantation. Blood. 2002;100:3908–3918. doi: 10.1182/blood-2002-04-1033. [DOI] [PubMed] [Google Scholar]
- 17.Lipscombe RJ, Sumiya M, Hill AV, et al. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet. 1992;1:709–715. doi: 10.1093/hmg/1.9.709. [DOI] [PubMed] [Google Scholar]
- 18.Madsen HO, Garred P, Thiel S, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155:3013–3020. [PubMed] [Google Scholar]
- 19.Sastry K, Herman GA, Day L, et al. The human mannose-binding protein gene. Exon structure reveals its evolutionary relationship to a human pulmonary surfactant gene and localization to chromosome 10. J Exp Med. 1989;170:1175–1189. doi: 10.1084/jem.170.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner MW. Mannose-binding lectin (MBL) in health and disease. Immunobiology. 1998;199:327–339. doi: 10.1016/S0171-2985(98)80037-5. [DOI] [PubMed] [Google Scholar]
- 21.Steffensen R, Thiel S, Varming K, et al. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J Immunol Methods. 2000;241:33–42. doi: 10.1016/s0022-1759(00)00198-8. [DOI] [PubMed] [Google Scholar]
- 22.Madsen HO, Garred P, Kurtzhals JA, et al. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics. 1994;40:37–44. doi: 10.1007/BF00163962. [DOI] [PubMed] [Google Scholar]
- 23.Mead R, Jack D, Pembrey M, et al. Mannose-binding lectin alleles in a prospectively recruited UK population. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. Lancet. 1997;349:1669–1670. doi: 10.1016/s0140-6736(05)62635-9. [DOI] [PubMed] [Google Scholar]
- 24.Minchinton RM, Dean MM, Clark TR, et al. Analysis of the relationship between mannose-binding lectin (MBL) genotype, MBL levels and function in an Australian blood donor population. Scand J Immunol. 2002;56:630–641. doi: 10.1046/j.1365-3083.2002.01167.x. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham D, Pyrhönen S, James RD, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998;352:1413–1418. doi: 10.1016/S0140-6736(98)02309-5. [DOI] [PubMed] [Google Scholar]
- 26.Rougier P, Van Cutsem E, Bajetta E, et al. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet. 1998;352:1407–1412. doi: 10.1016/S0140-6736(98)03085-2. [DOI] [PubMed] [Google Scholar]
- 27.de Jong FA, van der Bol JM, Mathijssen RH, et al. Renal function as a predictor of irinotecan-induced neutropenia. Clin Pharmacol Ther. 2008;84:254–262. doi: 10.1038/sj.clpt.6100513. [DOI] [PubMed] [Google Scholar]
- 28.Innocenti F, Kroetz DL, Schuetz E, et al. Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J Clin Oncol. 2009;27:2604–2614. doi: 10.1200/JCO.2008.20.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kehrer DF, Mathijssen RH, Verweij J, et al. Modulation of irinotecan metabolism by ketoconazole. J Clin Oncol. 2002;20:3122–3129. doi: 10.1200/JCO.2002.08.177. [DOI] [PubMed] [Google Scholar]
- 30.Rosner GL, Panetta JC, Innocenti F, et al. Pharmacogenetic pathway analysis of irinotecan. Clin Pharmacol Ther. 2008;84:393–402. doi: 10.1038/clpt.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Bol JM, Mathijssen RH, Loos WJ, et al. Cigarette smoking and irinotecan treatment: Pharmacokinetic interaction and effects on neutropenia. J Clin Oncol. 2007;25:2719–2726. doi: 10.1200/JCO.2006.09.6115. [DOI] [PubMed] [Google Scholar]
- 32.de Jong FA, Mathijssen RH, Xie R, et al. Flat-fixed dosing of irinotecan: Influence on pharmacokinetic and pharmacodynamic variability. Clin Cancer Res. 2004;10:4068–4071. doi: 10.1158/1078-0432.CCR-03-0591. [DOI] [PubMed] [Google Scholar]
- 33.Mathijssen RH, Verweij J, de Jonge MJ, et al. Impact of body-size measures on irinotecan clearance: Alternative dosing recommendations. J Clin Oncol. 2002;20:81–87. doi: 10.1200/JCO.2002.20.1.81. [DOI] [PubMed] [Google Scholar]
- 34.van der Bol JM, Mathijssen RH, Creemers GJ, et al. A CYP3A4 phenotype-based dosing algorithm for individualized treatment of irinotecan. Clin Cancer Res. 2010;16:736–742. doi: 10.1158/1078-0432.CCR-09-1526. [DOI] [PubMed] [Google Scholar]
- 35.de Jong FA, Kehrer DF, Mathijssen RH, et al. Prophylaxis of irinotecan-induced diarrhea with neomycin and potential role for UGT1A1*28 genotype screening: A double-blind, randomized, placebo-controlled study. The Oncologist. 2006;11:944–954. doi: 10.1634/theoncologist.11-8-944. [DOI] [PubMed] [Google Scholar]
- 36.Engels FK, de Jong FA, Sparreboom A, et al. Medicinal cannabis does not influence the clinical pharmacokinetics of irinotecan and docetaxel. The Oncologist. 2007;12:291–300. doi: 10.1634/theoncologist.12-3-291. [DOI] [PubMed] [Google Scholar]
- 37.Mathijssen RH, de Jong FA, van Schaik RH, et al. Prediction of irinotecan pharmacokinetics by use of cytochrome P450 3A4 phenotyping probes. J Natl Cancer Inst. 2004;96:1585–1592. doi: 10.1093/jnci/djh298. [DOI] [PubMed] [Google Scholar]
- 38.Goodman CS, Limbird CE, Milinoff PB, et al., editors. Eighth Edition. Columbus, OH: McGraw-Hill, Inc.; 1990. The Pharmacological Basis of Therapeutics, Antimicrobial Agents, Neomycin; pp. 1112–1113. [Google Scholar]
- 39.Mullighan CG, Marshall SE, Welsh KI. Mannose binding lectin polymorphisms are associated with early age of disease onset and autoimmunity in common variable immunodeficiency. Scand J Immunol. 2000;51:111–122. doi: 10.1046/j.1365-3083.2000.00697.x. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan KE, Wooten C, Goldman D, et al. Mannose-binding protein genetic polymorphisms in black patients with systemic lupus erythematosus. Arthritis Rheum. 1996;39:2046–2051. doi: 10.1002/art.1780391214. [DOI] [PubMed] [Google Scholar]
- 41.Innocenti F, Undevia SD, Iyer L, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol. 2004;22:1382–1388. doi: 10.1200/JCO.2004.07.173. [DOI] [PubMed] [Google Scholar]
- 42.Madsen HO, Videm V, Svejgaard A, et al. Association of mannose-binding-lectin deficiency with severe atherosclerosis. Lancet. 1998;352:959–960. doi: 10.1016/S0140-6736(05)61513-9. [DOI] [PubMed] [Google Scholar]
- 43.Pine SR, Mechanic LE, Ambs S, et al. Lung cancer survival and functional polymorphisms in MBL2, an innate-immunity gene. J Natl Cancer Inst. 2007;99:1401–1409. doi: 10.1093/jnci/djm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freifeld A, Marchigiani D, Walsh T, et al. A double-blind comparison of empirical oral and intravenous antibiotic therapy for low-risk febrile patients with neutropenia during cancer chemotherapy. N Engl J Med. 1999;341:305–311. doi: 10.1056/NEJM199907293410501. [DOI] [PubMed] [Google Scholar]
- 45.Slobbe L, Waal L, Jongman LR, et al. Three-day treatment with imipenem for unexplained fever during prolonged neutropaenia in haematology patients receiving fluoroquinolone and fluconazole prophylaxis: A prospective observational safety study. Eur J Cancer. 2009;45:2810–2817. doi: 10.1016/j.ejca.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 46.Schafranski MD, Stier A, Nisihara R, et al. Significantly increased levels of mannose-binding lectin (MBL) in rheumatic heart disease: A beneficial role for MBL deficiency. Clin Exp Immunol. 2004;138:521–525. doi: 10.1111/j.1365-2249.2004.02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma A, Lokeshwar N. Febrile neutropenia in haematological malignancies. J Postgrad Med. 2005;51(suppl 1):S42–S48. [PubMed] [Google Scholar]
- 48.Klostergaard A, Steffensen R, Møller JK, et al. Sepsis in acute myeloid leukaemia patients receiving high-dose chemotherapy: No impact of chitotriosidase and mannose-binding lectin polymorphisms. Eur J Haematol. 2010;85:58–64. doi: 10.1111/j.1600-0609.2010.01443.x. [DOI] [PubMed] [Google Scholar]
- 49.Frakking FN, van de Wetering MD, Brouwer N, et al. The role of mannose-binding lectin (MBL) in paediatric oncology patients with febrile neutropenia. Eur J Cancer. 2006;42:909–916. doi: 10.1016/j.ejca.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 50.Frakking FN, Brouwer N, van de Wetering MD, et al. Safety and pharmacokinetics of plasma-derived mannose-binding lectin (MBL) substitution in children with chemotherapy-induced neutropaenia. Eur J Cancer. 2009;45:505–512. doi: 10.1016/j.ejca.2008.11.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.