This study evaluated whether human epidermal growth factor receptor 2 status is an independent, poor prognostic marker in patients with small (≤1 cm), node-negative breast cancer and whether a subgroup of patients with these small tumors who might benefit from adjuvant systemic therapy could be identified.
Keywords: HER-2–positive tumor, Prognosis, Small breast cancer, Triple-negative tumor
Abstract
Long-term outcomes and hence the role of adjuvant therapy in patients with small (≤1 cm), node-negative breast cancer remain unclear. This study's objective was to evaluate whether human epidermal growth factor receptor (HER)-2 status is an independent, poor prognostic marker in patients with these tumors and to identify a subgroup of patients with these small tumors who might benefit from adjuvant systemic therapy. All patients with a diagnosis of a node-negative breast tumor measuring ≤1 cm and available HER-2 test results between January 1, 2001, and December 31, 2005, at the three Mayo Clinic sites were identified. Clinicopathologic data were compared in three groups: HER-2−, HER-2+, and triple-negative (TN) tumors. Of the 421 tumors identified, 364 (86.5%) were HER-2−, 28 (6.7%) were HER-2+, and 29 (6.9%) were TN. The median follow-up time was 1,015 days (range, 1–2,549 days). Groups were balanced in terms of patient age and tumor histology. Eleven patients with HER-2− tumors (3.0%), seven with HER-2+ tumors (25.0%), and eight with TN tumors (27.6%) received adjuvant chemotherapy. Follow-up data were available for 357, 28, and 28 patients in the three groups, respectively. Death rates in the three groups were 6.4% (23 of 357) (one recurrence-related death), 0% (0 of 28), and 7.1% (2 of 28) (one recurrence-related death), respectively. During follow-up, the tumor recurred in nine patients: four were HER-2− tumors (1.1%), two were HER-2+ tumors (7.1%), and three were TN tumors (10.7%). Patients with small, node-negative breast tumors have an excellent prognosis, but HER-2+ and TN tumors appear to have a higher recurrence rate, warranting consideration for broad use and optimization of systemic adjuvant treatments.
Introduction
Mammographic screening has made the diagnosis of small breast cancers a reality [1]. Tumor size and axillary lymph node status play an important part in predicting outcome in patients with this disease. The long-term prognosis for patients with small (≤1 cm), node-negative breast cancer was thought to be excellent, with an 88% recurrence-free survival rate at 10 years [2]. However, breast cancer recurrence and death are known to occur in patients with T1N0M0 breast cancer. This uncertainty about the long-term prognosis of patients with small (≤1 cm), node-negative tumors prompted a few retrospective evaluations worthy of consideration. The analysis of data from five National Surgical Adjuvant Breast and Bowel Project (NSABP) randomized clinical trials in the 1990s included 235 estrogen receptor (ER)− and 1,024 ER+, node-negative tumors, of which 40% measured ≤1 cm [3]. Eight-year follow-up data demonstrated that 23% of women with ER− tumors and 18% of women with ER+ tumors treated with surgery alone had a breast cancer recurrence or a contralateral breast cancer and an 8% mortality rate [3]. Eight-year data probably underestimate the long-term recurrence rates for small tumors, because breast cancer may recur beyond an 8-year period [4].
In a report of 10-year breast cancer outcomes in a Canadian population-based cohort of 326 patients with small (≤1 cm), node-negative breast cancer, 21 (6.4%) had human epidermal growth factor receptor (HER)-2+ disease, and those patients showed a trend toward worse recurrence-free survival but no difference in overall survival, compared with HER-2− patients. Among the subgroup of 225 patients with tumors 0.6–1.0 cm who did not receive adjuvant therapy, the 13 HER-2+ patients trended toward a worse outcome (10-year recurrence-free survival rate, 68.4% versus 81.8%; p = .312) [5].
These studies add to the premise that the prognosis for patients with tumors measuring ≤1 cm, although better than that for women with larger tumors, may not be good enough to exclude them as candidates for systemic adjuvant therapy. Although adjuvant therapy is now fairly routinely recommended for patients with node-positive breast cancer or node-negative breast cancer >1 cm, its potential role in small (≤1 cm), node-negative tumors needs to be better defined. If tumor characteristics associated with aggressive disease could be identified, a subgroup of these patients could potentially be counseled and considered for adjuvant therapy.
The goal of this study was to test the hypothesis that HER-2 status is an independent, poor prognostic marker in node-negative tumors measuring ≤1 cm and to identify a subgroup of patients with these small tumors who have a recurrence rate >10% at 5 years based on ER, progesterone receptor (PR), and HER-2 status, making them eligible for consideration of adjuvant systemic treatment.
Materials and Methods
All patients with small (≤1 cm), node-negative breast cancer diagnosed at the three Mayo Clinic sites (Jacksonville, FL, Scottsdale, AZ, and Rochester, MN) between January 1, 2001, and December 31, 2005, were identified. Pathologic data regarding tumor characteristics were derived from pathology reports archived in tumor registry data files. Cases that had HER-2 testing (using HercepTest®; Dako, Carpinteria, CA, or PathVysion® fluorescence in situ hybridization [FISH]; Vysis, Downers Grove, IL) were included. HER-2+ status was defined as an immunohistochemistry (IHC) score of 3+ (defined by the manufacturer's guidelines of >10% of tumor cells showing strong complete membrane staining) or a FISH HER-2/CEP17 (chromosome 17 centromere) ratio ≥2.0 based on regulatory agency approvals of these tests. Two hundred forty-three cases were excluded because they lacked or had incomplete HER-2 testing, and funds were not available to prospectively test these samples for HER-2 status. Patients with an IHC score of 2+ for HER-2 but with no available FISH test results were excluded from this study, because we could not define whether these tumors were truly HER-2− or HER-2+ (separate numbers not available). Additionally, ER and PR status were evaluated using standard IHC techniques, and at least 10% nuclear staining was needed to deem the specimen positive. All cases referred from outside facilities were reviewed by a Mayo Clinic pathologist. All patients had HER-2, ER, and PR testing done at Mayo Clinic.
Patients were divided into three groups:
The HER-2− group comprised patients whose tumors were ER+PR+HER-2−, ER+PR−HER-2−, or ER− PR+HER-2−.
The HER-2+ group comprised patients whose tumors were ER+PR+HER-2+, ER+PR−HER-2+, ER− PR+HER-2+, or ER−PR−HER-2+.
The triple-negative (TN) group comprised patients whose tumors were ER−PR−HER-2−.
Patients were followed up every 4–6 months for the first 5 years, then every 12 months, with a physical examination with or without routine laboratory studies. Mammography was performed every year or initially after 6 months if the patient received radiation therapy.
All patients had sentinel lymph node evaluation during surgery. Individual sentinel lymph nodes were macrosectioned in 1- to 2-mm slices, microscopically examined using three hematoxylin-eosin sections at different levels through the block, and further examined by cytokeratin AE1/AE3 staining at one level with one level negative control. Patients with positive lymph nodes, even those with microinvasive disease, were excluded. Data collected from medical records included age, grade and histologic type of the tumor, type of adjuvant treatment (radiation, chemotherapy, hormonal therapy), the recurrence rate, recurrence-free survival rate, and overall survival duration. Recurrence-free survival was defined as the time from diagnosis to the time of recurrence. Overall survival was calculated using the time from surgery until the time of death or most recent follow-up.
The study was approved by the Mayo Clinic Institutional Review Board.
Statistical Methods
The demographic and clinical characteristics of eligible patients were tabulated using number and percentage for categorical characteristics and median and range for continuous characteristics. Clinicopathologic data, recurrence, and survival were evaluated among the three study groups using Fisher's exact test to compare categorical variables and the Wilcoxon rank sum test to compare continuous variables. The median survival time was estimated using the Kaplan–Meier approach. Because of the large difference in the number of patients in each group and the small number of observed events, a descriptive approach was used in lieu of formal statistical comparisons of survival and recurrence time.
Results
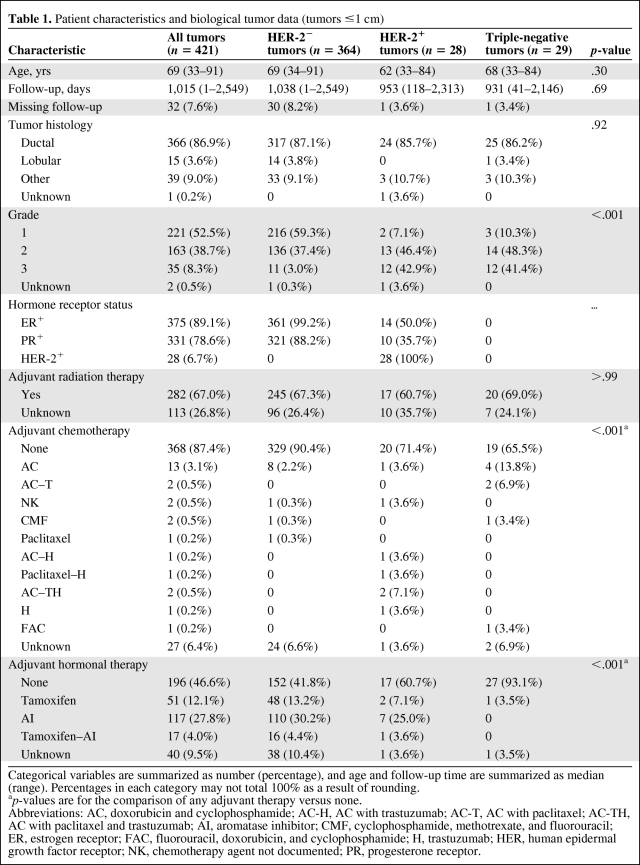
As described in Table 1, 421 tumors in 413 patients met the inclusion criteria for this study: 364 (86.4%) were HER-2−, 28 (6.7%) were HER-2+, and 29 (6.9%) were TN. The HER-2− tumors constituted the reference group. Table 1 also describes demographic characteristics for both the overall sample and the three defined groups. Eight participants had multiple tumors; because those tumors had different characteristics, they were all included in the analysis. ER, PR, and HER-2 were tested separately in each tumor. Thus, the analysis was performed on a per-tumor basis, instead of a per-patient basis. The median patient age was 69 years (range, 33–91 years). There was no difference in age (median, 69, 62, and 68 years, respectively; p = .30) or histology (p = .92) (invasive ductal carcinoma was the commonest subtype, with 87.1%, 85.7%, and 86.2% prevalence rates, respectively) across the three groups.
Table 1.
Patient characteristics and biological tumor data (tumors ≤1 cm)
Categorical variables are summarized as number (percentage), and age and follow-up time are summarized as median (range). Percentages in each category may not total 100% as a result of rounding.
ap-values are for the comparison of any adjuvant therapy versus none.
Abbreviations: AC, doxorubicin and cyclophosphamide; AC-H, AC with trastuzumab; AC-T, AC with paclitaxel; AC-TH, AC with paclitaxel and trastuzumab; AI, aromatase inhibitor; CMF, cyclophosphamide, methotrexate, and fluorouracil; ER, estrogen receptor; FAC, fluorouracil, doxorubicin, and cyclophosphamide; H, trastuzumab; HER, human epidermal growth factor receptor; NK, chemotherapy agent not documented; PR, progesterone receptor.
There were more grade 2 and 3 tumors in the HER-2+ and TN groups (89.3%, 89.7%) than in the HER-2− group (40.4%) (p < .001). Overall, 67.0% of tumors received adjuvant radiation therapy; this did not change notably across groups (p > .99). The rate of adjuvant chemotherapy administration varied among the groups: 3.0% (11 or 364) of HER-2− tumors, 25.0% (seven of 28) of HER-2+ tumors, and 27.6% (eight of 29) of TN tumors were treated with adjuvant chemotherapy (p < .001). Five patients (17.9%) with HER-2+ tumors received adjuvant trastuzumab (only one received trastuzumab alone). One hundred seventy-four HER-2− tumors (47.8%), 10 HER-2+ tumors (35.7%), and one TN tumor (3.5%) were treated with hormonal therapy. The follow-up range was 1–2,549 days (median, 1,015 days [33.8 months]).
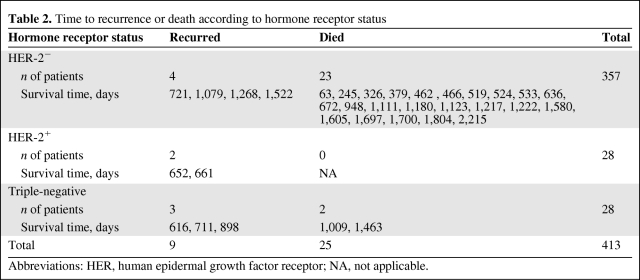
Table 2 shows the mortality and recurrence incidence rates for the three groups. During the follow-up period, 23 of the 357 patients with HER-2− tumors (6.4%; 95% confidence interval [CI], 4.1%–9.5%), none of the 28 patients with HER-2+ tumors (0%; 95% CI, 0%–12.3%), and two of 28 patients with TN tumors (7.1%; 95% CI, 0.9%–23.5%) died. During the follow-up period, the tumor recurred in nine patients: 4 were HER-2− tumors (1.1%; 95% CI, 0.3%–2.8%), 2 were HER-2+ tumors (7.1%; 95% CI, 0.9%–23.5%), and three were TN tumors (10.7%; 95% CI, 2.3%–28.2%). Death was recurrence related in one patient with a HER-2− tumor (0.3%; 95% CI, 0.01%–1.6%), no patient with a HER-2+ tumor (0%; 95% CI, 0%–12.3%), and one patient with a TN tumor (3.6%; 95% CI, 0.1%–18.4%). Only two of the nine patients who had recurrences received adjuvant chemotherapy. One of these patients had a HER-2+ tumor. Neither of the two patients with HER-2+ tumors who had recurrences received trastuzumab; one had a local relapse treated with surgery and hormonal therapy and the other had a systemic relapse treated with chemotherapy and trastuzumab. Both those patients had lumpectomy followed by adjuvant radiation therapy as their primary treatment. Five patients had systemic recurrences, three had local recurrences, and one had a recurrence in the opposite breast. The one recurrence in the contralateral breast occurred in the HER-2− group and was treated with surgery, radiation therapy, and chemotherapy.
Table 2.
Time to recurrence or death according to hormone receptor status
Abbreviations: HER, human epidermal growth factor receptor; NA, not applicable.
In the HER-2+ group, 12 cases were ER− and PR−. Of these, two patients had a relapse, one local and one distant. Thus, both the relapses in the HER-2+ group occurred in patients with hormone receptor–negative disease.
The Kaplan–Meier curves for overall survival and recurrence-free survival for the three groups are shown in Figures 1 and 2, respectively.
Figure 1.
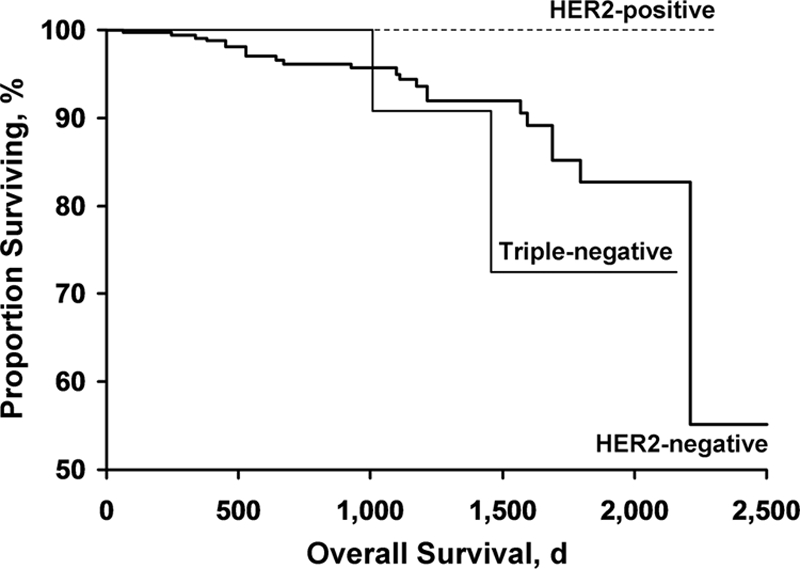
Kaplan–Meier curves for overall survival for the three groups.
Abbreviation: HER-2, human epidermal growth factor receptor 2.
Figure 2.
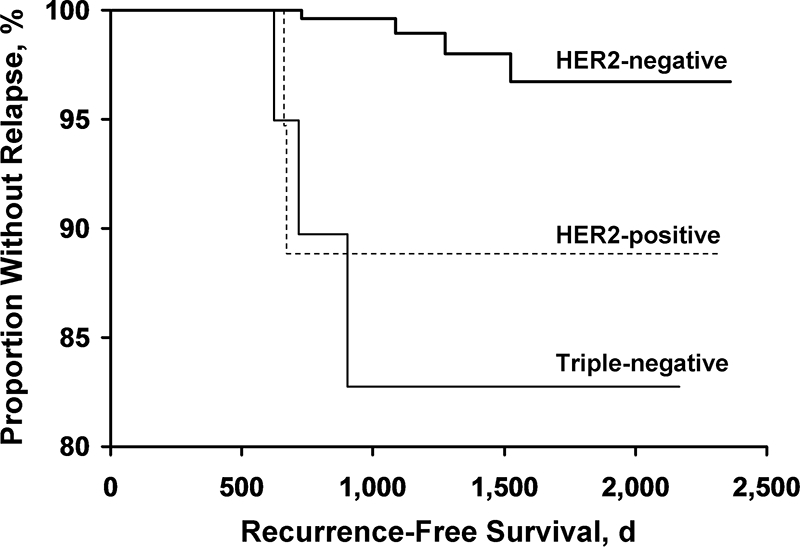
Kaplan–Meier curves for recurrence-free status for the three groups.
Abbreviation: HER-2, human epidermal growth factor receptor 2.
Discussion
The availability and effectiveness of adjuvant breast cancer therapy have led to improvements in disease outcomes but have also raised several questions. (a) Should all patients, irrespective of tumor size, receive adjuvant therapy? (b) Is there a role for adjuvant trastuzumab therapy for small (≤1 cm), node-negative tumors? (c) Should TN tumors be treated more aggressively, with a lower threshold for adjuvant treatment?
ER and HER-2 have been shown to be fairly reliable indicators of outcome in patients with invasive breast cancer >1 cm. However, the impact of tumor size on the decision to recommend systemic adjuvant therapies would be better predicted if there was a better understanding of the natural history of these small tumors. Several targeted treatments have a high likelihood of modifying the outcome of treatment in patients with small, node-negative breast cancer. These include antiestrogens, anti–HER-2 treatments, and chemotherapy. Various studies have reported that HER-2 status is an adverse prognostic marker in node-negative breast cancer patients, with higher risks for recurrence and death [6–9], but its prognostic importance in the subset of patients with small (≤1 cm) tumors is not clearly documented.
The development of trastuzumab, a humanized monoclonal antibody against HER-2, has been a major advance in the treatment of HER-2+ breast cancer [10–12]. Adjuvant trastuzumab therapy has become the standard of care for node-positive or high-risk node-negative breast cancers based on reports of recent trials. Two of those trials were led by the NSABP and the North Central Cancer Treatment Group (NCCTG), and the combined results were published in October 2005 and updated in 2007 [13, 14]. Both studies included patients with histologically proven, node-positive disease and high-risk node-negative disease (defined as ER+/PR+ tumors >2 cm in diameter or ER−/PR− tumors >1 cm in diameter). Only 191 patients (5.7%) included in the study were node negative, and only three of those patients had an event at the time of the analysis; hence, the effect of trastuzumab in this subgroup could not be commented upon.
The Herceptin® Adjuvant (HERA) trial was the third recently reported trial evaluating trastuzumab in the adjuvant setting in patients with node-positive tumors and node-negative tumors >1 cm [15, 16]. There were 1,100 node-negative cases (33% of all the patients enrolled) included in that study, with an observed benefit with trastuzumab (number of events in the trastuzumab versus observation arm, 20 versus 40; hazard ratio, 0.51).
The interim analysis of Breast Cancer International Research Group (BCIRG) protocol 006 compared the control arm of chemotherapy with two experimental arms evaluating the benefit of adding trastuzumab to chemotherapy. Trastuzumab led to a 51% higher recurrence-free survival rate when added to doxorubicin and cyclophosphamide followed by docetaxel and a 39% higher recurrence-free survival rate when used in the docetaxel and carboplatin plus concurrent trastuzumab combination, compared with the control arm [17]. Unlike the HERA trial and the NCCTG N9831 trial, the BCIRG 006 trial did include some tumors measuring <1 cm, although the exact numbers have not yet been disclosed. The BCIRG 006 trial also included patients with node-negative tumors, although the inclusion criteria were different from those for the NCCTG N9831 trial and the HERA trial.
Since these trials, the role of trastuzumab in the adjuvant setting has been well established, although these treatment recommendations cannot be routinely extended to patients with node-negative tumors that measure ≤1 cm. The prognostic importance of HER-2 positivity in small (≤1 cm), node-negative tumors and the potential benefit of extending trastuzumab to this group of patients remain to be evaluated.
A recently published study [18] reviewed the outcome of 965 patients with node-negative breast tumors measuring ≤1 cm. Patients with HER-2+ tumors had a higher risk for recurrence at 5 years (hazard ratio, 2.68; 95% CI, 1.44–5.0; p = .002) than those with HER-2− tumors. They did not report the effects of adjuvant chemotherapy or trastuzumab therapy or data on TN tumors separately.
Recently, another group of patients with hormone receptor–negative and HER-2− tumors was identified, who seem to have an overall worse outcome as a subset [19]. Whether this poor outcome extends to smaller tumors with this phenotype is unknown.
Our study provides insight into the natural history of small, node-negative tumors based on biological characteristics. Although as a class they seem to have an excellent outcome, certain subgroups (HER-2+ and TN tumors) in our dataset showed a significantly higher recurrence rate than others.
Of 28 patients with HER-2+ disease, seven (25%) received adjuvant chemotherapy, although only five of those regimens included trastuzumab. Two patients in this group had recurrences, one locally and the other systemically; neither of those two patients had received adjuvant trastuzumab.
Data on toxicity of adjuvant systemic therapy were not collected in our study. Although patients with HER-2+ and TN tumors were more likely to receive adjuvant chemotherapy, their outcome was worse than that of the HER-2− group. Hence, the question remains whether these patients derive any benefit from adjuvant therapy or if the risks of treatment outweigh the benefits. Larger studies with data on treatment toxicity and longer follow-up are needed to throw light on this issue.
Although provocative, these data are retrospective, patient numbers are fairly small, and the follow-up time is too short to make any sweeping recommendations or control for potential confounders such as treatment regimen. However, it is appropriate to conclude that traditional prognostic markers like tumor size and grade are no longer enough on their own to prognosticate accurately. Molecular profiling of cancers is the next frontier, and individualized therapy based on not only morphology but also cancer genotype is likely the future of oncology.
Acknowledgment
Presented as a poster at the 30th annual San Antonio Breast Cancer Symposium, San Antonio, Texas, December 13–16, 2007.
Author Contributions
Conception/Design: Surabhi Amar, Edith A. Perez, Ann E. McCullough, Winston Tan
Provision of study material or patients: Surabhi Amar, Frances M. Palmieri, Ann E. McCullough, Winston Tan, Judy C. Boughey, Rebecca B. McNeil
Collection and/or assembly of data: Surabhi Amar, Frances M. Palmieri, Ann E. McCullough, Xochiquetzal J. Geiger, Judy C. Boughey, Rebecca B. McNeil, Kyle E. Coppola
Data analysis and interpretation: Surabhi Amar, Sarah A. McLaughlin, Edith A. Perez, Winston Tan, Rebecca B. McNeil
Manuscript writing: Surabhi Amar, Sarah A. McLaughlin, Edith A. Perez, Ann E. McCullough, Winston Tan, Judy C. Boughey, Rebecca B. McNeil
Final approval of manuscript: Surabhi Amar, Sarah A. McLaughlin, Frances M. Palmieri, Edith A. Perez, Ann E. McCullough, Winston Tan, Judy C. Boughey, Rebecca B. McNeil
References
- 1.Tabar L, Yen MF, Vitak B, et al. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361:1405–1410. doi: 10.1016/S0140-6736(03)13143-1. [DOI] [PubMed] [Google Scholar]
- 2.Rosen PP, Groshen S, Saigo PE, et al. Pathological prognostic factors in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma: A study of 644 patients with median follow-up of 18 years. J Clin Oncol. 1989;7:1239–1251. doi: 10.1200/JCO.1989.7.9.1239. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Dignam J, Tan-Chiu E, et al. Prognosis and treatment of patients with breast tumors of one centimeter or less and negative axillary lymph nodes. J Natl Cancer Inst. 2001;93:112–120. doi: 10.1093/jnci/93.2.112. [DOI] [PubMed] [Google Scholar]
- 4.Rosen PR, Groshen S, Saigo PE, et al. A long-term follow-up study of survival in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma. J Clin Oncol. 1989;7:355–366. doi: 10.1200/JCO.1989.7.3.355. [DOI] [PubMed] [Google Scholar]
- 5.Chia S, Norris B, Speers C, et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol. 2008;26:5697–5704. doi: 10.1200/JCO.2007.15.8659. [DOI] [PubMed] [Google Scholar]
- 6.Ro JS, el-Naggar A, Ro JY, et al. c-erbB-2 amplification in node-negative human breast cancer. Cancer Res. 1989;49:6941–6944. [PubMed] [Google Scholar]
- 7.Gullick WJ, Love SB, Wright C, et al. c-erbB-2 protein overexpression in breast cancer is a risk factor in patients with involved and uninvolved lymph nodes. Br J Cancer. 1991;63:434–438. doi: 10.1038/bjc.1991.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Press MF, Bernstein L, Thomas PA, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: Poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997;15:2894–2904. doi: 10.1200/JCO.1997.15.8.2894. [DOI] [PubMed] [Google Scholar]
- 9.Allred DC, Clark GM, Tandon AK, et al. HER-2/neu in node-negative breast cancer: Prognostic significance of overexpression influenced by the presence of in situ carcinoma. J Clin Oncol. 1992;10:599–605. doi: 10.1200/JCO.1992.10.4.599. [DOI] [PubMed] [Google Scholar]
- 10.Burstein HJ. The distinctive nature of HER2-positive breast cancers. N Engl J Med. 2005;353:1652–1654. doi: 10.1056/NEJMp058197. [DOI] [PubMed] [Google Scholar]
- 11.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 12.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 13.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 14.Perez EA, Romond EH, Suman VJ, et al. Updated results of the combined analysis of NCCTG N9831 and NSABP B-31 adjuvant chemotherapy with/without trastuzumab in patients with HER2-positive breast cancer [abstract] J Clin Oncol. 2007;25(18 suppl):512. [Google Scholar]
- 15.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 16.Smith I, Procter M, Gelber RD, et al. HERA study team. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 17.Slamon D, Eiermann W, Pienkowski T, et al. BCIRG 006 Investigators. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG 006 study [abstract] Breast Cancer Res Treat. 2005;94(suppl 1):S5. [Google Scholar]
- 18.Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27:5700–5706. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakha EA, El-Sayed ME, Green AR, et al. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]