This review evaluates the available evidence and explores the role and importance of various modern chemotherapy regimens in the treatment of advanced non-small cell lung cancer, with the aim of optimizing treatment selection and combination with biological agents.
Keywords: Non-small cell lung carcinoma, Chemotherapy, Docetaxel, Treatment algorithm
Abstract
Despite recent progress in the development of new molecularly targeted agents, the chemotherapy regimens considered standard at the end of the last century—that is, two-drug combinations consisting of either cisplatin or carboplatin plus a third-generation agent (docetaxel, paclitaxel, gemcitabine, or vinorelbine)—remain the primary treatment option for advanced non-small cell lung cancer (NSCLC) patients. Most recently, the existing standard of care has been amended to reflect the significant survival advantage of cisplatin–pemetrexed over cisplatin–gemcitabine as first-line treatment of nonsquamous NSCLC. The addition of a biological drug (bevacizumab, cetuximab) or the use of a single-agent epidermal growth factor receptor inhibitor may further improve outcomes in selected patients.
It has become increasingly clear, primarily through recent meta-analyses, that although the therapeutic equivalence of any combination of a platinum agent plus either gemcitabine, vinorelbine, docetaxel, or paclitaxel has been long accepted, each regimen has different side effects and therapeutic outcomes that allow clinicians to select the most appropriate treatment for chemotherapy-naïve patients with stage IIIB/IV NSCLC. In this review, we evaluate the available evidence and explore the role and importance of various modern chemotherapy regimens, with the aim of optimizing treatment selection and combination with biological agents. Emphasis is placed on the role of taxanes (docetaxel versus paclitaxel) in this changing landscape.
Third-Generation Regimens
Historical Comparison
Since the end of the 1990s, it has been widely accepted that the efficacy of treatments for advanced non-small cell lung cancer (NSCLC) has reached a plateau [1], and that any combination of a platinum and a third-generation agent will produce similar survival results, despite efforts to modify treatment toward further improving outcomes. This belief was based on the findings of several randomized trials showing the objective equivalence of those regimens in terms of both efficacy and tolerability [2]. However, those trials were designed to detect substantial survival differences, thus reducing the possibility of discovering subtle, but relevant, outcome improvements in a disease for which survival is measured in months.
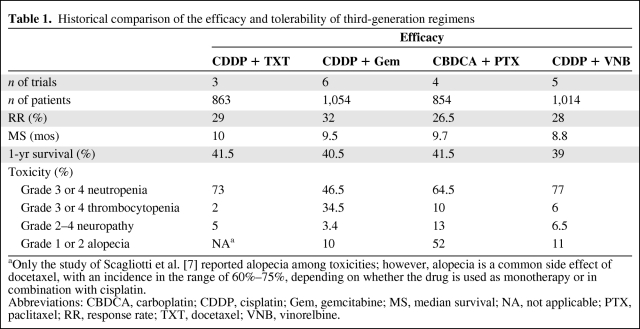
Taken together, the response rates (RRs) in trials of cisplatin (Platinol®; Bristol-Myers Squibb, Princeton, NJ) plus docetaxel (Taxotere®; Aventis Pharmaceuticals Inc., Bridgewater, NJ) and cisplatin plus gemcitabine (Gemzar®; Eli Lilly, Indianapolis, IN) appear to compare favorably with those of carboplatin (Paraplatin®; Bristol-Myers Squibb) plus paclitaxel (Taxol®; Bristol-Myers Squibb) [3–10]. Safety profiles vary, with some drugs associated with usually manageable toxicities such as neutropenia (vinorelbine [Navelbine®; GlaxoSmithKline, Philadelphia], docetaxel) and alopecia (paclitaxel, docetaxel), whereas others are associated with side effects such as thrombocytopenia (gemcitabine) and neurotoxicity (paclitaxel), which may require postponing scheduled treatments, reducing drug doses, or limiting treatment duration (Table 1) [3–10]. Furthermore, neurotoxicity often continues after the completion of chemotherapy and may have a detrimental effect on a patient's quality of life (QoL). Several randomized trials comparing docetaxel with vinca alkaloids showed that anemia, which also can negatively influence QoL, is less severe with docetaxel regimens [3, 11]. Considering that some trials that evaluated the use of erythropoiesis-stimulating agents during cancer treatment revealed inferior overall survival (OS) and progression-free survival (PFS) results [12, 13], anemia is an important factor in choosing the most appropriate chemotherapy regimen.
Table 1.
Historical comparison of the efficacy and tolerability of third-generation regimens
aOnly the study of Scagliotti et al. [7] reported alopecia among toxicities; however, alopecia is a common side effect of docetaxel, with an incidence in the range of 60%–75%, depending on whether the drug is used as monotherapy or in combination with cisplatin.
Abbreviations: CBDCA, carboplatin; CDDP, cisplatin; Gem, gemcitabine; MS, median survival; NA, not applicable; PTX, paclitaxel; RR, response rate; TXT, docetaxel; VNB, vinorelbine.
Docetaxel is associated with a high incidence of neutropenia, which represents its dose-limiting toxicity. Nonetheless, a recent meta-analysis has shown that the incidence of febrile neutropenia is 6% in NSCLC patients treated with second-line docetaxel [14], a relatively low proportion according to current European and American guidelines on the use of G-CSF. Docetaxel also yields significant nonhematologic toxicities, mainly grade 3–4 asthenia, with an incidence in the range of 12%–18% [15]. However, the docetaxel–platinum combination has demonstrated broad QoL benefits for chemotherapy-naïve patients with advanced NSCLC, which were not observed with other platinum or taxane–platinum combinations [16].
Overall, the historical comparison offers sufficient evidence of some differences in activity—although not in survival—and allows the characterization of toxicity profiles that have different implications for routine clinical practice.
Beyond historical observations, which should be interpreted with caution, hints of promising differences among modern regimens may emerge from two randomized clinical trials. In study TAX 326 [4], which compared cisplatin–vinorelbine with either cisplatin–docetaxel or carboplatin–docetaxel, survival estimates favored cisplatin–docetaxel, with a median survival time of 11.3 months—among the longest reached in similar trials—and a more promising 2-year survival rate (21% versus 14%). On the other hand, in study E1594 [5], comparing carboplatin–paclitaxel, cisplatin–docetaxel, and cisplatin–gemcitabine with a reference regimen of cisplatin–paclitaxel, the gemcitabine regimen showed a significantly longer time to progression and a trend toward a higher 2-year survival rate.
Evidence from Meta-Analyses
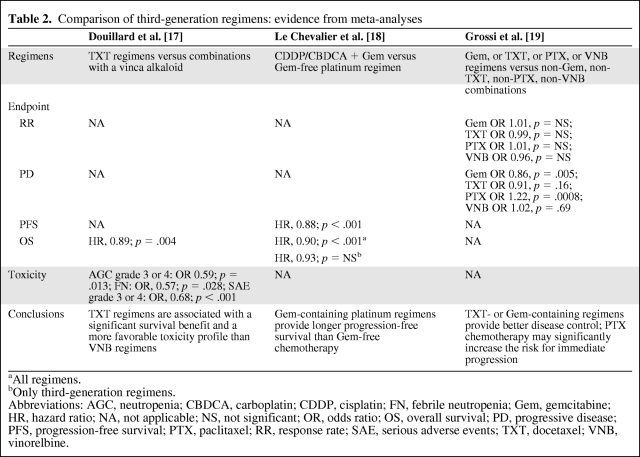
The suggestion that differences exist among the various third-generation regimens was confirmed by several recent meta-analyses (Table 2). Douillard and colleagues compared docetaxel-containing regimens with combinations including a vinca alkaloid, mainly vinorelbine [17]. That analysis showed that docetaxel-containing regimens provided significant benefits, both in terms of OS (hazard ratio [HR], 0.89; 95% confidence interval [CI], 0.82–0.96; p = .004) and tolerability, with a 41% lower rate of grade 3–4 neutropenia (p = .013) and a 43% lower rate of febrile neutropenia (p = .028), which was associated with less frequent use of G-CSF and fewer dose reductions. These benefits were maintained in all subgroup analyses. Accordingly, docetaxel emerged as the first drug to clearly establish its superior efficacy and tolerability over another third-generation agent. The findings of study E1594 were confirmed by a meta-analysis comparing platinum–gemcitabine chemotherapy with any nongemcitabine platinum regimen [18]. That analysis showed that platinum–gemcitabine regimens led to significantly longer PFS (HR, 0.88; 95% CI, 0.82–0.93; p < .001) and OS (HR, 0.90; 95% CI, 0.84–0.96; p < .001) times, although the OS benefit was no longer statistically significant when third-generation regimens were considered separately. The analysis included, almost exclusively, trials of vinorelbine and paclitaxel regimens; in only one study, patients on the comparator arm received a docetaxel-based therapy.
Table 2.
Comparison of third-generation regimens: evidence from meta-analyses
aAll regimens.
bOnly third-generation regimens.
Abbreviations: AGC, neutropenia; CBDCA, carboplatin; CDDP, cisplatin; FN, febrile neutropenia; Gem, gemcitabine; HR, hazard ratio; NA, not applicable; NS, not significant; OR, odds ratio; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PTX, paclitaxel; RR, response rate; SAE, serious adverse events; TXT, docetaxel; VNB, vinorelbine.
A recent meta-analysis [19] evaluated the RRs and progression rates in 18 randomized trials of platinum and nonplatinum doublets containing a third-generation agent versus regimens without the same third-generation drug (i.e., gemcitabine, paclitaxel, docetaxel, or vinorelbine-based doublets versus gemcitabine, paclitaxel, docetaxel, or vinorelbine-free combinations). The analysis found no statistically significant difference in RR; however, a significant 14% lower risk for immediate progression was observed, favoring gemcitabine over nongemcitabine regimens (odds ratio [OR], 0.86; 95% CI, 0.77–0.95; p = .005), which is in line with the PFS benefit observed by Le Chevalier and colleagues [18]. Docetaxel combinations exhibited a nonsignificant trend toward a lower risk for progression (OR, 0.91; 95% CI, 0.80–1.04; p = .16), whereas patients receiving paclitaxel had a 22% higher risk for immediate progression (OR, 1.22; 95% CI, 1.09–1.37; p = .0008). The risk for progression was similar with vinorelbine-containing and vinorelbine-free regimens. A possible limitation of the study is that, in most trials, gemcitabine and docetaxel were combined with cisplatin whereas paclitaxel was usually combined with carboplatin. Besides, additional analyses are necessary to show whether disease control (nonprogression) is related to a survival benefit and thus a valid surrogate endpoint.
In summary, the above cited meta-analyses [17–19] suggest that: docetaxel has superior efficacy and a better safety profile than vinorelbine [17], gemcitabine regimens may offer a significantly longer PFS time than nongemcitabine combinations [18], and gemcitabine and docetaxel are associated with better disease control, whereas patients receiving first-line paclitaxel combinations are at a significantly higher risk for progression as their best response [19]. In view of these results, cisplatin plus gemcitabine or docetaxel may be considered slightly superior to carboplatin–paclitaxel and cisplatin–vinorelbine.
Confusion over the role of carboplatin–paclitaxel stems from both the selection of paclitaxel, whose lower efficacy than other third-generation agents was demonstrated at least in terms of disease control [19], and from the unequivocal inferiority of carboplatin compared with cisplatin. A recent meta-analysis [20] of individual patient data from nine randomized trials definitively established that cisplatin-based third-generation regimens are associated with a higher RR (30% versus 24%; p < .001) and significant survival advantage (HR, 1.11; 95% CI, 1.01–1.21), compared with carboplatin combinations. Cisplatin produces higher rates of nausea/vomiting and nephrotoxicity, whereas carboplatin results in significantly more thrombocytopenia. Of note, contrary to previous recommendations for a 24-hour hospitalization following cisplatin infusion to reduce the risk for nephrotoxicity, several randomized trials and a retrospective analysis have confirmed that intermediate- to high-dose cisplatin may be safely administered in an outpatient setting using a short hydration regimen [21, 22].
Considering the evidence presented thus far, cisplatin–gemcitabine and cisplatin–docetaxel appear to be the best third-generation regimens to offer chemotherapy-naïve patients with advanced NSCLC. Because the only difference is in the drug that is combined with cisplatin, it is important to characterize differences among the regimens in terms of toxicity, administration schedule, and selection of patients. Differences in the docetaxel and gemcitabine safety profiles, with their diverse implications for routine management of patients, have already been discussed. The docetaxel schedule is easier to administer, with a single dose every 3 weeks instead of every 2 weeks, which may simplify the management of nonresident patients.
Individualized Treatment and New Agents for Patients with NSCLC
Individualized Treatment Approaches
Recently, more attention has been focused on selecting patients and individualizing treatment approaches, both through the use of biomarker-based targeted therapies and the customization of traditionally nonspecific approaches such as chemotherapy. In this regard, the very recent phase III trial comparing cisplatin–gemcitabine with cisplatin-pemetrexed (Alimta®; Eli Lilly and Company, Indianapolis, IN) provided some intriguing new evidence [23]. In that noninferiority trial, which reached the primary endpoint of OS, the most surprising findings emerged from a prospectively planned subgroup analysis, showing a survival benefit for pemetrexed over gemcitabine in patients with nonsquamous (adenocarcinomas, large-cell) tumors (median OS, 11.8 months versus 10.4 months; HR, 0.81; 95% CI, 0.70–0.94; p = .005); conversely, a longer survival time was associated with gemcitabine treatment in squamous tumors (10.8 months versus 9.4 months; HR, 1.23; 95% CI, 1.00–1.51; p = .05). These results probably relate to the different levels of thymidylate synthase (TS) expression among different histology subtypes [24]. Because tumor TS levels are inversely proportional to pemetrexed activity, different enzyme expression may contribute to significant variations in response [25].
This registration study established a new standard of care in the first-line treatment of nonsquamous NSCLC; accordingly, the existing second-line monotherapy indication of pemetrexed was amended to exclude the treatment of patients with predominantly squamous cell histology [26].
Although no head-to-head study has been performed to compare the efficacy and safety of cisplatin–pemetrexed with that of cisplatin–docetaxel in chemotherapy-naïve NSCLC patients, some supportive evidence may come both from the results of the phase III trial of pemetrexed versus docetaxel—which led to the registration of second-line pemetrexed—and from two retrospective analyses [27, 28] (Douillard JY, unpublished data).
In the study by Hanna and colleagues, pemetrexed benefits were similar to those of docetaxel in terms of the RR and median PFS and OS times, but pemetrexed was better tolerated, producing significantly less severe neutropenia and febrile neutropenia, alopecia, and peripheral neuropathy. Patients in the experimental arm also required significantly fewer hospitalizations and less need for G-CSF support [27].
In a retrospective analysis of that study, significant treatment-by-histology interactions indicated longer PFS and OS times for nonsquamous patients treated with pemetrexed and for squamous patients receiving docetaxel, respectively. The survival benefit of pemetrexed appeared most pronounced in the comparison of patients with large-cell tumors with patients with squamous-cell tumors (median OS, 12.8 months and 6.2 months, respectively), whereas the difference between pemetrexed and docetaxel was not evident in patients with adenocarcinoma (median OS, 9 months versus 9.2 months, respectively) [28]. Overall, differently from pemetrexed, the activity and efficacy of docetaxel did not appear to be influenced by tumor histology (median survival time in patients with squamous versus nonsquamous tumors receiving docetaxel, 7.4 months versus 8 months, respectively) (Table 3) [28]. Similarly, data from the docetaxel versus vinorelbine meta-analysis [17] show that survival varies according to histology for vinorelbine (10.4 months and 9.69 months in nonsquamous and squamous tumors, respectively; p = .018), whereas the difference is not significant in patients receiving docetaxel (p = .15) (Douillard JY, unpublished data). Therefore, it seems that histology does not exert any influence on docetaxel efficacy, as opposed to gemcitabine, pemetrexed, or vinorelbine,
Table 3.
Retrospective analysis of the randomized phase II study of pemetrexed versus docetaxel in previously treated non-small cell lung cancer patients: treatment-by-histology interaction
Abbreviations: CI, confidence interval; HR, hazard ratio; MS, median survival; NOS, not otherwise specified.
Taken together, this evidence supports the preferential, although not exclusive, use of cisplatin–pemetrexed in adenocarcinomas and large-cell tumors, for both safety reasons and efficacy, whereas cisplatin–docetaxel may represent the regimen of choice in the first-line treatment of not otherwise specified (NOS) tumors. With respect to squamous tumors, considerations of the docetaxel and gemcitabine safety profiles, as well as the flexibility of their different schedules, have already been presented.
Very recently, the randomized phase III IRESSA Non-small cell lung cancer Trial Evaluating Response and Survival against Taxotere (INTEREST) study compared second-line docetaxel with gefitinib (Iressa®; AstraZeneca Pharmaceuticals, Wilmington, DE), a biological agent targeting the tyrosine kinase associated with the epidermal growth factor receptor (EGFR), in 1,466 patients with locally advanced or metastatic NSCLC. In the overall population, no statistically significant difference was observed for the OS and PFS times and RR; however, the PFS results significantly favored gefitinib treatment in patients with EGFR activating mutations, whereas the OS results did not [29]. Gefitinib was also associated with lower rates of treatment-related adverse events, and significantly more patients on gefitinib had a sustained and clinically relevant improvement in QoL, as assessed by Functional Assessment of Cancer Therapy–Lung questionnaires.
In another phase III open-label trial (Iressa Pan-Asia Study [IPASS]), comparing gefitinib with carboplatin–paclitaxel in 1,217 chemotherapy-naïve patients with selected features (Asian ethnicity, adenocarcinoma histology, never-smokers or light ex-smokers), gefitinib achieved a superior PFS results, compared with chemotherapy (HR, 0.74; 95% CI, 0.65–0.85; p < .001) [30]; the treatment effect was not constant over time, favoring chemotherapy during the first 6 months and gefitinib for the remaining 16 months of follow-up. This variation is likely a result of different PFS outcomes according to mutation status, with the PFS time significantly longer for gefitinib in EGFR mutation–positive patients (HR, 0.48; 95% CI, 0.36–0.64; p < .001) and significantly longer for carboplatin–paclitaxel in EGFR mutation–negative patients (HR, 2.85; 95% CI, 2.05–3.98; p < .001). Analysis of OS data is pending [30].
A very recent study compared first-line gefitinib with cisplatin–docetaxel in 177 Asian patients selected on the basis of EGFR mutation status [31]. Overall, patients treated with gefitinib had a significantly longer PFS duration than those treated with cisplatin plus docetaxel (HR, 0.489; 95% CI, 0.336–0.710; p < .0001). Both groups experienced adverse events, with myelosuppression, alopecia, and fatigue reported more frequently in the chemotherapy group, whereas skin toxicity, liver dysfunction, and diarrhea were more frequent in the gefitinib group. The data for the OS duration are still immature, and follow-up is ongoing [31].
In April 2009, the European Medicines Agency (EMEA) granted marketing authorization for the use of gefitinib in NSCLC patients showing EGFR activating mutations [32], and the strategy of identifying such patients and steering them to targeted therapy has become a novel standard of care. Nonetheless, some open issues remain, particularly the feasibility of detecting EGFR mutations in routine clinical practice and the reproducibility of these findings in white patients, showing a much lower frequency of EGFR mutations, 10%–15%.
New Drugs
Recent studies evaluated the effects of adding biological agents to standard first-line chemotherapy in advanced NSCLC, mainly cetuximab (Erbitux®; ImClone Systems, Inc., New York), a monoclonal antibody targeting EGFR, and bevacizumab (Avastin®; Genentech Inc., South San Francisco, CA), which acts by blocking the vascular endothelial growth factor (VEGF) signaling pathway. The randomized phase III First-Line Erbitux in Lung Cancer (FLEX) trial [33] evaluated cisplatin–vinorelbine with or without cetuximab in 1,125 patients with EGFR+ tumors. Treatment duration was for a maximum of six cycles and cetuximab was administered as maintenance therapy until either disease progression or unacceptable toxicity. There was a significant benefit in favor of the cetuximab arm in terms of the OS time (median, 11.3 months versus 10.1 months; HR, 0.871; 95% CI, 0.762–0.996; p = .044), RR, and time to treatment failure.
Noticeably, no PFS benefit was seen in favor of the experimental group in the FLEX trial, a surprising finding because the trend of PFS is expected to mirror and precede that of OS. Furthermore, considering that the rates of grade 3–4 adverse events and febrile neutropenia were 91% versus 86% (p = .01) and 22% versus 15% (p = .0086) with cisplatin–vinorelbine with and without cetuximab, respectively, it appears that cisplatin–vinorelbine may not be the best combination chemotherapy to use with cetuximab.
In a parallel randomized phase III trial (BMS 099), 676 chemotherapy-naïve patients, without restrictions by histology or EGFR expression, were treated with carboplatin plus a taxane (either paclitaxel or docetaxel depending on investigator preference) with or without cetuximab [34]. Despite a significant benefit in RR, no statistically significant benefit was observed with respect to either PFS (HR, 0.902; 95% CI, 0.761–1.069; p = .236) or OS (HR, 0.890; 95% CI, 0.754–1.051; p = .169) [34]. Even though the study probably lacked the statistical power to show an OS benefit, the magnitude of the OS benefit was similar in the FLEX and BMS 099 trials. The role of cetuximab combined with first-line chemotherapy remains to be established, and additional trials are planned with other platinum-based combinations.
Bevacizumab was the first molecularly targeted agent to offer a significant survival benefit when added to standard chemotherapy in the first-line treatment of selected patients with advanced nonsquamous NSCLC. The Eastern Cooperative Oncology Group randomized phase III study (E4599) of 878 patients treated with carboplatin–paclitaxel with or without bevacizumab (15 mg/kg) showed a significantly higher RR and longer PFS and OS times in the experimental arm [35]. These data were not confirmed by a subsequent European trial (Avastin in Lung cancer [AVAiL]), which evaluated two doses of bevacizumab (7.5 mg/kg or 15 mg/kg) added to cisplatin–gemcitabine versus cisplatin–gemcitabine alone, showing a significantly longer PFS interval without any OS benefit [36]. Taken together with the results of the FLEX study [33], which appear numerically identical to those seen in the TAX 326 study [4], these findings suggest that the addition of a biologic agent might strengthen a weaker chemotherapy, but offers no further benefit when good disease control is provided by a well-selected cytotoxic regimen. Still, given the randomized nature of these studies, other possible explanations should be considered, such as differences in patient characteristics or the impact of further treatments on OS. Paclitaxel has also been shown to inhibit angiogenesis and tumor metastasis [37]; hence, simultaneously targeting VEGF could amplify the antitumor effects of chemotherapy.
However, the results of the above cited meta-analyses may assist in the selection of the best regimen to combine with bevacizumab. The CISplatin versus CArboplatin (CISCA) meta-analysis [20] favored the use of cisplatin over carboplatin in combination with third-generation agents and in patients with nonsquamous tumors (the subset for which the use of bevacizumab is approved). As for third-generation drugs, considering that paclitaxel and vinorelbine are inferior to gemcitabine and docetaxel [17–19], selection should be limited to the latter. Still, in the AVAiL trial [36], no OS benefit was seen when bevacizumab was added to cisplatin–gemcitabine, and in this regard cisplatin–docetaxel could emerge as a reasonable alternative combination chemotherapy.
A phase II feasibility study presented at the 2010 Congress of the American Society of Clinical Oncology showed an acceptable toxicity profile for this regimen, with a promising 67% objective RR, a median OS of 12.7 months, and a 1-year survival of 53.46% [38]. One could challenge the idea that cisplatin–pemetrexed is superior to cisplatin–gemcitabine in nonsquamous tumors [23] and should then be considered as the ideal chemotherapy doublet for a randomized study with bevacizumab. This combination was studied by Patel and colleagues in a trial of 50 patients that included a maintenance phase of bevacizumab plus pemetrexed, and that showed high activity (RR, 55%; disease control rate, 88%), good tolerability, and a promising median PFS duration (7.8 months) and OS time (14.1 months). Based on the statistical design of the study, the regimen warrants further consideration [39].
Yet, the independence of docetaxel efficacy from a specific histological type [28] (Douillard JY, unpublished data) should be taken into account when considering that most bevacizumab studies are currently intended to broaden the use of the drug to selected squamous-cell tumors. Furthermore, there is a strong preclinical rationale for combining docetaxel and bevacizumab, given the observed in vitro and in vivo inhibition of angiogenesis by docetaxel, which is fourfold higher than that of paclitaxel. Because high VEGF levels may protect tumor cells from the antiangiogenic properties of the taxane, the association with a VEGF blocker may be a strategy worthy of further evaluation [40].
Finally, the use of cisplatin–pemetrexed plus bevacizumab could be limited by cost constraints. It has been estimated that, given the longer survival observed in study E4599, the addition of bevacizumab to chemotherapy costs about U.S. $350,000 per year of life gained [41]. As for pemetrexed, the cost per cycle is high, almost $4,000 in the U.S., particularly when compared with a cost of $1,500 for one cycle of docetaxel [42].
Future Perspectives for First-Line Docetaxel: Sequential and Consolidation Chemotherapy
Recently, in an attempt to improve outcomes using traditional chemotherapy, alternative treatment schedules were proposed, particularly, sequential and maintenance/consolidation therapy. Sequential chemotherapy involves the administration of noncrossresistant chemotherapy, either as a single agent or in a combination regimen, in succession for a defined number of cycles. The switch from one treatment to another does not require documented progression and is generally independent of the response to the first part of the treatment sequence. This approach allows the delivery of a higher number of noncrossresistant drugs, both to optimize doses and to limit toxicities [43].
Several phase II studies have evaluated the activity and toxicity of a sequential versus a combination regimen (usually platinum based) followed by a single agent (often paclitaxel or docetaxel). Overall, RRs are encouraging, in the range of 21%–55%, with mild toxicities [43]. Based on the results of a phase II study of gemcitabine–vinorelbine followed by sequential docetaxel [44], Kubota and colleagues conducted a randomized phase III study comparing a sequential regimen of first-line vinorelbine–gemcitabine followed by docetaxel with standard treatment with carboplatin–paclitaxel [45]. Although results do not support either a PFS (5.5 months versus 5.8 months; p = .742) or an OS (13.6 months versus 14.1 months; p = .97) benefit for the experimental nonplatinum arm, the toxicity profile favors sequential treatment, with significantly lower rates of myelosuppression, neuropathy, pain, and myalgia [45].
The maintenance/consolidation approach implies that nonprogressing patients after standard first-line chemotherapy receive additional treatment, either for a defined number of cycles (consolidation) or until evidence of progression (maintenance). Maintenance/consolidation chemotherapy can incorporate a drug that was also included in the induction regimen or a noncrossresistant agent [43]. In this framework, Fidias and colleagues recently published the results of a pivotal phase III study, in which chemotherapy-naïve patients not progressing after four cycles of carboplatin–gemcitabine were randomized to second-line 3-weekly docetaxel either immediately after completing first-line treatment or at disease progression [46]. Results showed that docetaxel hematological toxicity was not influenced by the timing of treatment, with grade 3–4 neutropenia and febrile neutropenia rates of 27.6% and 28.6% and 3.5% and 2% in the immediate and delayed groups, respectively. No significant difference in the primary endpoint of OS was observed (12.3 months versus 9.7 months; p = .0853), but the proportion of patients alive at 12 months favored the maintenance arm over the control arm (51.1% versus 43.5%, respectively). Furthermore, the PFS interval was longer in the immediate group (median, 5.7 months versus 2.7 months; p = .0001). That study showed that first-line docetaxel, although used in a sequential approach, is more convenient than administration at disease progression.
Ciuleanu and colleagues recently published a phase III study of pemetrexed versus placebo in patients with stage IIIB/IV NSCLC who had not progressed on four cycles of a platinum doublet without pemetrexed [47]. In the 663 randomized patients, pemetrexed resulted in both a longer PFS interval (4.3 months versus 2.6 months; HR, 0.5; 95% CI, 0.42–0.61; p < .0001) and a longer OS time (13.4 months versus 10.6 months; HR, 0.79; 95% CI, 0.65–0.95; p = .012). Such improvements were observed primarily in patients with nonsquamous histology (HR, 0.47 and 0.7 for progression and death, respectively). Maintenance pemetrexed was well tolerated, although the rate of drug-related grade 3 and 4 toxicities was higher in the experimental arm (16% versus 4%; p < .0001) [47]. Based on these results, both the U.S. Food and Drug Administration (FDA) and the EMEA Agency have now approved pemetrexed as maintenance therapy for patients with locally advanced or metastatic NSCLC having at least stable disease after four cycles of platinum-based first-line chemotherapy. The indication is restricted to patients with other than predominantly squamous-cell histology [48, 49].
The phase III Sequential Tarceva in Unresectable NSCLC trial (SATURN) evaluated the efficacy and tolerability of erlotinib (Tarceva®; Genentech, Inc.) as maintenance therapy in patients who completed initial treatment with conventional chemotherapy [50]. Maintenance erlotinib provided a 41% longer PFS time, the primary endpoint, than placebo (HR, 0.71; 95% CI, 0.62–0.82; p = .000003). The median OS time was also longer in the experimental arm (12 months versus 11 months), with a 10% absolute difference between the treatment and placebo groups at 3 years [51]. A prospective molecular marker analysis showed that the PFS and OS benefits were not driven by EGFR status and may also be seen in patients with wild-type EGFR tumors [51]. In April 2010, the U.S. FDA approved erlotinib as maintenance treatment for patients with locally advanced or metastatic NSCLC whose disease had not progressed after four cycles of platinum-based first-line chemotherapy [52]. At the same time, the EMEA Committee for Medicinal Products for Human Use adopted a positive opinion recommending a variation in the erlotinib indication to include the maintenance treatment of advanced NSCLC patients with stable disease after four cycles of standard platinum-based first-line chemotherapy [53].
A literature-based meta-analysis of 14 randomized trials (including trials of both maintenance and consolidation chemotherapy, and comparisons of four cycles with six cycles of the same chemotherapy) explored the optimal duration of first-line chemotherapy. The analysis showed a substantial PFS benefit with a longer treatment duration (HR, 0.75; 95% CI, 0.69–0.81; p < .00001) and a moderate OS benefit (HR, 0.92; 95% CI, 0.86–0.99; p = .03), at the cost of a higher rate of adverse events [54].
Conclusions: A Decision Algorithm for Treating Patients with Advanced NSCLC
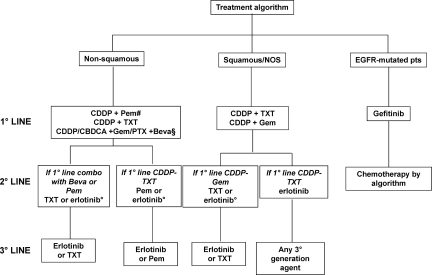
Despite the existence of an approximate therapeutic equivalence across different third-generation regimens, the results of recent meta-analyses and randomized studies allow the identification of an optimum treatment strategy that takes into account clinical and preclinical evidence, characteristics of the patients, disease biology and histology, and treatment choices in the first-line setting (Fig. 1).
Figure 1.
A possible decision algorithm for the first-, second-, and third-line treatment of “fit” patients with advanced non-small cell lung cancer (NSCLC).
#At present, pemetrexed may be used for maintenance treatment of patients with advanced nonsquamous NSCLC whose disease has not progressed after four cycles of a pemetrexed-free platinum doublet. Maintenance erlotinib may be an option in all advanced NSCLC patients with stable disease after four cycles of platinum-based chemotherapy.
§In selected cases.
°Preferably in tumors harboring EGFR mutations.
Abbreviations: Beva, bevacizumab; CBDCA, carboplatin; CDDP, cisplatin; EGFR-TKIs, epidermal growth factor receptor tyrosine kinase inhibitors; Gem, gemcitabine; NOS, not otherwise specified; Pem, pemetrexed; PTX, paclitaxel; TXT, docetaxel.
The algorithm is specifically designed for the treatment of young, fit (performance status score of 0–1) patients with advanced NSCLC. In nonmutated patients, it recommends the first-line use of a cisplatin doublet, with either docetaxel or gemcitabine in tumors with unspecified or squamous histology, and pemetrexed or docetaxel for nonsquamous tumors. Patients with nonsquamous tumors who have not progressed after four cycles of a pemetrexed-free platinum doublet may be offered maintenance pemetrexed until progression or unacceptable toxicity. In very selected patients, the option of adding bevacizumab to a platinum agent plus gemcitabine or paclitaxel should also be considered.
At the time of disease progression, patients with squamous and NOS tumors may receive erlotinib, in the case of first-line treatment with docetaxel, and either docetaxel or erlotinib if gemcitabine was used upfront. However, erlotinib should be selected preferably based on the presence of EGFR mutation. Docetaxel or pemetrexed or erlotinib represent appropriate second-line agents for the management of nonsquamous tumors, depending on previous treatment and on the presence of EGFR mutation.
Finally, both erlotinib and docetaxel represent an optimal third-line option in tumors of any histology, unless used formerly; in that case, pemetrexed may be an appropriate alternative for nonsquamous tumors, whereas any third-generation agent may be administered to patients with squamous-cell or NOS tumors.
An alternative strategy is suggested for the treatment of EGFR-mutated patients. Based on data from the IPASS trial, it is now reasonable to conclude that, in such patients, first-line treatment with an EGFR tyrosine kinase inhibitor (TKI) may be preferable to chemotherapy. Upon disease progression, patients who maintain a good performance status may receive additional treatment based on the algorithm and tumor histology. Specific and irreversible inhibitors of EGFR carrying second mutations are in clinical development and may represent a promising new approach to EGFR mutation–positive patients with disease progression following front-line EGFR TKI therapy and second-line chemotherapy [55].
Acknowledgment
English-language editing was provided by Sanofi-Aventis.
Author Contributions
Conception/Design: Francesco Grossi
Collection and/or assembly of data: Francesco Grossi, Federico Cappuzzo, Filippo de Marinis, Cesare Gridelli, Marianna Aita, Jean-Yves Douillard, Kaoru Kubota
Data analysis and interpretation: Francesco Grossi, Federico Cappuzzo, Filippo de Marinis, Cesare Gridelli, Marianna Aita, Jean-Yves Douillard, Kaoru Kubota
Manuscript writing: Marianna Aita
Final approval of manuscript: Francesco Grossi, Federico Cappuzzo, Filippo de Marinis, Cesare Gridelli, Marianna Aita, Jean-Yves Douillard, Kaoru Kubota
References
- 1.Shepherd FA. Chemotherapy for non-small cell lung cancer: Have we reached a new plateau? Semin Oncol. 1999;26(suppl 4):3–11. [PubMed] [Google Scholar]
- 2.Grossi F, Gridelli C, Aita M, et al. Identifying an optimum treatment strategy for patients with advanced non-small cell lung cancer. Crit Rev Oncol Hematol. 2008;67:16–26. doi: 10.1016/j.critrevonc.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Kubota K, Watanabe K, Kunitoh H, et al. Phase III randomized trial of docetaxel plus cisplatin versus vindesine plus cisplatin in patients with stage IV non-small-cell lung cancer: The Japanese Taxotere Lung Cancer Study Group. J Clin Oncol. 2004;22:254–261. doi: 10.1200/JCO.2004.06.114. [DOI] [PubMed] [Google Scholar]
- 4.Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: The TAX 326 study group. J Clin Oncol. 2003;21:3016–3024. doi: 10.1200/JCO.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 5.Schiller JH, Harrington D, Belani C, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 6.Kelly K, Crowley J, Bunn PA, Jr, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: A Southwest Oncology Group trial. J Clin Oncol. 2001;19:3210–3218. doi: 10.1200/JCO.2001.19.13.3210. [DOI] [PubMed] [Google Scholar]
- 7.Scagliotti GV, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–4291. doi: 10.1200/JCO.2002.02.068. [DOI] [PubMed] [Google Scholar]
- 8.Smit EF, van Meerbeeck JP, Lianes P, et al. Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer: A phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group—EORTC 08975. J Clin Oncol. 2003;21:3909–3917. doi: 10.1200/JCO.2003.03.195. [DOI] [PubMed] [Google Scholar]
- 9.Alberola V, Camps C, Provencio M, et al. Cisplatin plus gemcitabine versus a cisplatin-based triplet versus nonplatinum sequential doublets in advanced non-small-cell lung cancer: A Spanish Lung Cancer Group phase III randomized trial. J Clin Oncol. 2003;21:3207–3213. doi: 10.1200/JCO.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 10.Kubota K, Nishiwaki Y, Ohashi Y, et al. The Four-Arm Cooperative Study (FACS) for advanced non-small-cell lung cancer (NSCLC) J Clin Oncol. 2004;22(suppl 14):616. [Google Scholar]
- 11.Douillard JY, Gervais R, Dabouis G, et al. Sequential two-line strategy for stage IV non-small-cell lung cancer: Docetaxel-cisplatin versus vinorelbine-cisplatin followed by cross-over to single-agent docetaxel or vinorelbine at progression: Final results of a randomised phase II study. Ann Oncol. 2005;16:81–89. doi: 10.1093/annonc/mdi013. [DOI] [PubMed] [Google Scholar]
- 12.Henke M, Laszig R, Rübe C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 13.Leyland-Jones B BEST Investigators and Study Group. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol. 2003;4:459–460. doi: 10.1016/s1470-2045(03)01163-x. [DOI] [PubMed] [Google Scholar]
- 14.Wailoo A, Sutton A, Morgan A. The risk of febrile neutropenia in patients with non-small-cell lung cancer treated with docetaxel: A systematic review and meta-analysis. Br J Cancer. 2009;100:436–441. doi: 10.1038/sj.bjc.6604863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Marinis F, Grossi F. Clinical evidence for second- and third-line treatment options in advanced non-small cell lung cancer. The Oncologist. 2008;13(suppl 1):14–20. doi: 10.1634/theoncologist.13-S1-14. [DOI] [PubMed] [Google Scholar]
- 16.Rigas JR. Taxane-platinum combinations in advanced non-small cell lung cancer: A review. The Oncologist. 2004;9(suppl 2):16–23. doi: 10.1634/theoncologist.9-suppl_2-16. [DOI] [PubMed] [Google Scholar]
- 17.Douillard JY, Laporte S, Fossella F, et al. Comparison of docetaxel- and vinca alkaloid-based chemotherapy in the first-line treatment of advanced non-small cell lung cancer: A meta-analysis of seven randomized clinical trials. J Thorac Oncol. 2007;2:939–946. doi: 10.1097/JTO.0b013e318153fa2b. [DOI] [PubMed] [Google Scholar]
- 18.Le Chevalier T, Scagliotti G, Natale R, et al. Efficacy of gemcitabine plus platinum chemotherapy compared with other platinum containing regimens in advanced non-small-cell lung cancer: A meta-analysis of survival outcomes. Lung Cancer. 2005;47:69–80. doi: 10.1016/j.lungcan.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Grossi F, Aita M, Defferrari C, et al. Impact of third-generation drugs on the activity of first-line chemotherapy in advanced non-small cell lung cancer: A meta-analytical approach. The Oncologist. 2009;14:497–510. doi: 10.1634/theoncologist.2008-0260. [DOI] [PubMed] [Google Scholar]
- 20.Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: An individual patient data meta-analysis. J Natl Cancer Inst. 2007;99:847–857. doi: 10.1093/jnci/djk196. [DOI] [PubMed] [Google Scholar]
- 21.Mor V, Stalker MZ, Gralla R, et al. Day hospital as an alternative to inpatient care for cancer patients: A random assignment trial. J Clin Epidemiol. 1988;41:771–785. doi: 10.1016/0895-4356(88)90164-3. [DOI] [PubMed] [Google Scholar]
- 22.Tiseo M, Martelli O, Mancuso A, et al. Short hydration regimen and nephrotoxicity of intermediate to high-dose cisplatin-based chemotherapy for outpatient treatment in lung cancer and mesothelioma. Tumori. 2007;93:138–144. doi: 10.1177/030089160709300205. [DOI] [PubMed] [Google Scholar]
- 23.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 24.Ceppi P, Volante M, Saviozzi S, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer. 2006;107:1589–1596. doi: 10.1002/cncr.22208. [DOI] [PubMed] [Google Scholar]
- 25.Giovannetti E, Mey V, Nannizzi S, et al. Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small-cell lung cancer cells. Mol Pharmacol. 2005;68:110–118. doi: 10.1124/mol.104.009373. [DOI] [PubMed] [Google Scholar]
- 26.European Medicines Agency. Alimta: EPAR-Product Information. [accessed August 28, 2010]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000564/WC500025611.pdf.
- 27.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 28.Peterson P, Park K, Fossella F, et al. Is pemetrexed more effective in adenocarcinoma and large cell lung cancer than in squamous cell carcinoma? A retrospective analysis of a phase III trial of pemetrexed vs docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC) [abstract P2–328] J Thorac Oncol. 2007;2(suppl 4):S851. [Google Scholar]
- 29.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): A randomised phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 30.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 31.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 32.European Medicines Agency. Public Assessment Report for Iressa. [accessed August 28, 2010]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001016/WC500036358.pdf.
- 33.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): An open-label randomised phase III trial. Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 34.Lynch TJ, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: Results of the randomized multicenter phase III trial BMS099. J Clin Oncol. 2010;28:911–917. doi: 10.1200/JCO.2009.21.9618. [DOI] [PubMed] [Google Scholar]
- 35.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 36.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 37.Belotti D, Vergani V, Drudis T, et al. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res. 1996;2:1843–1849. [PubMed] [Google Scholar]
- 38.Cobo M, Ferrer N, Paredes A, et al. Phase II study of bevacizumab in combination with cisplatin and docetaxel as first-line treatment of patients (p) with metastatic non-squamous non-small cell lung cancer (NSCLC): Final report. J Clin Oncol. 2010;28(15 suppl):e18009. [Google Scholar]
- 39.Patel JD, Hensing TA, Rademaker A, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer. J Clin Oncol. 2009;27:3284–3289. doi: 10.1200/JCO.2008.20.8181. [DOI] [PubMed] [Google Scholar]
- 40.Herbst RS, Khuri FR. Mode of action of docetaxel - a basis for combination with novel anticancer agents. Cancer Treat Rev. 2003;29:407–415. doi: 10.1016/s0305-7372(03)00097-5. [DOI] [PubMed] [Google Scholar]
- 41.Grusenmeyer PA, Gralla RJ. Examining the cost and cost-effectiveness of adding bevacizumab to carboplatin and paclitaxel in advanced non-small cell lung cancer [abstract 6057] J Clin Oncol. 2006;24(18 suppl):314s. [Google Scholar]
- 42.Muhsin M, Gricks C, Kirkpatrick P. Pemetrexed disodium. Nat Rev Drug Discov. 2004;3:825–826. doi: 10.1038/nrd1528. [DOI] [PubMed] [Google Scholar]
- 43.Grossi F, Aita M, Follador A, et al. Sequential, alternating, and maintenance/consolidation chemotherapy in advanced NSCLC: A review of the literature. The Oncologist. 2007;12:451–464. doi: 10.1634/theoncologist.12-4-451. [DOI] [PubMed] [Google Scholar]
- 44.Hosoe S, Komuta K, Shibata K, et al. Gemcitabine and vinorelbine followed by docetaxel in patients with advanced non-small-cell lung cancer: A multi-institutional phase II trial of nonplatinum sequential triplet combination chemotherapy (JMTO LC00–02) Br J Cancer. 2003;88:342–347. doi: 10.1038/sj.bjc.6600723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kubota K, Kawahara M, Ogawara M, et al. Vinorelbine plus gemcitabine followed by docetaxel versus carboplatin plus paclitaxel in patients with advanced non-small-cell lung cancer: A randomised, open-label, phase III study. Lancet Oncol. 2008;9:1135–1142. doi: 10.1016/S1470-2045(08)70261-4. [DOI] [PubMed] [Google Scholar]
- 46.Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:591–598. doi: 10.1200/JCO.2008.17.1405. [DOI] [PubMed] [Google Scholar]
- 47.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: A randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 48.U.S. Food and Drug Administration. Center for Drug Evaluation and Research. Alimta® [label]. Approved on 07/02/2009, NDA no. 021462. [accessed October 16, 2009]. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021462s021lbl.pdf.
- 49.European Medicines Agency. Alimta-H-564-II-15: EPAR-Scientific Discussion-Variation. [accessed August 28, 2010]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion_-_Variation/human/000564/WC500025610.pdf.
- 50.Cappuzzo F, Coudert BP, Wierzbicki R, et al. Efficacy and safety of erlotinib as first-line maintenance in NSCLC following non-progression with chemotherapy: Results from the phase III SATURN study. Presented at the 13th World Conference on Lung Cancer; July 31 – August 4, 2009; San Francisco, CA. [Google Scholar]
- 51.Boughton B. New SATURN Results Show Survival Benefit From Erlotinib in NSCLC. [accessed October 16, 2009]. Available at http://www.medscape.com/viewarticle/706862.
- 52.U.S. Food and Drug Administration. Tarceva® (erlotinib) [label] [accessed April 25, 2010]. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021743s14s16lbl.pdf.
- 53.European Medicines Agency. Tarceva: EPAR-Assessment Report- Variation. [accessed August 28, 2010]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000618/WC500090680.pdf.
- 54.Soon Y, Stockler MR, Askie LM, et al. Duration of chemotherapy for advanced non-small cell lung cancer: A systematic review and meta-analysis of randomized trials. J Clin Oncol. 2009;27:3277–3283. doi: 10.1200/JCO.2008.19.4522. [DOI] [PubMed] [Google Scholar]
- 55.Riely GJ. Second-generation epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. J Thorac Oncol. 2008;3(suppl 2):S146–S149. doi: 10.1097/JTO.0b013e318174e96e. [DOI] [PubMed] [Google Scholar]