A review of the literature was used to compare the tolerability, efficacy, and safety profiles of pegylated liposomal doxorubicin in combination with carboplatin with those of gemcitabine–carboplatin for the treatment of patients with platinum-sensitive recurrent ovarian cancer.
Keywords: Ovarian cancer, Platinum, Pegylated liposomal doxorubicin, Gemcitabine, Carboplatin
Abstract
Objective.
To compare the tolerability, efficacy, and safety profiles of pegylated liposomal doxorubicin in combination with carboplatin (PLD–Carbo) with those of gemcitabine–carboplatin (Gem–Carbo) for the treatment of patients with platinum-sensitive recurrent ovarian cancer (PSROC) by reviewing the published literature.
Methods.
Using the PubMed database, a systematic review of peer-reviewed literature published between January 2000 and September 2009 was undertaken to identify studies related to the treatment of patients with PSROC with PLD–Carbo or Gem–Carbo. Studies reporting either response rate, progression-free survival (PFS), and/or overall survival (OS) were included. Treatment regimens, efficacy endpoints, and safety profiles were compared between the two combination therapies.
Results.
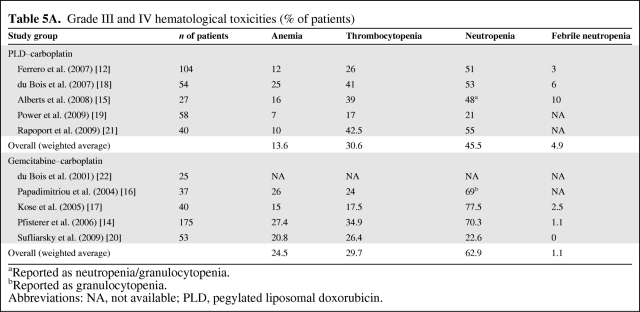
Ten studies evaluating 608 patients (PLD–Carbo: 5 studies, 278 patients; Gem–Carbo: 5 studies, 330 patients) were identified. The mean planned doses were: PLD, 34.8 mg/m2 and Gem, 993 mg/m2. The dose intensity reported in Gem trials was lower (75% of the planned dose) than the dose intensity reported in PLD trials (93.7% of the planned dose), suggesting better tolerability for the PLD–Carbo regimen. Among patients receiving PLD–Carbo, 60.2% achieved a response (complete, 27.0%; partial, 33.2%), versus 51.4% of patients treated with Gem–Carbo (complete, 19.2%; partial, 32.2%). The median PFS times were 10.6 months and 8.9 months in the PLD–Carbo and the Gem–Carbo populations, respectively. The median OS was longer for the PLD–Carbo regimen (27.1 months) than for the Gem–Carbo regimen (19.7 months). The hematological safety profiles were comparable in the two groups, although grade III or IV anemia (PLD–Carbo, 13.6%; Gem–Carbo, 24.5%) and neutropenia (PLD–Carbo, 45.5%; Gem–Carbo, 62.9%) were more common in patients receiving Gem–Carbo.
Conclusion.
Results from this systematic analysis of peer-reviewed literature suggest that PLD–Carbo therapy is a rational alternative to Gem–Carbo for the treatment of patients with PSROC.
Introduction
Ovarian cancer is the leading cause of death attributed to gynecologic malignancies for women in the U.S. [1]. The standard approach for treating ovarian cancer is debulking surgery followed by combination platinum and taxane chemotherapy, usually carboplatin and paclitaxel [2–5]. Intraperitoneal cisplatin and intravenous paclitaxel have further augmented response and time to progression, and pushed the median survival to >5 years for optimally resected patients [6]. Although significant progress in the treatment of ovarian cancer has been recognized, the majority of patients experience disease recurrence and receive additional second- and third-line chemotherapy regimens. Patients with ovarian cancer who experience a relapse >6 months following completion of first-line platinum-based chemotherapy treatment are considered to have “platinum-sensitive” ovarian cancer. These patients are considered good candidates for second-line platinum-based chemotherapy regimens because they often achieve a second response, with the likelihood of response related to the time interval from completion of first-line therapy or the progression-free interval [7, 8]. Although the paclitaxel–carboplatin combination is an established regimen for first-line therapy of ovarian cancer, the cumulative neurotoxicity of both platinum and paclitaxel raises important concerns about the retreatment of patients with recurrent disease using the same combination [4, 5, 9–11].
Several antitumor agents with novel mechanisms of action possess significant documented clinical activity in the treatment of patients with platinum-sensitive recurrent ovarian cancer (PSROC). These agents include gemcitabine (Gemzar®; Eli Lilly and Company, Indianapolis, IN), a nucleoside analog, and pegylated liposomal doxorubicin (PLD) (Doxil®; Ortho Biotech Products, L.P., Raritan, NJ; known as Caelyx® outside the U.S.), a nucleic acid synthesis inhibitor with a longer circulation time than conventional doxorubicin, which allows for a lower peak plasma concentration and greater tumor doxorubicin concentration [9, 12]. The use of these agents in combination with platinum agents has shown better progression-free survival (PFS) and overall survival (OS) results compared with carboplatin alone [9, 12–15]. Based on the reported longer PFS time than seen with single-agent carboplatin, the U.S. Food and Drug Administration recently approved gemcitabine in combination with carboplatin for the treatment of patients with advanced ovarian cancer who relapsed ≥6 months after completion of platinum-based therapy. Furthermore, several prospective phase II studies have evaluated the tolerability and efficacy profiles of the PLD–carboplatin and gemcitabine–carboplatin combination therapies in recurrent ovarian cancer patients, highlighting their efficacy and suggesting that these combination therapies are safe and effective for the treatment of PSROC [12, 16–18].
Although the PLD–carboplatin and gemcitabine–carboplatin combinations have been studied in many clinical trials, no prospective, randomized, phase III comparison trials of their relative efficacy and safety profiles have been conducted. The objective of the current study was to compare the tolerability, efficacy, and safety profiles of PLD plus carboplatin with those of gemcitabine plus carboplatin for the treatment of patients with PSROC by reviewing published literature.
Methods
Search Strategy
Using the PubMed database, we performed a systematic search of peer-reviewed literature published between January 2000 and September 2009 for studies related to the PLD–carboplatin and gemcitabine–carboplatin combinations in patients with PSROC. The search was performed using the following boolean search: “ovarian” AND ((“doxorubicin” and “carboplatin”) OR (“gemcitabine” and “carboplatin”)). Results of the search were initially analyzed in title and abstract format. Reference sections from the selected publications were checked for additional studies. Items were selected based on an initial search for a full-text analysis to determine the eligibility of the article for our analysis.
Criteria for Inclusion of Studies for Analysis
We included all studies that reported either the response rate, time to disease progression, and/or OS in patients receiving the PLD–carboplatin or gemcitabine–carboplatin combination for the treatment of PSROC. Studies reported in languages other than English were included in this review if the relevant data for the analysis were available from the abstract.
Data Extraction
Two reviewers, P.L. and F.V., independently applied the inclusion criteria and assessed the quality of the data collected. Each reviewer evaluated relevant data from the eligible studies and entered the information into a Microsoft Excel data collection form with prespecified fields. If relevant data were reported only graphically, values were estimated from the graphs. Quality control was done by comparing the two independent datasets, and any differences were reconciled by a third party, R.W.H., referencing to the original sources.
Information regarding the study design, patient characteristics, outcomes, and safety were collected for each eligible study. This included the number of patients enrolled, relevant aspects of the study design (e.g., definition of response), baseline characteristics of the patients (e.g., age, treatment-free interval), dosing regimen (e.g., planned dose, number of cycles, dose intensity), efficacy endpoints (e.g., PFS, OS), and proportion of patients experiencing toxicities (hematological and nonhematological).
Analytical Approach
Studies included in the analysis were stratified based on whether patients were treated with the PLD–carboplatin or gemcitabine–carboplatin combination. The main outcome measures upon which the comparisons across treatment group were based were response rate (overall, complete, and partial response), PFS, OS, and proportion of patients with grade III or IV hematological and nonhematological toxicities. Three definitions were used to determined response rate in the eligible studies: the World Health Organization (WHO) criteria, the Response Evaluation Criteria in Solid Tumors (RECIST), and the Southwest Oncology Group (SWOG) criteria, with or without the addition of serum cancer antigen 125 levels. The time to disease progression (or PFS) and OS time were both reported as the median number of months. Finally, endpoint results for each treatment group were pooled together using a weighted average.
Pooled univariate statistics were generated for the PLD–carboplatin and the gemcitabine–carboplatin studies. Frequency counts and percentages were used to summarize categorical variables whereas means or medians were used for continuous variables. Because of the limited number of studies considered and, more importantly, the absence of information regarding the variability of the measurements for some of the endpoints, no formal statistical comparisons between groups were conducted.
Results
Selection of Studies
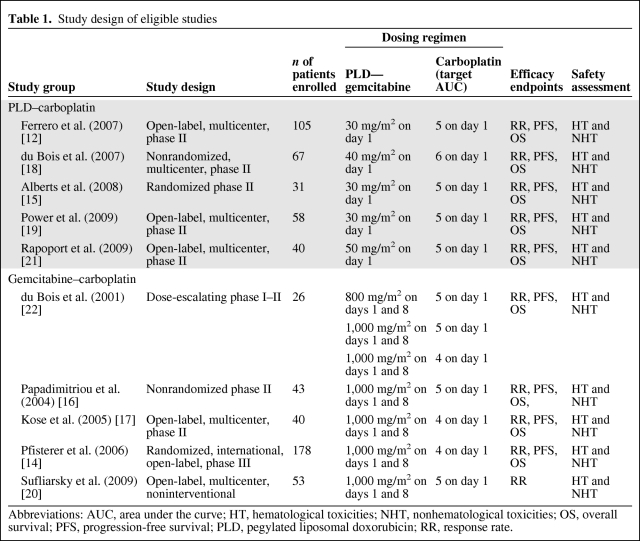
The initial search yielded 311 studies from PubMed or the reference sections of identified PubMed publications. In total, 10 studies were eligible for the current analysis [12, 14–22]. Reviews of the existing literature, letters to the editor, editorials, practice guidelines, case reports, and duplicate reports (n = 137); in vitro studies (n = 31); and studies not in English (n = 1) were excluded. In addition, studies in which the treatment regimens of interest were not considered (n = 79) or were not given as first-line therapy (n = 5) or in which the efficacy endpoints of interest were not considered (n = 48) were also excluded. The remaining study set included five unique PLD–carboplatin studies with nonoverlapping patient populations and five studies of the gemcitabine–carboplatin regimen. Table 1 summarizes the study designs of the eligible 10 studies.
Table 1.
Study design of eligible studies
Abbreviations: AUC, area under the curve; HT, hematological toxicities; NHT, nonhematological toxicities; OS, overall survival; PFS, progression-free survival; PLD, pegylated liposomal doxorubicin; RR, response rate.
Patient Characteristics
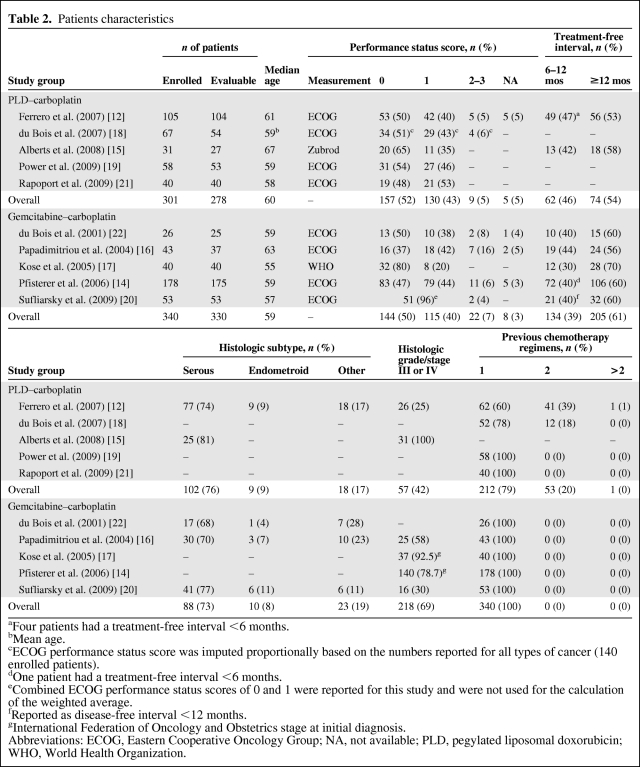
In total, 278 patients treated with PLD–carboplatin and 330 patients treated with gemcitabine–carboplatin were evaluable. Table 2 describes their baseline characteristics. Patients in both treatment groups had similar median ages (PLD–carboplatin, 60 years; gemcitabine–carboplatin, 59 years) and Eastern Cooperative Oncology Group (ECOG) performance status scores, whereas the treatment-free interval was slightly longer in gemcitabine–carboplatin-treated patients (≥12 months: PLD–carboplatin, 54%; gemcitabine–carboplatin, 61%) at baseline. Few studies reported the histological subtype of tumor. Among those that reported this information, a similar proportion of patients in both treatment groups had serous (PLD–carboplatin, 76%; gemcitabine–carboplatin, 73%) or endometroid (PLD–carboplatin, 9%; gemcitabine–carboplatin, 8%) tumors. A greater proportion of patients in the gemcitabine–carboplatin group had tumor grade III or IV than in the PLD–carboplatin group (69% versus 42%).
Table 2.
Patients characteristics
aFour patients had a treatment-free interval <6 months.
bMean age.
cECOG performance status score was imputed proportionally based on the numbers reported for all types of cancer (140 enrolled patients).
dOne patient had a treatment-free interval <6 months.
eCombined ECOG performance status scores of 0 and 1 were reported for this study and were not used for the calculation of the weighted average.
fReported as disease-free interval <12 months.
gInternational Federation of Oncology and Obstetrics stage at initial diagnosis.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NA, not available; PLD, pegylated liposomal doxorubicin; WHO, World Health Organization.
Treatment Regimens, Tolerability, and Efficacy
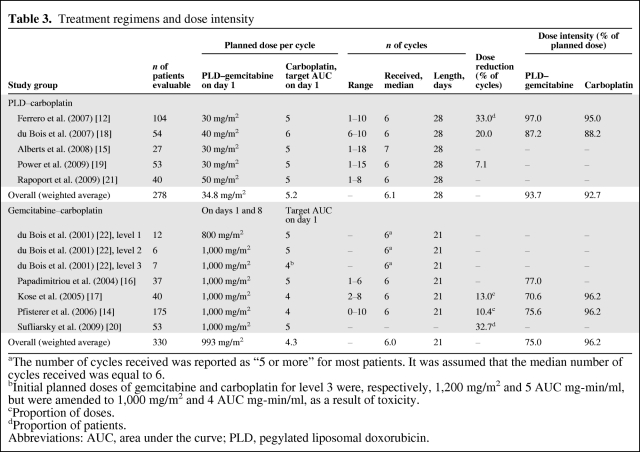
Table 3 describes the treatment regimens. The mean planned target carboplatin areas under the curve (AUC) were, respectively, 5.2 (range, 5–6) and 4.3 (range, 4–5) with the PLD–carboplatin and the gemcitabine–carboplatin regimens. The mean planned doses were 34.8 mg/m2 (day 1 range, 30–50 mg/m2) for PLD and 993 mg/m2 (days 1 and 8 range, 800–1,000 mg/m2) for gemcitabine. Overall, the dose intensity reported in gemcitabine trials was lower (75.0% of the planned dose) than that in PLD trials (93.7% of the planned dose).
Table 3.
Treatment regimens and dose intensity
aThe number of cycles received was reported as “5 or more” for most patients. It was assumed that the median number of cycles received was equal to 6.
bInitial planned doses of gemcitabine and carboplatin for level 3 were, respectively, 1,200 mg/m2 and 5 AUC mg-min/ml, but were amended to 1,000 mg/m2 and 4 AUC mg-min/ml, as a result of toxicity.
cProportion of doses.
dProportion of patients.
Abbreviations: AUC, area under the curve; PLD, pegylated liposomal doxorubicin.
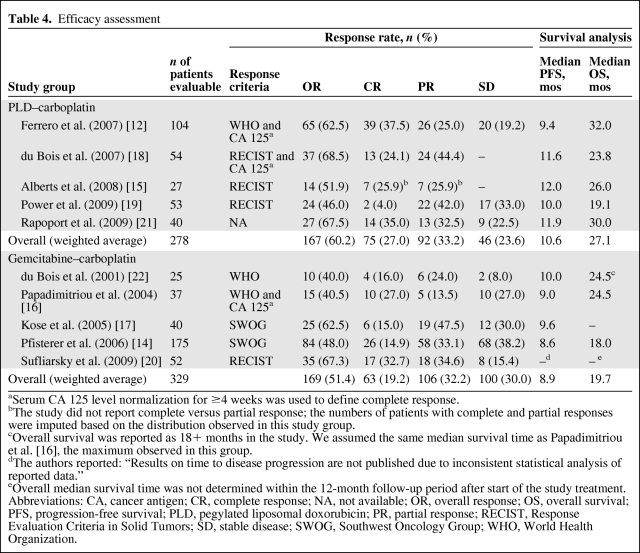
Among patients receiving the PLD–carboplatin combination, 60.2% achieved a response (complete, 27.0%; partial, 33.2%), versus 51.4% of patients treated with the gemcitabine–carboplatin regimen (complete, 19.2%; partial, 32.2%) (Table 4). The proportion of patients with stable disease was greater in the gemcitabine–carboplatin group than in the PLD–carboplatin group (30.0% versus 23.6%). The median PFS intervals were 10.6 months and 8.9 months in the PLD–carboplatin and the gemcitabine–carboplatin populations, respectively. The median OS time was longer for patients treated with the PLD–carboplatin regimen (27.1 months) than for those treated with the gemcitabine–carboplatin regimen (19.7 months).
Table 4.
Efficacy assessment
aSerum CA 125 level normalization for ≥4 weeks was used to define complete response.
bThe study did not report complete versus partial response; the numbers of patients with complete and partial responses were imputed based on the distribution observed in this study group.
cOverall survival was reported as 18+ months in the study. We assumed the same median survival time as Papadimitriou et al. [16], the maximum observed in this group.
dThe authors reported: “Results on time to disease progression are not published due to inconsistent statistical analysis of reported data.”
eOverall median survival time was not determined within the 12-month follow-up period after start of the study treatment.
Abbreviations: CA, cancer antigen; CR, complete response; NA, not available; OR, overall response; OS, overall survival; PFS, progression-free survival; PLD, pegylated liposomal doxorubicin; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; SWOG, Southwest Oncology Group; WHO, World Health Organization.
Safety
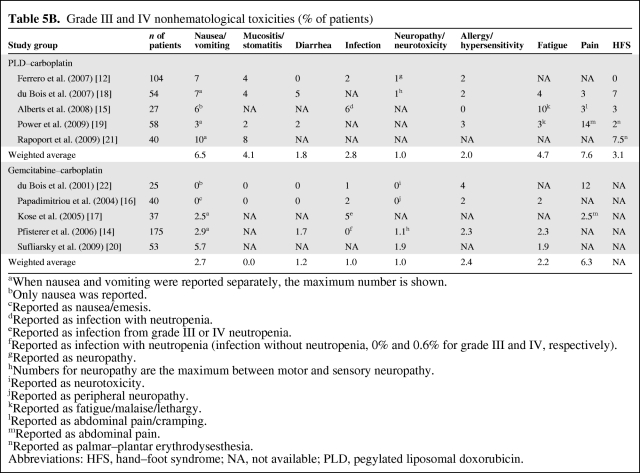
The hematological safety profiles (anemia, thrombocytopenia, and neutropenia) were comparable in the two groups, although grade III or IV anemia (PLD–carboplatin, 13.6%; gemcitabine–carboplatin, 24.5%) and neutropenia (PLD–carboplatin, 45.5%; gemcitabine–carboplatin, 62.9%) were more common in patients receiving gemcitabine–carboplatin (Table 5A). The mean proportion of patients with grade III or IV hand–foot syndrome (HFS) was 3.1% in PLD–carboplatin studies (information not reported in gemcitabine–carboplatin studies) (Table 5B). The incidences of other grade III or IV nonhematological toxicities were comparable between the two treatment regimens (Table 5B).
Table 5A.
Grade III and IV hematological toxicities (% of patients)
aReported as neutropenia/granulocytopenia.
bReported as granulocytopenia.
Abbreviations: NA, not available; PLD, pegylated liposomal doxorubicin.
Table 5B.
Grade III and IV nonhematological toxicities (% of patients)
aWhen nausea and vomiting were reported separately, the maximum number is shown.
bOnly nausea was reported.
cReported as nausea/emesis.
dReported as infection with neutropenia.
eReported as infection from grade III or IV neutropenia.
fReported as infection with neutropenia (infection without neutropenia, 0% and 0.6% for grade III and IV, respectively).
gReported as neuropathy.
hNumbers for neuropathy are the maximum between motor and sensory neuropathy.
iReported as neurotoxicity.
jReported as peripheral neuropathy.
kReported as fatigue/malaise/lethargy.
lReported as abdominal pain/cramping.
mReported as abdominal pain.
nReported as palmar–plantar erythrodysesthesia.
Abbreviations: HFS, hand–foot syndrome; NA, not available; PLD, pegylated liposomal doxorubicin.
Discussion
This current systematic review of the published literature is the first to compare the relative tolerability, efficacy, and safety profiles of the PLD–carboplatin and the gemcitabine–carboplatin combinations in patients with PSROC. These novel platinum-based regimens have been well received by the clinical community, considering the limited choice of second-line treatments for ovarian cancer patients. Indeed, the cumulative neurotoxicity of the widely used platinum and taxane-based regimen in the first-line therapy of ovarian cancer limits its use in recurrent disease. In a study of 87 patients with gynecologic malignancies receiving initial dosing of carboplatin at an AUC of 5 or 6 and paclitaxel at a dose of 175 mg/m2 (3-hour infusion), 25% of patients developed peripheral neuropathy, of whom 13% were considered to be a grade ≥2 in severity [23].
Platinum sensitivity and the platinum-free interval are the cornerstone factors for tumor response in PSROC patients [12]. Results from the current review suggest that patients treated with the PLD–carboplatin combination had a better response rate than those treated with the gemcitabine–carboplatin regimen (60.2% versus 51.4%), despite the slightly lower proportion of patients with a treatment-free interval ≥12 months observed in the PLD–carboplatin group (54% versus 61%). Whereas the PFS times were comparable in the two groups (10.6 months and 8.9 months in the PLD–carboplatin and the gemcitabine–carboplatin populations, respectively), the OS time was 7 months longer in the PLD–carboplatin group. It is possible that the higher delivered dose intensity for PLD than for gemcitabine may have influenced these findings. Despite the observed trend in favor of the PLD–carboplatin regimen, we acknowledge that OS might not be the most appropriate measure of efficacy in second-line therapy. That is, in trials involving patients with a poor cancer prognosis, data on survival are likely to be confounded by poststudy treatments [14]. This situation is further exacerbated by the fact that standardized treatments are difficult to achieve in patients with recurrent ovarian cancer [24].
The higher delivered dose intensity for PLD than for gemcitabine suggests better tolerability for the PLD–carboplatin regimen in light of comparable efficacies in the two groups. This result is strengthened by the fact that the mean target carboplatin AUC was higher with the PLD–carboplatin regimen than with the gemcitabine–carboplatin regimen (5.2 versus 4.3). The suggested lower tolerability of the gemcitabine–carboplatin combination might explain the observed differences in the incidences of anemia and neutropenia in patients receiving this treatment regimen. There seems, however, to be a trend toward a higher incidence of nonhematologic adverse events with the PLD–carboplatin regimen. Considering that second-line therapies are rarely curative for patients with recurrent ovarian cancer, other factors, such as chemotherapy tolerability and quality of life, must be taken into account. This is particularly relevant for patients who have experienced substantial toxicities during their first-line treatments. However, although tolerability could be compared between the two groups, a lack of data prevented an analysis of the impact on quality of life of these two regimens.
Of note, the current study findings for the PLD–carboplatin regimen are consistent with recent results (CALYPSO trial) presented at the 2009 annual meeting of the American Society of Clinical Oncology. The CALYPSO study, an open-label, international, multicenter, randomized phase III trial, evaluated the efficacy and safety of PLD (30 mg/m2 on day 1) and carboplatin (AUC of 5 on day 1) with those of paclitaxel–carboplatin in patients with epithelial ovarian cancer in late relapse (>6 months) [25]. The CALYPSO trial enrolled a total of 466 patients in the PLD–carboplatin arm. The median age was 60.5 years; 61%, 34%, and 3% of patients had an ECOG performance status score of 0, 1, and 2, respectively. The median treatment duration was 21 weeks, with a dose intensity of 99.0% for both PLD and carboplatin, versus 93.7% and 92.7% in our study, respectively (Table 3). The median PFS interval reported in the CALYPSO (11.3 months) was slightly longer than what we observed in the current study (10.6 months) (Table 4). Finally, the incidences of both hematological and nonhematological toxicities of grades III and IV reported in the CALYPSO trial were lower than what we found: anemia, 8%; thrombocytopenia, 16%; neutropenia, 35%; febrile neutropenia, 2%; nausea/vomiting, 4%; mucositis/stomatitis, 2%; diarrhea, 2%; infection, 3%; fatigue, 7%; and HFS, 2%.
This review has several limitations. First, the number of studies considered for each regimen was relatively low. In addition, no PLD–carboplatin versus gemcitabine–carboplatin head-to-head clinical trial was available in the literature. Second, the lack of primary source data from the original studies and the absence of a reported variance in most of the studies prevented proper statistical comparisons between the two groups for most endpoints. In addition, because of the absence of a reported variance, other commonly used pooling methods, such as meta-analysis with fixed or random effects, could not be applied. Third, although all studies were carefully selected from peer-reviewed journals, we did not assess the quality or reporting of the original studies. Fourth, in this analysis, pooled results were weighted based on the number of evaluable patients in each study, which favors studies with larger sample sizes. Fifth, it is possible that the availability of PLD–carboplatin and gemcitabine–carboplatin in countries where the studies were conducted may have influenced the population recruited. However, the selection of patients was primarily dictated by trial protocol, and as such, patient inclusion/exclusion criteria were similar across studies. Sixth, pooled results for the gemcitabine–carboplatin group were dependent on the Pfisterer et al. [14] study because of its relatively large population compared with the other gemcitabine–carboplatin studies. Lastly, we acknowledge that the criteria used to evaluate response rates were not consistent across studies, and therefore all patients might not have been evaluated for response on the same basis. This is especially true because the RECIST criteria were used in most of the PLD–carboplatin trials whereas the SWOG criteria were used in the gemcitabine–carboplatin trials. However, although it is generally accepted that the WHO, the RECIST, and the SWOG criteria do not give the exact same response rates, there seems to be no indication of important biases toward lower or greater response rates with one set of criteria over the other [26–29]. Additionally, other reported outcomes (OS and PFS) were not influenced by differences in response criteria. In light of these limitations, the results from the current study must be seen as hypothesis-generating.
Conclusion
Results from this systematic analysis of peer-reviewed literature suggest that PLD–carboplatin therapy is a rational and clinically reasonable alternative to gemcitabine–carboplatin for the treatment of PSROC patients. The delivered dose intensity for the PLD–carboplatin regimens was higher than that for the gemcitabine–carboplatin regimens. The PFS intervals appeared to be similar for two treatments, but the incidence of grade III or IV hematologic toxicity was higher in the gemcitabine–carboplatin trials. A phase III, randomized clinical trial is needed to evaluate the relative efficacy and safety profiles of PLD–carboplatin versus gemcitabine–carboplatin in PSROC patients.
Acknowledgments
This research was supported by Centocor Ortho Biotech Services, LLC.
Parts of this work were presented at the 40th Society of Gynecologic Oncologists (SGO) Annual Meeting on Women's Cancer, San Antonio, TX, February 5–8, 2009.
Author Contributions
Conception/Design: Patrick Lefebvre, Robert W. Holloway, Edward C. Grendys, Scott McMeekin
Collection and/or assembly of data: Patrick Lefebvre, Francis Vekeman
Data analysis and interpretation: Patrick Lefebvre, Robert W. Holloway, Edward C. Grendys, Francis Vekeman, Scott McMeekin
Manuscript writing: Patrick Lefebvre, Robert W. Holloway, Edward C.
Grendys, Francis Vekeman, Scott McMeekin
Final approval of manuscript: Patrick Lefebvre, Robert W. Holloway, Edward C. Grendys, Francis Vekeman, Scott McMeekin
References
- 1.Ries LAG, Melbert D, Krapcho M, et al. Bethesda, MD: National Cancer Institute; 2008. [accessed June 25, 2009]. SEER Cancer Statistics Review, 1975–2004. Available at http://seer.cancer.gov/csr/1975_2005/ [Google Scholar]
- 2.Berek JS, Bertelsen K, Du Bois A, et al. Advanced epithelial ovarian cancer: 1998 consensus statements. Ann Oncol. 1999;10(suppl 1):87–92. doi: 10.1023/a:1008323922057. [DOI] [PubMed] [Google Scholar]
- 3.Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma in the platinum era: A meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 4.du Bois A, Lück HJ, Meier W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–1329. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 5.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gyncologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 7.Gore ME, Fryatt I, Wiltshaw E, et al. Treatment of relapsed carcinoma of the ovary with cisplatin or carboplatin following initial treatment with these compounds. Gynecol Oncol. 1990;36:207–211. doi: 10.1016/0090-8258(90)90174-j. [DOI] [PubMed] [Google Scholar]
- 8.Markman M, Rothman R, Hakes T, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991;9:389–393. doi: 10.1200/JCO.1991.9.3.389. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong DK. Relapsed ovarian cancer: Challenges and management strategies for a chronic disease. The Oncologist. 2002;7(suppl 5):20–28. doi: 10.1634/theoncologist.7-suppl_5-20. [DOI] [PubMed] [Google Scholar]
- 10.du Bois A, Schlaich M, Lück HJ, et al. Evaluation of neurotoxicity induced by paclitaxel second-line chemotherapy. Support Care Cancer. 1999;7:354–361. doi: 10.1007/s005200050275. [DOI] [PubMed] [Google Scholar]
- 11.Markman M. Managing taxane toxicities. Support Care Cancer. 2003;11:144–147. doi: 10.1007/s00520-002-0405-9. [DOI] [PubMed] [Google Scholar]
- 12.Ferrero JM, Weber B, Geay JF, et al. Second-line chemotherapy with pegylated liposomal doxorubicin and carboplatin is highly effective in patients with advanced ovarian cancer in late relapse: A GINECO phase II trial. Ann Oncol. 2007;18:263–268. doi: 10.1093/annonc/mdl376. [DOI] [PubMed] [Google Scholar]
- 13.Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: The ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–2106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 14.Pfisterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: An intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–4707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 15.Alberts DS, Liu PY, Wilczynski SP, et al. Randomized trial of pegylated liposomal doxorubicin (PLD) plus carboplatin versus carboplatin in platinum-sensitive (PS) patients with recurrent epithelial ovarian or peritoneal carcinoma after failure of initial platinum-based chemotherapy (Southwest Oncology Group Protocol S0200) Gynecol Oncol. 2008;108:90–94. doi: 10.1016/j.ygyno.2007.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadimitriou CA, Fountzilas G, Aravantinos G, et al. Second-line chemotherapy with gemcitabine and carboplatin in paclitaxel-pretreated, platinum-sensitive ovarian cancer patients. A Hellenic Cooperative Oncology Group study. Gynecol Oncol. 2004;92:152–159. doi: 10.1016/j.ygyno.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Kose MF, Sufliarsky J, Beslija S, et al. A phase II study of gemcitabine plus carboplatin in platinum-sensitive, recurrent ovarian carcinoma. Gynecol Oncol. 2005;96:374–380. doi: 10.1016/j.ygyno.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 18.du Bois A, Pfisterer J, Burchardi N, et al. Combination therapy with pegylated liposomal doxorubicin and carboplatin in gynecologic malignancies: A prospective phase II study of the Arbeitsgemeinschaft Gynäekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and Kommission Uterus (AGO-K-Ut) Gynecol Oncol. 2007;107:518–525. doi: 10.1016/j.ygyno.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Power P, Stuart G, Oza A, et al. Efficacy of pegylated liposomal doxorubicin (PLD) plus carboplatin in ovarian cancer patients who recur within six to twelve months: A phase II study. Gynecol Oncol. 2009;114:410–414. doi: 10.1016/j.ygyno.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 20.Sufliarsky J, Chovanec J, Svetlovska D, et al. Gemcitabine and carboplatin treatment in patients with relapsing ovarian cancer. Neoplasma. 2009;56:291–297. doi: 10.4149/neo_2009_04_291. [DOI] [PubMed] [Google Scholar]
- 21.Rapoport BL, Vorobiof DA, Slabber C, et al. Phase II study of pegylated liposomal doxorubicin and carboplatin in patients with platinum-sensitive and partially platinum-sensitive metastatic ovarian cancer. Int J Gynecol Cancer. 2009;19:1137–1141. doi: 10.1111/IGC.0b013e3181a8b938. [DOI] [PubMed] [Google Scholar]
- 22.du Bois A, Lück HJ, Pfisterer J, et al. Second-line carboplatin and gemcitabine in platinum sensitive ovarian cancer—a dose-finding study by the Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) Ovarian Cancer Study Group. Ann Oncol. 2001;12:1115–1120. doi: 10.1023/a:1011605008922. [DOI] [PubMed] [Google Scholar]
- 23.Markman M, Kennedy A, Webster K, et al. Neurotoxicity associated with a regimen of carboplatin (AUC 5–6) and paclitaxel (175 mg/m2 over 3 h) employed in the treatment of gynecologic malignancies. J Cancer Res Clin Oncol. 2001;127:55–58. doi: 10.1007/s004320000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thigpen T, Stuart G, du Bois A, et al. Clinical trials in ovarian carcinoma: Requirements for standard approaches and regimens. Ann Oncol. 2005;16(suppl 8):viii13–viii19. doi: 10.1093/annonc/mdi962. [DOI] [PubMed] [Google Scholar]
- 25.Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol. 2010;28:3323–3329. doi: 10.1200/JCO.2009.25.7519. [DOI] [PubMed] [Google Scholar]
- 26.Gehan EA, Tefft MC. Will there be resistance to the RECIST (Response Evaluation Criteria in Solid Tumors)? J Natl Cancer Inst. 2000;92:179–181. doi: 10.1093/jnci/92.3.179. [DOI] [PubMed] [Google Scholar]
- 27.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 28.James K, Eisenhauer E, Christian M, et al. Measuring response in solid tumors: Unidimensional versus bidimensional measurement. J Natl Cancer Inst. 1999;91:523–528. doi: 10.1093/jnci/91.6.523. [DOI] [PubMed] [Google Scholar]
- 29.Therasse P, Le Cesne A, Van Glabbeke M, et al. RECIST vs. WHO: Prospective comparison of response criteria in an EORTC phase II clinical trial investigating ET-743 in advanced soft tissue sarcoma. Eur J Cancer. 2005;41:1426–1430. doi: 10.1016/j.ejca.2005.04.005. [DOI] [PubMed] [Google Scholar]