Abstract
Yeast cells lacking a functional p24 complex accumulate a subset of secretory proteins in the endoplasmic reticulum (ER) and increase the extracellular secretion of HDEL-containing ER residents such as Kar2p/BiP. We report that a loss of p24 function causes activation of the unfolded protein response (UPR) and leads to increased KAR2 expression. The HDEL receptor (Erd2p) is functional and traffics in p24 deletion strains as in wild-type strains, however the capacity of the retrieval pathway is exceeded. Other conditions that activate the UPR and elevate KAR2 expression also lead to extracellular secretion of Kar2p. Using an in vitro assay that reconstitutes budding from the ER, we detect elevated levels of Kar2p in ER-derived vesicles from p24 deletion strains and from wild-type strains with an activated UPR. Silencing the UPR by IRE1 deletion diminished Kar2p secretion under these conditions. We suggest that activation of the UPR plays a major role in extracellular secretion of Kar2p.
INTRODUCTION
In eukaryotic cells, secretory proteins and lipids are synthesized at the endoplasmic reticulum (ER) and then transported to intracellular organelles or the plasma membrane via the secretory pathway (reviewed by Kaiser et al., 1997). Several lines of evidence indicate protein sorting occurs during export from the ER such that anterograde secretory cargo is selected for export in comparison to ER resident proteins (Balch et al., 1994; Rexach et al., 1994; Bednarek et al., 1995). Current models suggest a coat protein complex, termed COPII, mediates this sorting event by forming vesicles and including the desired set of cargo molecules by direct or indirect interaction (Springer et al., 1999). In addition to coat-dependent selection into vesicles, mechanisms of ER retention (Sato et al., 1996) and retrieval (Semenza et al., 1990) operate to maintain overall compartmental organization of the secretory pathway.
A family of transmembrane proteins, known as the p24 proteins, form heteromeric complexes and influence sorting during transport through the early secretory pathway. Initially discovered as abundant proteins contained on ER membranes (Wada et al., 1991), and then identified on COPI vesicles (Stamnes et al., 1995) and COPII vesicles (Schimmoller et al., 1995), the p24 proteins have been proposed to function as cargo receptors (Schimmoller et al., 1995; Muñiz et al., 2000), as negative regulators of vesicle budding (Elrod-Erickson and Kaiser, 1996), or as structural components of vesicles (Bremser et al., 1999), ER (Lavoie et al., 1999), and Golgi (Rojo et al., 2000). Deletion of all eight p24 genes in yeast produces viable cells that display phenotypes (Springer et al., 2000) exhibited by the single deletion of EMP24 (Schimmoller et al., 1995) or ERV25 (Belden and Barlowe, 1996). These single deletions appear to destabilize heteromeric p24 complexes, leading to a general loss of p24 function (Marzioch et al., 1999). Under this loss of function condition, the secretory proteins Gas1p and invertase are transported at reduced rates and partially accumulate in the ER. In contrast, ER resident proteins that contain an HDEL retrieval sequence (e.g., Kar2/BiP) escape the early secretory pathway and are secreted into the extracellular medium. In addition, loss of p24 function suppresses a deletion of SEC13, an essential gene that encodes a subunit of the COPII vesicle coat (Elrod-Erickson and Kaiser, 1996). Although some of these phenotypes seem consistent with current models for p24 function (Kaiser, 2000), others are less clear. In this report we examine mechanisms underlying secretion of Kar2p to provide insight into the role of p24 proteins in membrane trafficking. We find that extracellular secretion of Kar2p involves the induction of a stress response pathway.
An intracellular signaling pathway known as the unfolded protein response (UPR) controls ER homeostasis and protein folding in eukaryotic cells (reviewed by Chapman et al., 1998). In yeast, the UPR transcriptionally regulates >300 genes, including ER-resident chaperones such as Kar2p, Pdi1p (protein disulfide isomerase), Fkb2p (peptidy-prolyl cis-trans isomerase), and a PDI-like protein encoded by EUG1 (Sidrauski et al., 1998; Travers et al., 2000). The UPR also regulates ER lipid synthesis by negatively affecting the Opi1p repressor of lipid synthesis (Cox et al., 1997). The UPR is activated when unfolded proteins accumulate in the ER and this can be triggered experimentally by treating cells with compounds that interfere with protein folding in the ER (e.g., tunicamycin and β-mercaptoethanol). An ER-localized kinase, Ire1p, is thought to somehow sense the accumulation of unfolded proteins in the ER and then acts as a specific endoribonuclease in splicing the HAC1 mRNA. The translation product of spliced HAC1 mRNA then acts as a transcriptional activator for a set of genes that contain an upstream UPR element (UPRE), including the KAR2 gene (Sidrauski and Walter, 1997). In this report, we find that deletion of p24 genes leads to activation of the UPR and that secretion of Kar2p is due in large part to activation of this pathway.
MATERIALS AND METHODS
Strains, Media, and Growth Conditions
Yeast strains used in this study were grown in rich media (1% Bacto-yeast extract, 2% Bacto-peptone, and 2% dextrose) or selective media (0.67% yeast nitrogen base without amino acids, 2% dextrose, and required supplements). These growth conditions and other standard genetic methods used have been described (Sherman, 1991). When indicated, cultures were treated with 15 mM β-mercaptoethanol to activate the UPR (Cox and Walter, 1996). The optical densities of cell cultures were measured at 600 nm in a Beckman DU40 model spectrophotometer.
Strain Construction
All strains used in this report are listed in Table 1. Strains expressing a c-myc–tagged version of Erd2p were generated by transformation with the plasmid pJS209 (Semenza et al., 1990). An isogenic set of strains containing the ire1Δ allele was made by repeated backcrosses of MS3548 (Beh and Rose, 1995) with FY834 and then CBY114 or CBY99 (Belden and Barlowe, 1996). Strains with the UPRE-LacZ reporter construct were generated by transformation with pJC31 (Cox and Walter, 1996). Overexpression of KAR2 was achieved by transformation with a 2 μ plasmid containing the KAR2 gene (pMR109) as previously described (Rose et al., 1989).
Table 1.
Strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| FY833 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 | Winston et al. (1995) |
| FY834 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 | Winston et al. (1995) |
| CBY99 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 emp24Δ::LEU2 | Belden and Barlowe (1996) |
| CBY112 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 emp24Δ::LEU2 erv25Δ::HIS3 | Belden and Barlowe (1996) |
| CBY114 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 erv25Δ::HIS3 | Belden and Barlowe (1996) |
| RSY279 | MATα his4Δ619 ura3-52 sec22-3 | Kaiser and Schekman (1990) |
| RSY263 | MATα ura3-52 sec12-4 | Kaiser and Schekman (1990) |
| MS3548 | MATα ura3-52 leu2Δ1 ade2 ire1Δ::URA3 | Beh and Rose (1995) |
| CBY398 | FY834 with pJS209 (2μ-URA3-TPI-ERD2-myc) | This study |
| CBY419 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 erv25Δ::HIS3 with pJS209 | This study |
| CBY423 | MATα his4-619 ura3-52 sec22-3 with pJS209 | This study |
| CBY425 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 erv25Δ::HIS3 ire1Δ::URA3 | This study |
| CBY427 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 ire1Δ::URA3 | This study |
| CBY428 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 emp24Δ::LEU2 ire1Δ::URA3 | This study |
| CBY548 | MATα his3Δ200 ura3-52 trp1Δ63 sec12-4 with pJS209 | This study |
| CBY549 | MATα his3Δ200 ura3-52 leu2Δ1 trp1Δ63 erv25Δ::HIS3 with pJS209 | This study |
| CBY550 | MATα his3Δ200 ura3-52 leu2Δ1 trp1Δ63 erv25Δ:: HIS3 sec12-4 with pJS209 | This study |
| CBY635 | FY834 with pJC31 (UPRE-LacZ in pRS314) | This study |
| CBY636 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 emp24Δ::LEU2 with pJC31 | This study |
| CBY637 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 erv25Δ::HIS2 with pJC31 | This study |
| CBY748 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 sec22-3 with pJC31 | This study |
| CBY752 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 emp24Δ::LEU2 erv25Δ::HIS3 with pJC31 | This study |
| CBY750 | FY834 with pMR109 (2μ−URA3-KAR2) | This study |
| CBY751 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 erv25Δ::HIS3 with pMR109 | This study |
| CBY983 | FY834 with pJC31 and pMR109 | This study |
| CBY984 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 erv25Δ::HIS3 with pJC31 and pMR109 | This study |
Antibodies and Immunoblotting
Antibodies specific for Kar2p (Brodsky et al., 1993), Sec61p (Stirling et al., 1992), Erv25p (Belden and Barlowe, 1996), Emp24p (Schimmoller et al., 1995), Bos1p (Cao and Barlowe, 2000), Emp47p (Schroder et al., 1995), Gas1p (Frankhauser and Conzelmann, 1991), Gdi1p (Garrett et al., 1994), and c-myc (Evan et al., 1985) were used in this study at dilutions previously described. Protein samples were electophoretically separated on 12.5% polyacrylamide gels (Laemmli, 1970) and transferred to nitrocellulose membranes for immunoblotting (Towbin et al., 1979). Primary antibodies bound to nitrocellulose were detected using horseradish peroxidase-conjugated secondary antibody followed by chemiluminescence (Amersham-Pharmacia, Piscataway, NJ).
Kar2p Secretion
Extracellular Kar2p secretion was analyzed as previously described (Elrod-Erickson and Kaiser, 1996; Marzioch et al., 1999) with minor modifications. Stationary phase cultures were back diluted into rich media and grown to mid-logarithmic phase. Logarithmic stage cells were then harvested, washed, and resuspended in fresh rich medium at equivalent cell densities (OD600 = 0.5). After growth for 1 and 3 h, 1.5 ml of the cultures was centrifuged at 14,000 × g for 5 min and 1.35 ml of the supernatant fluids was collected. Proteins contained in this extracellular media were precipitated by adding 0.15 ml of 100% trichloroacetic acid (TCA) (Sigma Chemical, St Louis, MO) and incubated on ice for 20 min. The precipitated proteins were collected by centrifugation at 14,000 × g for 15 min at 4°C, washed with 100% acetone, dried at room temperature, and resuspended in 35 μl of SDS-PAGE sample buffer supplemented with 50 mM Tris pH 9.4. One-fifth of this sample was resolved by SDS-PAGE for immunoblots or one-half for silver staining.
Cell pellets from the above-mentioned 1.5-ml cultures were lysed in SDS-PAGE sample buffer or used to obtain whole cell membrane preparations. Briefly, cells were resuspended in 0.4 ml of lysis buffer (0.1 M sorbitol, 50 mM KOAc, 2 mM EDTA, 20 mM HEPES pH 7.5, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride) and vortexed in the presence of one-half volume of glass beads. The resulting lysates were subjected to a clearing spin at 5000 × g for 5 min to remove unlysed cells and 0.2 ml of this low-speed supernatant was transferred to a new tube and membranes were isolated by centrifugation at 100,000 × g in a TLA100.3 rotor (Beckman Instruments, Fullerton, CA) for 15 min. The high-speed pellet that contained whole cell membranes was resuspended in 35 μl of SDS-PAGE sample buffer and one-fifth was analyzed by immunoblot.
In Vitro Budding Assays
Vesicle budding from the ER was reproduced in vitro by incubation of microsomes (Wuestehube and Schekman, 1992) with purified COPII proteins (Sar1p, Sec23p complex, and Sec13p complex) as described (Barlowe et al., 1994). Where indicated, microsomes were prepared from cells grown in the presence of 15 mM β-mercaptoethanol for 1 h before harvesting cultures. To measure incorporation of proteins into COPII vesicles, a 15-μl aliquot of the total budding reaction and 150 μl of a supernatant fluid containing budded vesicles were centrifuged at 100,000 × g in a TLA100.3 rotor (Beckman Instruments) to collect membranes. The resulting membrane pellets were solubilized in 30 μl of SDS-PAGE sample buffer and 10–15 μl was resolved on 12.5% polyacrylamide gels. The percentages of individual proteins (Erd2p-myc, Erv25p, Bos1p, and Sec61p) packaged into vesicles from a total reaction were determined by densitometric scanning of immunoblots. Protease protected [35S]glyco-pro-α-factor ([35S]gp-α-F) packaged into budded vesicles was measured by precipitation with Concanavaline Sepharose after posttranslational translocation of [35S]-prepro-α-F into microsomes (Wuestehube and Schekman, 1992). In some experiments, [35S]gp-α-F was quantified by PhosphoImager analysis (Molecular Dynamics, Sunnyvale, CA) after transfer to nitrocellulose membranes and exposure to a phosphoscreen. For measurement of Kar2p contained in COPII vesicles, budded vesicles were treated with trypsin (100 μg/ml) for 10 min on ice followed by tyrpsin inhibitor (100 μg/ml) to ensure detection of a protease-protected species.
Cell Fractionation
Membrane fractions enriched in ER (p13) and Golgi (p100) were prepared from gently lysed cells as previously described (Wooding and Pelham, 1998) with minor modifications. Cell cultures (25 ml) in logrithmic growth phase at 30°C were shifted to 37°C for 30 min to invoke temperature-sensitive blocks. Cells were harvested, resuspended in a 4 ml of spheroplast buffer (0.7 M sorbitol, 10 mM Tris-Cl pH 7.4, 0.5% glucose), treated with lytic enzyme for 10 min at 37°C, chilled on ice, and spheroplasts collected by centrifugation. Spheroplasts were resuspended in lysis buffer (0.1 M sorbitol, 50 mM KOAc, 2 mM EDTA, 20 mM HEPES pH 7.5, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride) and lysed with a dounce homogenizer at 4°C. Unlysed cells were cleared at 2500 × g for 10 min and the supernatant fraction was centrifuged at 13,000 × g for 10 min to generate the p13 fraction. A p100 fraction was prepared from the p13 supernatant fluid after centrifugation at 100,000 × g for 15 min. Pellets were resuspended in 50 μl of 2× SDS-PAGE sample buffer and 15 μl was analyzed by immunoblot.
β-Galactosidase Assays
Yeast strains containing the UPRE-LacZ fusion construct, pJC31, were grown overnight in selective media to maintain selection of the plasmid and then back diluted into rich media to an OD600 = 0.2. After 6 h of growth, cells were harvested and β-galactosidase activity was measured as previously described (Asubel et al., 1997). Activity is expressed in Miller Units and SE and p values were calculated as described (Remington and Schork, 1985). Where indicated, 15 mM β-mercaptoethonol was added to cultures to activate the UPR 1 h before measuring β-galactosidase activity.
Northern Blots
Log phase cultures (25 ml) were grown in the absence or presence of 15 mM β-mercaptoethanol, harvested (OD600 = 0.5), washed and resuspended in 0.5 ml RNA extraction buffer (100 mM LiCl, 100 mM Tris-HCl pH 7.5, 0.1 mM EDTA), and lysed with glass beads. The RNA was extracted twice with phenol/chloroform and precipitated with 2 volumes of 100% ethanol. The RNA was resuspended in 0.05 ml of TE buffer and quantified for equal loading. DNaseI-treated RNA was separated on a 1.2% agarose, 0.625% formaldehyde gel in 1× 3-(N-morpholino)propanesulfonic acid running buffer. The RNA was transferred to a Nytran membrane (Schleicher & Schuell, Keene, NH) and probed for 2 h at 65°C in Perfecthyb Plus hybridization buffer (Sigma Chemical). The membranes were washed once in low-stringency buffer (1× SSC, 0.1% SDS) and twice in high-stringency buffer (0.1× SSC, 0.1% SDS) at 65°C, and then exposed to PhosphoImager screens. Probes were labeled with [α-32P]dATP (New England Nuclear, Boston, MA) by using RadPrime DNA Labeling System (Life Technologies, Grand Island, NY). DNA corresponding to HAC1 was polymerase chain reaction amplified from pJC835 (Cox and Walter, 1996) and a 1.0-kb EcoRI fragment of KAR2 was obtained from pMR109 (Rose et al., 1989).
RESULTS
Erd2p Traffics Independent of p24 Proteins
Loss of p24 function in yeast results in an ER accumulation of Gas1p and invertase as well as increasing the secretion of resident ER proteins (Schimmoller et al., 1995; Elrod-Erickson et al., 1996; Marzioch et al., 1999). We sought to examine mechanisms underlying secretion of the ER-resident protein Kar2p to provide insight into the function of p24 proteins. Kar2p and other soluble resident ER proteins are correctly localized in part because a specific retrieval system acts to return residents that have leaked out. An essential component of this retrieval system is the yeast HDEL-receptor encoded by the ERD2 gene. Erd2p binds to HDEL sequences present on the C terminus of ER resident proteins that have trafficked to the Golgi complex and subsequently returns them to the ER in a COPI-dependent manner (reviewed by Pelham, 1998). Previous studies suggested that loss of p24 function affected an ER-retention mechanism that was independent of the HDEL-retrieval pathway (Elrod-Erickson et al., 1996) although additional studies indicate mammalian p24 proteins participate in retrograde trafficking of KDEL-tagged proteins (Majoul et al., 1998). Indeed, many of the phenotypes associated with p24 deletion strains could be explained if the HDEL-retrieval system was impaired. To test whether the Erd2p retrieval pathway was functioning correctly in the absence of p24 proteins, we first monitored transport of Erd2p between the ER/Golgi in an erv25Δ strain.
Our previous studies have shown that deletion of EMP24 or ERV25 does not affect the formation of COPII-coated vesicles from ER membranes in vivo and in vitro (Belden and Barlowe, 1996). Furthermore, deletion of all eight p24 family members in yeast does not appear to influence the overall rates of COPII- or COPI-dependent budding (Springer et al., 2000). Some secretory proteins, however, are not efficiently exported from the ER (Schimmoller et al., 1995; Springer et al., 2000) apparently due to a decreased rate of incorporation into ER-derived vesicles (Muñiz et al., 2000). Erd2p may also depend on the p24 complex for efficient packaging into ER-derived vesicles therefore we directly measured Erd2p packaging in an in vitro assay that reconstitutes vesicle budding and cargo selection (Salama et al., 1993; Barlowe et al., 1994). Cellular membranes enriched in ER (microsomes) were isolated from wild-type and erv25Δ strains containing an epitope-tagged version of Erd2p (Semenza et al., 1990). The efficiency of Erd2p incorporation into ER-derived vesicles was measured after addition of purified COPII proteins and collection of budded vesicles (Belden and Barlowe, 1996). As seen in Figure 1A, the amounts of Erd2p packaged into COPII vesicles from wild-type and erv25Δ membranes were similar. As controls, Sec61p, an ER-resident protein required for membrane translocation, was not efficiently packaged into budded vesicles, whereas Bos1p, an ER/Golgi SNARE protein required for vesicle fusion, was efficiently incorporated. Approximately 3% of the total Erd2p was incorporated into ER-derived vesicles compared with 8 and 10% of Erv25p and Bos1p respectively. The differences in these percentages likely reflect the amount of each species that resides in the ER. Microsomal membrane preparations contain ER and Golgi membranes and because a majority of Erd2p is Golgi localized (Lewis and Pelham, 1992; Townsley et al., 1994), we surmise that only a small fraction of this receptor is cycling through the ER and would be available for packaging into COPII vesicles. Regardless, we detected COPII-dependent release of Erd2p and the amount of Erd2p packaged into COPII vesicles was equivalent whether the p24 complex was present or not. Therefore, we conclude that p24 proteins are not required for anterograde transport of Erd2p from the ER in COPII vesicles.
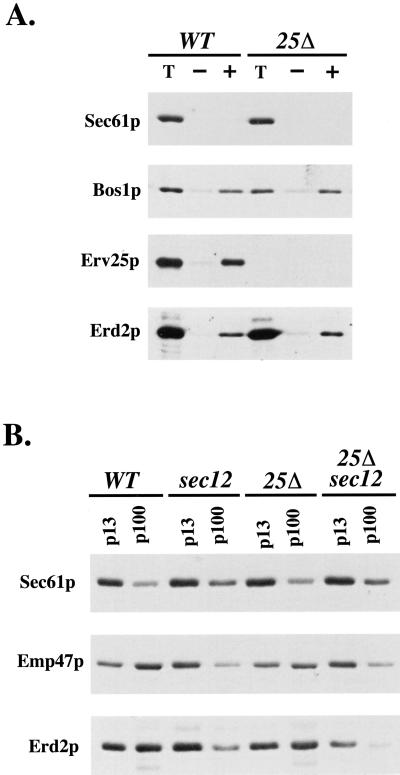
Figure 1.
Erd2p transport between the ER and Golgi compartments is independent of the p24 complex. (A) Reconstituted COPII budding reactions were performed on ER membranes isolated from CBY398 (WT) and CBY419 (erv25Δ) strains that express an epitope-tagged version of Erd2p. Lanes labeled T represent one-tenth of the total membranes used in a budding reaction, minus (−) lanes indicate the amount of vesicles formed in the absence of the purified COPII components, and plus (+) lanes indicate vesicles produced when COPII proteins are added. Total membranes and budded vesicles were collected by centrifugation, resolved on a polyacrylamide gel, and immunoblotted for indicated proteins. (B) ER and Golgi membrane fractions from CBY398 (WT), CBY548 (sec12), CBY549 (erv25Δ), and CBY550 (sec12, erv25Δ) strains that express the myc-tagged version of Erd2p. Cells were shifted to 37OC for 1 h before harvesting and lysis. ER membranes were pelleted by a medium-speed centrifugation (p13) and Golgi membranes were collected by a high-speed centrifugation (p100). Proteins contained in these fractions were monitored by immunoblot analysis.
Next we tested the hypothesis that p24 proteins are required for retrograde transport of Erd2p. Again, Kar2p secretion could be explained if Erd2p failed to enter COPI vesicles. To determine whether Erd2p is efficiently recycled back to the ER in an erv25Δ strain, we blocked COPII-dependent export with the temperature-sensitive sec12-4 allele to accumulate cycling proteins in the ER (Lewis and Pelham, 1996). If retrograde transport of Erd2p depended on the p24 complex, an erv25Δ sec12-4 double mutant strain should not accumulate Erd2p in the ER when shifted to a nonpermissive temperature. As seen in Figure 1B, Erd2p shifted equally to the ER in both ERV25 sec12-4 and erv25Δ sec12-4 strains. In this experiment, crude ER (p13) and Golgi (p100) were isolated from cells after incubation at 37°C for 30 min. As controls, Sec61p served as an ER marker and Emp47p served as a Golgi marker that cycles between these compartments as previously demonstrated (Schroder et al., 1995). In wild-type and erv25Δ strains, both Emp47 and Erd2p maintained a Golgi localization. However, in the presence of the sec12-4 allele, these Golgi-localized proteins shifted to the ER fraction in a manner that was independent of ERV25. We conclude that the p24 complex does not function in anterograde or retrograde traffic of Erd2p.
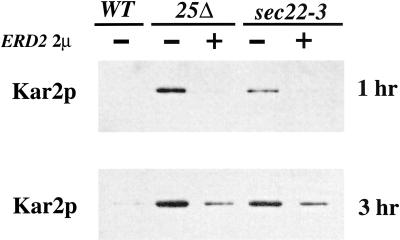
Although Erd2p appears to be cycling correctly, it may be incapable of binding HDEL proteins in the absence of a functional p24 complex. Previous reports have shown that increasing functional ERD2 expression can suppress trafficking mutants that secrete Kar2p (Semenza et al., 1990). Therefore, we tested whether increased expression of Erd2p could suppress the Kar2p secretion phenotype of an erv25Δ strain, indicating whether Erd2p was functional. As seen in Figure 2, an erv25Δ strain harboring a 2 μ version of ERD2 contained at least10-fold less Kar2p in the extracellular medium than an erv25Δ strain after 3 h of growth. As a positive control, the sec22-3 strain shown previously to secrete Kar2p, was suppressed approximately sixfold by overexpression of Erd2p at the 3-h time point. The data indicate ERD2 overexpression was able to increase the capacity of the HDEL retrieval system in erv25Δ and sec22-3 strains. These results suggested that the p24 complex was unlikely to play a role in promoting the association of HDEL proteins to Erd2p. Together with the trafficking studies, it appeared that Erd2p cycles properly and was capable of retrieving HDEL proteins in a p24 deletion strain but that the Erd2p-dependent retrieval pathway was saturated and could not prevent secretion of Kar2p.
Figure 2.
Overexpression of ERD2 partially suppresses the secretion of Kar2p in an erv25Δ deletion strain. The amount of extracellular Kar2p secreted after 1 and 3 h in erv25Δ and sec22-3 strains with and without an ERD2 2 μ plasmid. Proteins contained in the cell culture supernatant were concentrated by TCA precipitation and Kar2p was detected by immunoblot.
Deletion of ERV25 and IRE1 Reduces Growth Rate
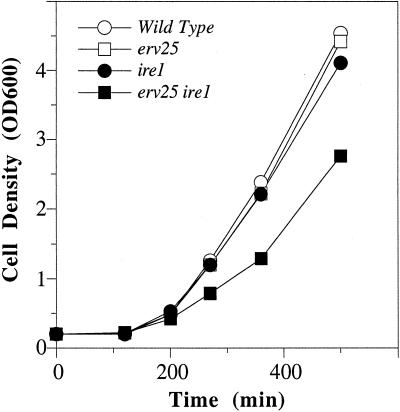
ERD2 is an essential gene suggesting that retrieval of HDEL proteins is vital. However, removal of the HDEL sequence from Kar2p (KAR2ΔHDEL) does not result in loss of cell viability. Because KAR2 is also an essential gene, cell viability appears to be maintained by increasing Kar2p synthesis (Semenza et al.,. 1990). This increased Kar2p synthesis depends on activation of the UPR because KAR2ΔHDEL displays a synthetic lethal relationship with ire1Δ, an ER localized transmembrane kinase that activates the UPR (Beh and Rose, 1995). In other words, a KAR2ΔHDEL strain survives because the cell compensates for loss of Kar2p through activation of the UPR and increased KAR2 expression. Based on these published findings, we sought to determine whether a p24 deletion strain would rely on IRE1 for growth because Kar2p was secreted from these strains. To test this possibility, we backcrossed erv25Δ::HIS3 and ire1Δ::URA3 strains and dissected individual asci. In all cases, dissection of asci resulted in four viable spores when grown on rich media at temperatures ranging from 25 to 37°C. Further quantitative analyses were performed by measuring the growth rates of an isogenic set of spores (WT, erv25Δ, ire1Δ, and erv25Δ ire1Δ) at 30°C. As seen in Figure 3, growth of the individual erv25Δ or ire1Δ strains did not differ from that of the wild-type, however, there was a detectable reduction in the growth rate of the erv25Δ ire1Δ double mutant strain. Based on exponential fitting of these data, the doubling time of the wild-type strain was calculated to be 92 min (R = 0.99) compared with 124 min (R = 0.99) for the double mutant. An almost identical decrease in growth rate was observed with an emp24Δ ire1Δ double mutant (our unpublished data), which displayed a growth rate of 123 min (R = 0.99). These results suggested that activation of the UPR was required for optimal growth although cell viability of p24 deletion strains did not depend on IRE1.
Figure 3.
Growth rate is decreased in an erv25Δ ire1 double mutant. The optical density at 600 nm was determined for FY834 (WT), CBY114 (erv25), CBY427 (ire1), and CBY425 (ire1 erv25) in rich media at 30OC and plotted as a function of time.
Loss of p24 Complex Activates the UPR
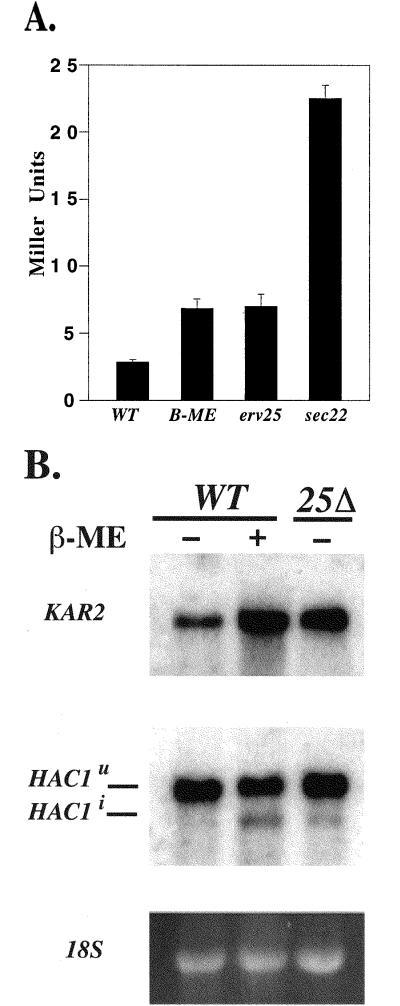
The properties on an erv25Δ ire1Δ double mutant strain suggested the UPR pathway was required for optimal growth in p24 deletion strains. To directly test whether the UPR was activated, we measured induction of the UPR from a reporter construct (pJC31) that contains the 22-bp UPRE from KAR2 fused to LacZ (Mori et al., 1992; Cox et al., 1993). The fold activation from the UPRE can be determined in strains harboring this reporter construct through assay of β-galactosidase activity. As seen in Figure 4A, wild-type cells exposed for 60 min to β-mercaptoethanol (15 mM), activated transcription from the UPRE, resulting in an increase in β-galactosidase activity (Cox and Walter, 1996). Under this condition, cell growth continued at near normal rates. Similarly, an erv25Δ strain displayed a >2.4-fold increase in the amount of β-galactosidase compared with an untreated wild-type strain (p < 0.0001), indicating activation of the UPRE. There was not a significant difference in activation between the erv25Δ strain with a wild-type strain exposured to β-mercaptoethanol for 60 min; however, longer treatments with this reducing agent resulted in significantly greater activation of the UPRE (our unpublished data). Furthermore, the amount of β-galactosidase activity measured in an emp24Δ erv25Δ strain was not significantly different from the erv25Δ strain alone (our unpublished data).
Figure 4.
(A) Deletion of ERV25 causes activation of the UPR. β-Galctosidase assays were performed on CBY635 (WT), CBY635 treated with 15 mM β-mercaptoethanol (β-ME) for 60 min, CBY637 (erv25Δ), and CBY748 (sec22-3). Activity is given in Miller Units and represents the average of seven independent determinations with SE. There is a significant difference (p < 0.0001) between the wild-type and erv25Δ strain. (B) Northern blot analysis of a FY834 (WT) and CBY114 (25Δ) with (+) or without (−) 15 mM β-mercaptoethanol (β-ME) treatment. Blots were probed for KAR2 and HAC1 messages with 32P-labeled probes. Spliced (HAC1i) and unspliced (HAC1U) were observed. Ethidium stained 18 S rRNA serves as a loading control.
Interestingly, a strain with the sec22-3 allele exhibited a strong activation of the UPR (>7-fold) as measured from this reporter construct. As reported previously (Semenza et al., 1990) and shown in Figure 2, sec22 strains secreted significant amounts of Kar2p into the medium and these cells apparently manage this situation through induction of the UPR. Sec22p may act in retrograde transport of proteins from the Golgi to the ER (Spang and Schekman, 1998); therefore, retrieval of HDEL proteins may be hindered in a sec22-3 strain and cause increased Kar2p secretion. Alternatively, an ER accumulation of secretory proteins in sec22-3 at permissive temperatures could lead to a proliferation of the ER and activation of the UPR. We favor this second interpretation because other sec mutants examined (e.g., uso1-1) exhibited varying degrees of UPR activation (our unpublished data). However, the amount of Kar2p secreted in an erv25Δ strain was slightly more than a sec22-3 strain (Figure 2), whereas activation of the UPR was modest in erv25Δ and strong in sec22-3. These results suggested the manner in which sec22 mutants and p24 deletion strains transport and/or dispose of up-regulated Kar2p expression may be distinct. These differences could be accounted for by a general secretory block in the sec22 strain grown at this temperature or distinct compensatory changes in gene expression under control of the UPR (Travers et al., 2000).
To provide further evidence for activation of the UPR in an erv25Δ strain, we monitored the levels of KAR2 and spliced HAC1 mRNA. Increases in these message levels are characteristic of an activated UPR (Sidrauski and Walter, 1997). As seen in Figure 4B, KAR2 message was significantly elevated and spliced HAC1 message was modestly elevated in an erv25Δ strain. A similar result was observed for wild-type cells treated with 15 mM β-mercaptoethanol. Based on these collective results, we conclude that loss of the p24 complex activates the UPR.
Activation of the UPR Increases Kar2p Secretion
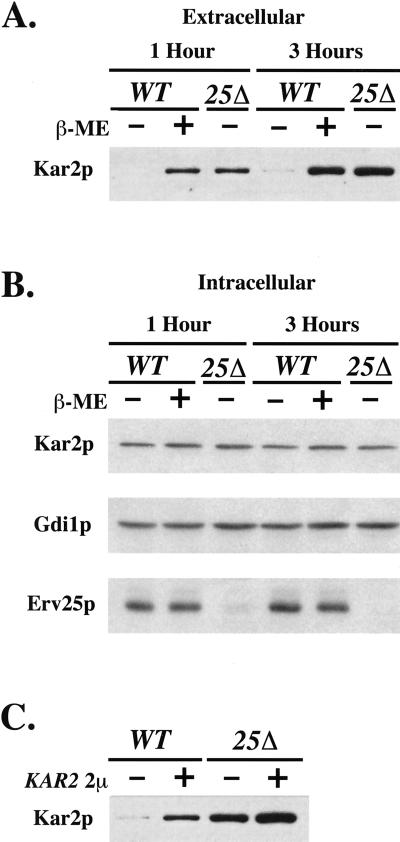
The p24 deletion mutants and certain ER/Golgi sec mutants have a constitutively active UPR and secrete Kar2p. We next considered the possibility that activation of the UPR would lead to increased secretion of HDEL proteins. To test this idea, we activated the UPR by exposure to 15 mM β-mercaptoethanol and measured Kar2p secretion (Figure 5A). The level of extracellular Kar2p from treated cells was at least 10-fold greater than untreated cells after 3 h and was comparable to the amount secreted from an erv25Δ strain. In contrast, the intracellular levels of Kar2p were relatively constant in wild-type, β-mercaptoethanol treated and erv25Δ strains compared with the cytosolic marker protein Gdi1p (Figure 5B). Importantly, the extracellular Kar2p detected from cells treated with β-mercaptoethanol represents secreted material and was not due to cell lysis because intracellular markers were not increased in the culture medium. We have also observed that treatment with other activators of the UPR (e.g., 5 μg/ml tunicamycin) increased secretion of Kar2p into the culture medium (our unpublished data). Therefore, Kar2p secretion appeared to be a general phenotype of UPR activation. We chose 15 mM β-mercaptoethanol for additional studies (see below) because this relatively mild UPR activator had a modest effect on growth rate and secretion.
Figure 5.
Activation of the UPR or overproduction of Kar2p increases extracellular Kar2p secretion. (A) Amount of secreted Kar2p from FY834 (WT) cells treated with (+) and without (−) 15 mM β-mercaptoethanol (β-ME) compared with CBY114 (25Δ). (B) Intracellular levels of Kar2p, Gdi1p, and Erv25p from equivalent amounts of cells (based on OD600) grown as in A. (C) Extracellular Kar2p from FY834 (WT) and CBY114 (25Δ) that were transformed (+) or not (−) with a 2 μ KAR2 plasmid. Proteins contained in the cell culture supernatant were concentrated by TCA precipitation and Kar2p was detected by immunoblot.
Based on these results, we speculated that UPR activation and subsequent up-regulation of HDEL proteins surpasses the capacity of both the ER retention and Erd2p-dependent retrieval processes. Previous studies have demonstrated that Erd2p-dependent retrieval is saturable when an HDEL-tagged version of a secretory protein (pro-α-factor) was expressed (Townsley et al., 1994). To determine whether overexpression of an endogenous HDEL protein was capable of saturating Erd2p retrieval, we transformed yeast strains with a 2 μ version of KAR2 and measured levels of Kar2p secreted into the culture medium. In Figure 5C, wild-type strains containing the KAR2 plasmid secreted at least 11-fold more Kar2p than untransformed strains. This result indicated that overproduced Kar2p failed to be retained in the ER and/or retrieved from post-ER compartments. Interestingly, we found that KAR2 overexpression alone activated the UPR as evidenced by a 2.1-fold increase in β-galactosidase activity from the UPRE reporter construct when cotransformed with the 2 μ version of KAR2 (strain CBY983). Presumably the UPR is activated under this condition because of a decrease in ER retention of other HDEL proteins (e.g., Pdi1p) that are replenished through UPR activation. Even higher levels of Kar2p were secreted from an erv25Δ strain containing the KAR2 plasmid than from an erv25Δ strain or a wild-type strain with the KAR2 plasmid (Figure 5C). The higher level of Kar2p secretion was approximately additive and was coincident with a greater activation of the UPR as revealed by a 3.7-fold increase in β-galactosidase activity when measured from the UPRE reporter construct in this strain (strain CBY984). These observations suggested the possibility that deletion of p24 genes causes Kar2p secretion as a consequence of UPR activation and up-regulation of HDEL proteins. Heightened activation of the UPR (addition of 2 μ KAR2) results in even greater levels of extracellular Kar2p when in p24 deletion strains.
Increased Kar2p in COPII Vesicles upon UPR Activation
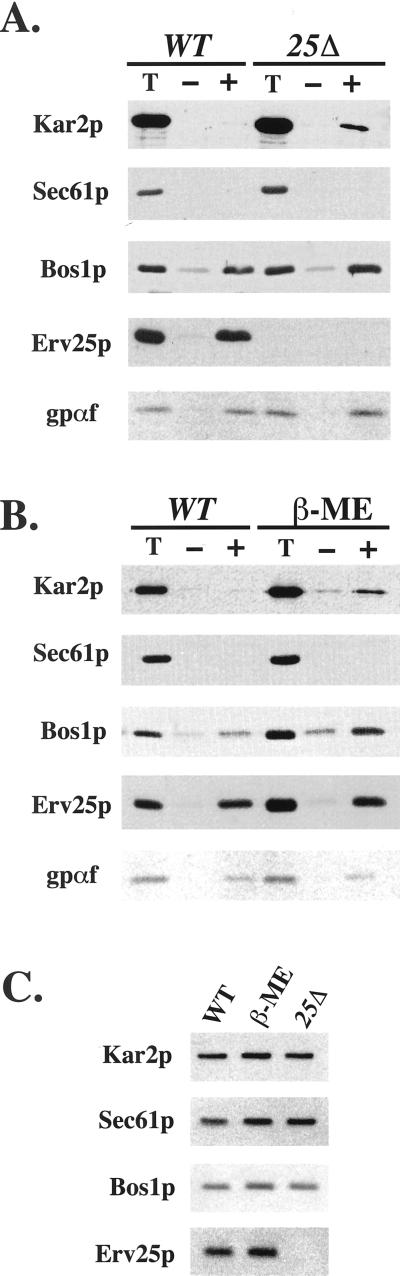
If UPR activation somehow saturates the capacity of ER retention, we predicted that the amount of HDEL proteins exiting the ER would increase when the UPR is activated. To directly measure this level, we performed in vitro assays that reproduce COPII budding and cargo selection from ER membranes as in Figure 1. For these experiments, microsomes were prepared from a wild-type strain, an erv25Δ strain, or from a wild-type strain treated with 15 mM β-mercaptoethanol for 1 h. COPII-budded vesicles from these microsomes were isolated and the level of individual proteins packaged into vesicles monitored by immunoblot (Figure 6). The efficiency of incorporation for each protein was calculated as a percentage of the total by densitometry. From wild-type microsomes, we detected minor amounts of Kar2p (0.4% of total) or Sec61p in ER-derived vesicles, whereas Bos1p (10%), Erv25p (9.3%), and gp-α-factor (16%) were efficiently packaged (Figure 6A). In contrast, microsomes prepared from an erv25Δ strain budded COPII vesicles that contained sixfold more Kar2p (2.4%) but similar levels of Bos1p (11%) and gp-α-factor (13%). For the analysis of Kar2p and gp-α-factor by this method, vesicle preparations were treated with trypsin to ensure detection of protease-protected lumenal species. Clearly, the percentage of exported Kar2p increased when vesicles were budded from p24 deletion membranes, but also the ratio of Kar2p to Bos1p was greater in the deletion strain (0.22) compared with the wild-type strain (0.04). A similar result was obtained if Kar2p was compared with other vesicle proteins (i.e., gp-α-factor). These results demonstrated that more Kar2p was incorporated per COPII vesicle marker when the membrane source was from an erv25Δ strain.
Figure 6.
Deletion of ERV25 or activation of the UPR increases the amount of Kar2p in COPII vesicles. (A) Reconstituted COPII budding reactions were performed on ER membranes isolated from FY834 (WT) and CBY114 (25Δ). Lanes labeled T represent one-tenth of the total membranes used in a budding reaction, minus (−) lanes indicate the amount of vesicles formed in the absence of the purified COPII components, and plus (+) lanes indicate vesicles produced when COPII proteins are added. Total membranes and budded vesicles were collected by centrifugation, resolved on polyacrylamide gels, and immunoblotted for indicated proteins. The Kar2p detected represents protease protected material (see MATERIALS AND METHODS) and [35S]-glyco-pro-α-factor (gpαf) was monitored by fluorography. (B) As in A except ER membranes were prepared from FY834 (WT) or FY834 treated with 15 mM β-mercaptoethanol (β-ME), to activate the UPR. (C) Equal amounts of ER membranes (microsomes) blotted with indicated markers to compare levels of Kar2p.
We performed a similar analysis on microsomes prepared from wild-type cells that had been treated with 15 mM β-mercaptoethanol for 1 h to activate the UPR. As seen in Figure 6B, microsomes remained competent for budding after this treatment and more Kar2p was contained in COPII vesicles prepared from treated than untreated cells. Again, the ratio of Kar2p to gp-α-factor or Bos1p indicated an increase in the level of Kar2p per vesicle. Notably, Erv25p was present and efficiently packaged into vesicles after β-mercaptoethanol treatment yet Kar2p was not excluded from these vesicles. Therefore, p24 proteins do not appear to prevent Kar2p from entering COPII vesicles when HDEL proteins are induced by activation of the UPR.
To determine whether the increase in Kar2p export from the ER correlated with an increase in ER levels of Kar2p, we directly compared microsomes prepared from wild-type, β-mercaptoethanol-treated, and erv25Δ strains (Figure 6C). Microsomal levels of Kar2p were monitored by immunoblot with Sec61p and Bos1p as loading controls. We did not detect significant Kar2p increases in microsomes prepared from an erv25Δ strain or a wild-type strain treated with β-mercaptoethanol. This result is consistent with our previous analysis of whole cells (Figure 5B). However, we probably would not be able to detect small changes in Kar2p levels by this method. One interpretation of these results is that Kar2p and other HDEL proteins are normally expressed at a threshold level that allows for efficient ER retention. When this level is exceeded, excess Kar2p is exported from the ER, surpasses Erd2p-dependent retrieval, and is secreted from cells. We would not expect to detect a 2% increase in Kar2p above endogenous levels in the ER fraction but can readily detect a 2% increase in Kar2p in COPII vesicles.
Further Analysis of the erv25Δ/ire1Δ Double Mutant
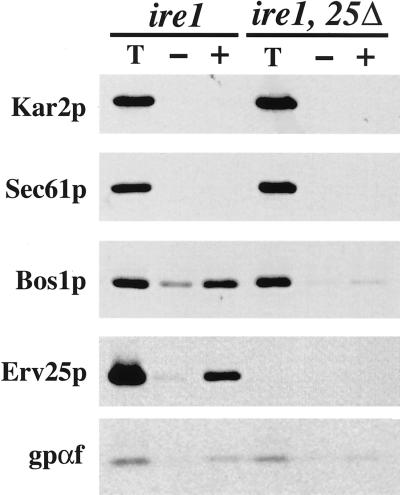
Loss of p24 function could cause a small amount of HDEL proteins to leak from the ER leading to activation of the UPR, or an ER accumulation of secretory proteins due to p24 deletion could activate the UPR. Either sequence of these events would apparently result in saturation of retention and retrieval processes leading to Kar2p secretion. The next series of experiments attempts to distinguish between these possibilities. If the increased level of Kar2p detected in COPII vesicles from an erv25Δ strain was due to activation of the UPR, we reasoned that silencing the UPR by IRE1 deletion should diminish Kar2p export. To experimentally test this idea, we first compared budding reactions from an ire1Δ and an erv25Δ ire1Δ double mutant (Figure 7). Microsomes from the ire1Δ strain were fully competent for COPII budding in vitro because [35S]-gp-α-factor, Erv25p, and Bos1p were efficiently packaged into vesicles. However, repeated isolation of microsomes from the erv25Δ ire1Δ double mutant strain yielded membranes that were not fully active in COPII budding reactions but were functional for translocation and core glycosylation of [35S]-prepro-α-factor. Budding from the double mutant as measured by the percentage of release of [35S]-gp-α-factor was approximately one-third the level observed for fully active microsomes. Elevating the concentration of COPII proteins or performing the budding reaction with semi-intact cell membranes (Baker et al., 1988) did not alleviate this defect (our unpublished results). Regardless, we did not observe COPII-dependent budding of Kar2p from erv25Δ ire1Δ microsomes and speculate that we would be able to detect this amount if comparable to an erv25Δ strain that budded at one-third the wild-type level. From these results, we conclude that silencing IRE1 in a p24 deletion strain somehow influences COPII budding from ER membranes. The experiment may indicate that Kar2p export from the ER is diminished in a p24 deletion strain when the UPR is silenced, however this interpretation is compromised because of the reduced budding efficiency.
Figure 7.
COPII budding is decreased in an erv25Δ ireΔ double mutant. (A) Reconstituted COPII budding reactions were performed as in Figure 6 except ER membranes were prepared form CBY427 (ire1 Δ) and CBY425 (ire1 Δ, 25Δ).
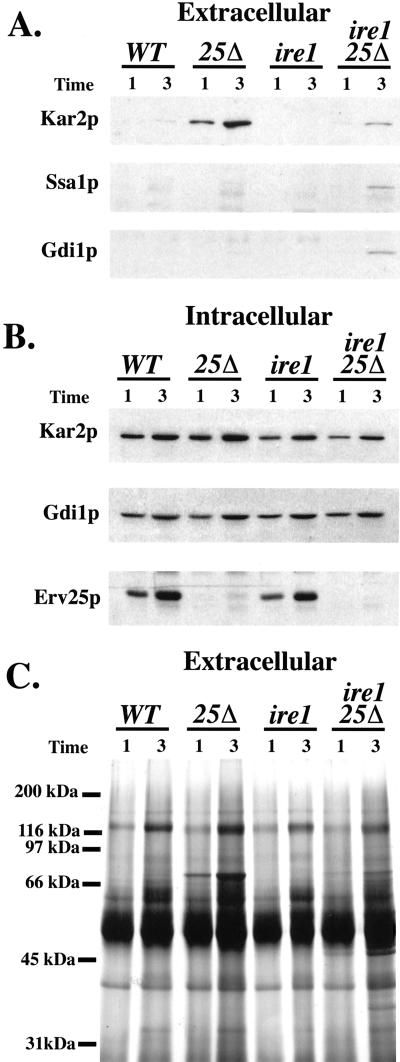
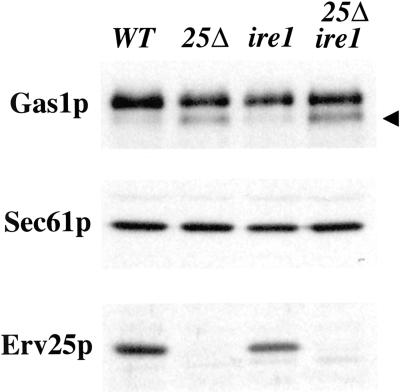
If deletion of IRE1 in the erv25Δ strain reduces the level of Kar2p export from the ER, we anticipated that less would be secreted into the extracellular medium. Indeed, immunoblot analysis of the culture medium from single and double mutant strains indicated combining the ire1Δ with erv25Δ blocked Kar2p secretion (Figure 8A) but did not alleviate ER accumulation of Gas1p (Figure 9). In the erv25Δ ire1Δ strain, some extracellular Kar2p could be detected above wild-type levels (Figure 8A). However, this may be true secretion or due to a small amount of cell lysis that occurred in the erv25Δ ire1Δ strain because minor amounts of cytosolic markers, such as Ssa1p (a heat shock protein 70 protein) and Gdi1p (a Rab-specific GDP dissociation inhibitor) were also detected in the culture fluid of this strain (Figure 8A). The general pattern observed by protein staining of extracellular proteins was similar in these four strains (Figure 8C) except that additional proteins and background staining appear in the erv25Δ ire1Δ strain, presumably due to cell lysis. The observed cell lysis of the double mutant may account for the decreased growth rate observed in Figure 3. However, the reduction in Kar2p secretion from the erv25Δ ire1Δ strain appears greater than the 1.3-fold reduction in growth rate exhibited by this strain. Furthermore, comparable amounts of the other major extracellular secretory proteins were detected in all four strains, indicating secretion was near normal in the double mutant. When we monitored intracellular levels of Kar2p at these time points, we did not detect any significant increases however modest decreases in Kar2p were observed in ire1Δ strains (Figure 8B). These observations suggest that most of the Kar2p secreted by an erv25Δ strain depends on IRE1.
Figure 8.
Deletion of IRE1 prevents Kar2p secretion from an erv25Δ strain. (A) Amount of extracellular Kar2p in cultures from FY834 (WT), CBY114 (erv25 Δ), CBY427 (ire1 Δ), and CBY425 (erv25 Δ ire1 Δ) after 1 and 3 h. Proteins contained in the cell culture supernatant were concentrated by TCA precipitation and Kar2p was detected by immunoblot. (B) Intracellular levels of Kar2p, Gdi1p, and Erv25p from equal volumes of cell cultures grown as in A. (C) The same samples of precipitated proteins as in A were resolved on 12.5% SDS-PAGE and silver stained.
Figure 9.
Immunoblot analysis of wild-type and deletion strains. Whole cell membranes prepared from FY834 (WT), CBY114 (erv25 Δ), CBY427 (ire1 Δ), and CBY425 (erv25 Δ ire1 Δ) strains after 3 h. Membrane proteins were resolved on polyacrylamide gels and immunoblotted for Gas1p, Sec61p (loading control), and Erv25p. The arrowhead indicates the ER form of Gas1p.
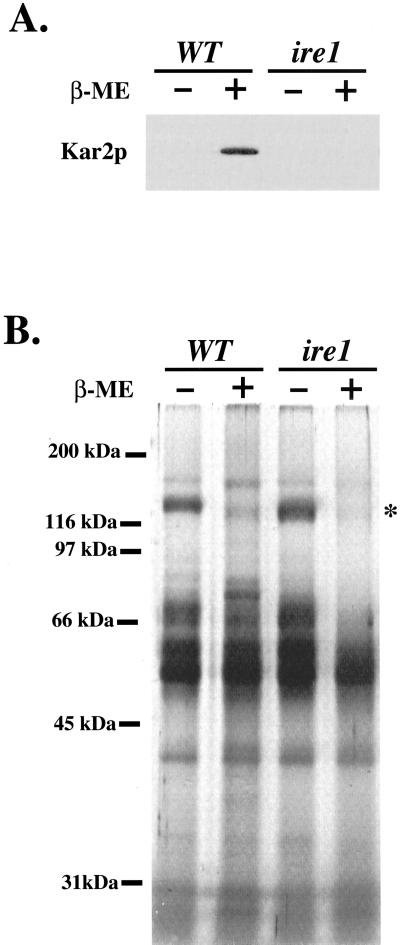
Finally, we measured the amount of Kar2p secreted by wild-type and ire1Δ strains that were treated with 15 mM β-mercaptoethanol to activate the UPR pathway. We reasoned that if general induction of the UPR causes Kar2p secretion, silencing this response by IRE1 deletion should also block the appearance of extracellular Kar2p. As seen in Figure 10A, IRE1 deletion blocked Kar2p secretion by cells treated with β-mercaptoethanol. This treatment did not cause cell lysis or stop secretion over the 3-h time course that we monitored because comparable amounts of most extracellular secretory proteins were detected in the culture supernatant (Figure 10B). Treatment with this reductant did decrease the level of some secretory proteins (note asterisk at 120 kDa) and may be due to impaired disulfide bond formation of this secretory protein in the ER. In summary, extracellular secretion of Kar2p caused by p24 deletion or treatment with reducing agents depended largely on IRE1.
Figure 10.
Deletion of IRE1 prevents Kar2p secretion from cells treated with β-mercaptoethanol. (A) Amount of extracellular Kar2p from FY834 (WT) or CBY427 (ire1 Δ) cells treated with (+) and without (−) 15 mM β-mercaptoethanol (β-ME) for 3 h. Proteins contained in the cell culture supernatant were concentrated by TCA precipitation and Kar2p was detected by immunoblot. (B) The same samples of precipitated proteins as in A were resolved on 12.5% SDS-PAGE and silver stained.
DISCUSSION
In this report, we investigated the pathways involved in extracellular secretion of Kar2p when cells lack a functional p24 complex. In erv25Δ strains, the Golgi-localized HDEL receptor Erd2p trafficked properly and was competent for returning HDEL proteins that had escaped the ER. In the absence of p24 function, however, the Erd2p-dependent retrieval pathway appeared saturated and failed to retrieve Kar2p, a condition that was partially alleviated by increasing the expression level of Erd2p. Reconstituted budding assays in an erv25Δ strain suggested that this saturation was due to an increase in the amount of Kar2p, and presumably other HDEL proteins, exported from the ER in COPII vesicles. We found that cells lacking a functional p24 complex exhibited an activated UPR. In addition, extracellular secretion of Kar2p in p24 deletion strains depended in large part on activation of the UPR because silencing this pathway through IRE1 deletion greatly diminished Kar2p secretion. Finally, we report that other known activators of the UPR caused extracellular secretion of Kar2p and that Kar2p secretion is probably a hallmark of UPR activation.
Our experiments were performed in yeast cells that lack Erv25p and/or Emp24p and we find that these single or double mutants produced identical results. In the course of these studies, yeast strains that lack four (Marzioch et al., 1999) or all eight (Springer et al., 2000) of the yeast p24 family members were characterized and displayed secretion phenotypes that are indistinguishable from the single ERV25 or ERV24 deletions. Deletion of ERV25 or EMP24 destabilizes additional members of the heteromeric p24 complex and apparently leads to a complete loss of p24 function (Belden and Barlowe, 1996; Marzioch et al., 1999; Springer et al., 2000). We do not believe that destabilized p24 protein subunits in single deletion strains cause activation of the UPR because deletion of ERV25 or all eight p24 genes produces identical phenotypes with respect to extracellular secretion of Kar2p (Springer et al., 2000) and presumably UPR activation. Therefore, we speculate that an accumulation of secretory cargo in the ER activates the UPR although we cannot exclude the possibility that there are other non-p24 components of the hetromeric p24 complex that accumulate as unfolded species in the octuple mutant and influence the UPR pathway.
Two recent reports suggested that disruption of p24 function did not induce the UPR pathway in yeast (Springer et al., 2000) or mammalian cells (Rojo et al., 2000). We provide five experimental results that support a connection between the UPR pathway and p24 function in yeast. First, we find that the growth rate of p24 deletion strains was significantly reduced when IRE1 was deleted (Figure 3). Second, in an erv25Δ strain we detected a greater than 2-fold activation of the UPR when β-galactosidase activity was measured from a reporter construct that places lacZ under the control of the UPRE (Figure 4). Third, we document that KAR2 and spliced HAC1 message levels are elevated in an erv25Δ strain (Figure 4B). Fourth, we find that microsomal membranes prepared from an erv25Δ ire1Δ double mutant strain were specifically compromised for vesicle budding in vitro, however, neither of these single mutations caused a defect (Figure 7). Fifth, silencing the UPR by IRE1 deletion blocked Kar2p secretion from p24 deletion strains (Figure 8). In accord with previously reported findings (Springer et al., 2000), we were unable to detect UPR activation in our p24 deletion strains from the lacZ reporter on X-Gal plates and we did not detect a temperature-sensitive phenotype when combining the p24 deletions with ire1Δ. However, when we measured β-galactosidase activity from cell lysates or followed the logarithmic phase growth rates of specific strains, reproducible and statistically significant effects were observed. Furthermore, we detected striking differences in the amount of Kar2p secreted by an erv25Δ strain compared with an erv25Δ ire1Δ strain and the in vitro budding efficiency of this double mutant was clearly distinct from either of the single mutants. Therefore, we believe these discrepancies can be accounted for by the assays used to detect UPR induction or that different strain backgrounds will influence these observations.
We observed that a general feature of UPR activation was extracellular secretion of Kar2p and probably other soluble HDEL-containing ER proteins that are induced by this stress response pathway. Treatment with chemical agents that interfere with protein folding in the ER (β-mercaptoethanol or tunicamycin) led to induction of the UPR and extracellular secretion of Kar2p. Overexpression of KAR2 from a 2 μ plasmid also induced the UPR and caused Kar2p secretion. Based on these observations, we speculate that soluble ER residents are normally maintained at a threshold level and when elevated, ER retention and Erd2p-dependent retrieval mechanisms are surpassed. We are limited in our understanding of ER retention mechanisms but as previously suggested, IRE1 could coordinate ER protein with ER membrane biosynthesis under normal conditions to achieve efficient retention (Cox et al., 1997). Other conditions resulting in Kar2p secretion may represent an activation of the UPR. In keeping with this idea, we found that the sec22-3 and uso1-1 mutants induced the UPR and secreted extracellular Kar2p when grown at semipermissive temperatures. Other sec mutants that secrete Kar2p (Semenza et al., 1990) probably possess an activated UPR. Further work will be needed to distinguish whether secretion of ER-resident proteins is beneficial in coping with accumulated secretory cargo or is simply a consequence of UPR activation. In considering this question, it seems notable that IRE1 was required for optimal growth of p24 deletion strains, suggesting the UPR helps these cells manage a loss of p24 function. UPR induction in these strains could facilitate disposal of accumulated cargo through ER-associated protein degradation (McCracken and Brodsky, 1996) and/or accelerate transport from the ER for normal secretion or for degradation in the vacuole.
Do our findings provide insight on the function of p24 proteins? Several models have been offered on their role in the early secretory pathway (reviewed by Kaiser, 2000). First, the lumenal domains of these transmembrane proteins have been proposed to act as cargo receptors that bind to secretory cargo and link lumenal cargo to vesicle coat complexes (Schimmoller et al., 1995; Muñiz et al., 2000). Second, p24 proteins could act as negative regulators of vesicle budding, delaying the budding process to allow for more efficient segregation of cargo away from ER residents (Elrod-Erickson and Kaiser, 1996). Third, these molecules have been proposed to act as structural components of vesicles (Bremser et al., 1999), of ER (Lavoie et al., 1999), or of Golgi membranes (Rojo et al., 2000) that could create specialized packaging zones. Fourth, the p24 proteins could act as steric exclusion devices occupying space within the lumen of vesicles, thereby blocking entry of soluble ER residents (Springer et al., 2000; Kaiser, 2000). In considering the first and third models, our data seem consistent with the possibility that deletion of p24 proteins initially cause an accumulation of secretory cargo in the ER. Accumulated cargo may then elicit the UPR and increase the expression levels of ER chaperones, resulting in saturation of ER retention and post-ER retrieval processes. Our data may also be interpreted in a manner that is consistent with the second and fourth models, whereby p24 proteins act initially in retention of ER resident proteins. For example, Kar2p could initially leak from the ER in p24 deletion strains and trigger the UPR. An activated UPR could again lead to saturation of ER retention and retrieval processes. Regardless of initial cause, UPR activation appears to be critical for extracellular secretion of Kar2p in p24 deletion strains because of the observed influence of IRE1 deletion. In considering these models, it may be important to note that Kar2p secretion appeared to depend on IRE1 but was not strictly dependent on the presence or absence of p24 proteins. For example, treatment of wild-type strains with β-mercaptoethanol resulted in extracellular secretion of Kar2p and this level of secretion depended on IRE1. However, deletion of p24 proteins appeared to be doing something more than simply activating the UPR because ER forms of secretory cargo such as Gas1p accumulated in p24 deletion strains and this effect was not reversed by IRE1 deletion.
Clearly, further experimentation will be necessary to determine the function of p24 proteins in ER sorting. Support for the cargo receptor model has been provided by evidence demonstrating a direct association between Gas1p and the Emp24p-Erv25p complex (Muñiz et al., 2000). To further test this model, it will be informative to determine whether the secretory cargo invertase, which also accumulates in p24 deletion strains, possesses an affinity for the p24 complex. Alternatively, p24 proteins could be essential for setting up specialized membrane zones such as transitional ER (Lavoie et al., 1999) or lipid rafts (Bagnat et al., 2000) where cargo concentration might occur. Experimental methods to test these ideas are also available. For example, formation of transitional ER sites can be monitored in certain model organisms (Rossanese et al., 1999) after deletion of p24 genes. These and other approaches should lead us to an understanding of p24 function in the early secretory pathway.
ACKNOWLEDGMENTS
We thank Jeff Brodsky, Hugh Pelham, Mark Rose, and Peter Walter for providing strains and plasmids used in this study. Nicole Ballew and Jacqueline Powers are thanked for their comments on this work. This research was supported by the National Institutes of Health and the Pew Scholars Program. W.B. is supported by a predoctoral training grant from the National Institutes of Health.
REFERENCES
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1997. [Google Scholar]
- Bagnat M, Keranen S, Shevchenko A, Shevchenko A, Simons K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci USA. 2000;97:3254–3259. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, Hicke L, Rexach M, Schleyer M, Schekman R. Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell. 1988;54:335–344. doi: 10.1016/0092-8674(88)90196-1. [DOI] [PubMed] [Google Scholar]
- Balch WE, McCaffery JM, Plutner H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzsola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Bednarek SY, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Beh CT, Rose MD. Two redundant systems maintain levels of resident proteins within the yeast endoplasmic reticulum. Proc Natl Acad Sci USA. 1995;92:9820–9823. doi: 10.1073/pnas.92.21.9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden WJ, Barlowe C. Erv25p, a component of COPII-coated vesicles, forms a complex with Emp24p that is required for efficient endoplasmic reticulum to Golgi transport. J Biol Chem. 1996;271:26939–26946. doi: 10.1074/jbc.271.43.26939. [DOI] [PubMed] [Google Scholar]
- Bremser M, Nickel W, Schweikert M, Ravazzola M, Amherdt M, Hughes CA, Sollner TH, Rothman JE, Wieland FT. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 1999;96:495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- Brodsky JL, Hamamoto S, Feldheim D, Schekman R. Reconstitution of protein translocation from solubilized yeast membranes reveals topologically distinct roles for BiP and Cytosolic Hsc70. J Cell Biol. 1993;120:95–102. doi: 10.1083/jcb.120.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Barlowe C. Asymmetric requirements for a Rab GTPase and SNARE proteins in fusion of COPII vesicles with acceptor membranes. J Cell Biol. 2000;149:55–65. doi: 10.1083/jcb.149.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R, Sidrauski C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus. Annu Rev Cell Dev Biol. 1998;14:459–485. doi: 10.1146/annurev.cellbio.14.1.459. [DOI] [PubMed] [Google Scholar]
- Cox JS, Chapman R, Walter P. The unfolded protein response coordinates the production of endoplasmic reticulum membrane. Mol Biol Cell. 1997;8:1805–1814. doi: 10.1091/mbc.8.9.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- Cox JS, Walter P. A novel mechanism for regulating activity of a transcriptional factor that controls the unfolded protein response. Cell. 1996;87:391–404. doi: 10.1016/s0092-8674(00)81360-4. [DOI] [PubMed] [Google Scholar]
- Elrod-Erickson MJ, Kaiser CA. Genes that control the fidelity of endoplasmic reticulum to Golgi transport identified as suppressors of vesicle budding mutations. Mol Biol Cell. 1996;7:1043–1058. doi: 10.1091/mbc.7.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankhauser C, Conzelmann A. Purification, biosynthesis and cellular localization of a major 125-kDa glycophosphatidylinositol-anchored membrane glycoprotein of Saccharomyces cerevisiae. Eur J Biochem. 1991;195:439–448. doi: 10.1111/j.1432-1033.1991.tb15723.x. [DOI] [PubMed] [Google Scholar]
- Garrett MD, Zahner JE, Cheney CM, Novick PJ. GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 1994;13:1718–1728. doi: 10.1002/j.1460-2075.1994.tb06436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C. Thinking about p24 proteins and how transport vesicles select their cargo. Proc Natl Acad Sci USA. 2000;97:3783–3785. doi: 10.1073/pnas.97.8.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser CA, Gimeno RE, Shaywitz DA. Protein secretion, membrane biogenesis, and endocytosis. Yeast. 1997;3:91–227. [Google Scholar]
- Kaiser CA, Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavoie C, Paiement J, Dominguez M, Roy L, Dahan S, Gushue JN, Bergeron JJ. Roles for alpha(2)p24 and COPI in endoplasmic reticulum cargo exit site formation. J Cell Biol. 1999;146:285–299. doi: 10.1083/jcb.146.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MJ, Pelham HR. Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell. 1992;68:353–364. doi: 10.1016/0092-8674(92)90476-s. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Pelham HRB. SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell. 1996;85:205–215. doi: 10.1016/s0092-8674(00)81097-1. [DOI] [PubMed] [Google Scholar]
- Majoul I, Sohn K, Wieland FT, Pepperkok R, Pizza M, Hillemann J, Soling H-D. KDEL receptor (Erd2p)-mediated retrograde transport of the Cholera toxin A subunit from the Golgi involves COPI, p23, and the COOH terminus of Erd2p. J Cell Biol. 1998;143:601–612. doi: 10.1083/jcb.143.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzioch M, Henthorn DC, Herrmann JM, Wilson R, Thomas DY, Bergeron JJM, Solari RCE, Rowley A. Erp1p and Erp2p, partners for Emp24p and Erv25p in a yeast p24 complex. Mol Biol Cell. 1999;10:1923–1938. doi: 10.1091/mbc.10.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken AA, Brodsky JL. Assembly of ER-associated protein degradation in vitro: dependence on cytosol, calnexin, and ATP. J Cell Biol. 1996;132:291–298. doi: 10.1083/jcb.132.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Sant A, Kohno K, Normington K, Gething M-J, Sambrook JF. A 22 bp cis-acting element is necessary and sufficient for the induction of the yeast KAR2 (BiP) gene by unfolded proteins. EMBO J. 1992;11:2583–2593. doi: 10.1002/j.1460-2075.1992.tb05323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñiz M, Nuoffer C, Hauri H-P, Riezman H. The Emp24 complex recruits a specific cargo molecule into endoplasmic reticulum-derived vesicles. J Cell Biol. 2000;148:925–930. doi: 10.1083/jcb.148.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham HRB. Getting through the Golgi complex. Trends Cell Biol. 1998;8:45–49. doi: 10.1016/s0962-8924(97)01185-9. [DOI] [PubMed] [Google Scholar]
- Remington RD, Schork MA. Statistics with Application to the Biological and Health Sciences. Englewood Cliffs, NJ: Prentice Hall, Inc; 1985. [Google Scholar]
- Rexach MF, Latterich M, Schekman RW. Characteristics of endoplasmic reticulum-derived transport vesicles. J Cell Biol. 1994;126:1133–1148. doi: 10.1083/jcb.126.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo M, Emery G, Marjomaki V, McDowall AW, Parton RG, Gruenberg J. The transmembrane protein p23 contributes to the organization of the Golgi apparatus. J Cell Sci. 2000;113:1043–1057. doi: 10.1242/jcs.113.6.1043. [DOI] [PubMed] [Google Scholar]
- Rose MD, Misra LM, Vogle JP. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989;57:1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Rossanese OW, Soderholm J, Bevis BJ, Sears IB, O'Conner J, Williamson EK, Glick BS. Golgi structure correlates with transitional endoplasmic reticulum organization in Pichia pastoris and Saccharomyces cerevisiae. J Cell Biol. 1999;145:69–81. doi: 10.1083/jcb.145.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama NR, Yeung T, Schekman R. The Sec13p complex and reconstitution of vesicle budding from the ER with purified cytosolic proteins. EMBO J. 1993;12:4073–4082. doi: 10.1002/j.1460-2075.1993.tb06091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Sato K, Nakano A. Endoplasmic reticulum localization of Sec12p is achieved by two mechanisms: Rer1p-dependent retrieval that requires the transmembrane domain and Rer1p-independent retention that involves the cytoplasmic domain. J Cell Biol. 1996;134:279–294. doi: 10.1083/jcb.134.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmoller F, Singer-Kruger B, Schroder S, Kruger U, Barlowe C, Riezman H. The absence of Emp24p, a component of ER-derived COPII-coated vesicles, causes a defect in transport of selected proteins to the Golgi. EMBO J. 1995;14:1329–1339. doi: 10.1002/j.1460-2075.1995.tb07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder S, Schimmoller F, Singer-Kruger B, Riezman H. The Golgi-localization of yeast Emp47p depends on its di-lysine motif but is not affected by the ret1–1 mutation in α-COP. J Cell Biol. 1995;131:895–912. doi: 10.1083/jcb.131.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza JC, Hartwick KG, Dean N, Pelham HRB. ERD2, a yeast gene required for the receptor-mediated retrival of luminal ER proteins from the secretory pathway. Cell. 1990;61:1349–1357. doi: 10.1016/0092-8674(90)90698-e. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Sidrauski C, Chapman R, Walter P. The unfolded protein response: an intracellular signaling pathway with many surprising features. Trends Cell Biol. 1998;8:245–249. doi: 10.1016/s0962-8924(98)01267-7. [DOI] [PubMed] [Google Scholar]
- Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded proteins response. Cell. 1997;90:1031–1039. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- Spang A, Schekman R. Reconstitution of retrograde transport from the Golgi to the ER in vitro. J Cell Biol. 1998;143:589–599. doi: 10.1083/jcb.143.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer S, Chen E, Duden R, Marzioch M, Rowley A, Hamamoto S, Merchant S, Schekman R. The p24 proteins are not essential for vesicular transport in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2000;97:4034–4039. doi: 10.1073/pnas.070044097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer S, Spang A, Schekman R. A primer on vesicle budding. Cell. 1999;97:145–148. doi: 10.1016/s0092-8674(00)80722-9. [DOI] [PubMed] [Google Scholar]
- Stamnes MA, Craighead MW, Hoe MH, Lampen N, Geromanos S, Tempst P, Rothman JE. An integral membrane component of coatomer-coated transport vesicles defines a family of proteins involved in budding. Proc Natl Acad Sci USA. 1995;92:8011–8015. doi: 10.1073/pnas.92.17.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling CJ, Rothblatt J, Hosobuchi M, Deshaies R, Schekman R. Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Mol Biol Cell. 1992;3:129–142. doi: 10.1091/mbc.3.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- Towbin H, Straelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley FM, Frigerio G, Pelham HRB. Retrieval of HDEL proteins is required for growth of yeast cells. J Cell Biol. 1994;127:21–28. doi: 10.1083/jcb.127.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada I, Rindress D, Cameron PH, Ou W-J, Doherty JJ, Louvard D, Bell AW, Dignard D, Thomas DY, Bergeron JJ. SSRα and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J Biol Chem. 1991;266:19599–19610. [PubMed] [Google Scholar]
- Winston F, Dollard C, Ricuupero-Hovasse SL. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast. 1995;11:53–55. doi: 10.1002/yea.320110107. [DOI] [PubMed] [Google Scholar]
- Wooding S, Pelham HRB. The dynamics of Golgi protein traffic visualized in living yeast cells. Mol Biol Cell. 1998;9:2667–2680. doi: 10.1091/mbc.9.9.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuestehube LJ, Schekman R. Reconstitution of transport from the endoplasmic reticulum to the Golgi complex using an ER-enriched membrane fraction from yeast. Methods Enzymol. 1992;219:124–136. doi: 10.1016/0076-6879(92)19015-x. [DOI] [PubMed] [Google Scholar]