Studies on obesity and the risk for hematological malignancies are reviewed. The paper includes a discussion of the metabolic effects of obesity and their possible role in linking increased body fat to neoplasia.
Keywords: Obesity, Body mass index, Leukemia, Lymphoma, Myeloma, Cancer
Abstract
The aggregate of epidemiological studies indicates a significantly elevated risk for cancer in people with a high body mass index (BMI); a “dose–response” effect exists with increasing risk as BMI increases from the normal to overweight to obese categories. Successful sustained weight loss decreases future risk. The relationship of being overweight to the risk for leukemia in the aggregate has been supported in several large cohort studies and two meta-analyses of cohort and case–control studies. One meta-analysis found an elevated risk for each of the four major subtypes of leukemia. A significant association between the risk for non-Hodgkin's lymphoma and elevated BMI was supported by a meta-analysis of 13 cohort and nine case–control studies. The risk for diffuse large B-cell lymphoma may be especially significant. A high BMI increases the risk for myeloma, as judged by a meta-analysis of 11 cohort and four case–control studies. The biological relationship of obesity to the risk for cancer (biological plausibility) is unresolved. The two major causal final pathways could be “inductive” or “selective.” The metabolic, endocrinologic, immunologic, and inflammatory-like changes resulting from obesity may increase the cell mutation rate, dysregulate gene function, disturb DNA repair, or induce epigenetic changes, favoring the induction of neoplastic transformation (inductive). Alternatively, obesity may create an environment in which pre-existing clones that are dormant are permitted (selected) to emerge.
Introduction
The prevalence of obesity in the U.S. has increased, at least, over the last several decades. The National Health and Nutrition Examination Survey, which consists of a nationally representative sample of men and woman aged ≥20 years, indicates that, in the 2007–2008 sample, 33.8% (confidence interval [CI], 31.6%–36.0%) of Americans were obese as defined by the World Health Organization categorization, a body mass index (BMI) ≥ 30.0 kg/m2. Overweight, defined as a BMI of 25–29.9 kg/m2, was present in an additional 34.2% of the population. Thus, 68% of the U.S. population exceeded the upper limit of normal BMI (18.5–24.9 kg/m2) [1]. The World Health Organization has estimated that >1.6 billion people worldwide are overweight, of whom approximately one quarter are obese.
BMI, with the exception of some heavily muscled athletes, is closely correlated with an individual's amount of body fat. In children, BMI is adjusted for differences in body fat between males and females and for different ages in childhood. BMI is one of several indicators of body fat (others include skin-fold thickness, waist circumference, waist to hip circumference ratio, and several techniques requiring special instruments and operator expertise); it, however, has been accepted as the best and most practical screening index by the World Health Organization and the U.S. Public Health Service.
There are differences in rates of obesity among Americans of European, African, and Asian ancestry but the current prevalence of obesity in each group has increased over their historical standards. There are minor adjustments in the definition of overweight and obesity in some areas of the world. For example, in China, the BMI is about 2 kg/m2 lower for each body mass category: underweight, normal, overweight, and obese. Trends indicate a plateau in the prevalence rate of obesity in the U.S. in women and children over the last 10 years and in men over the last 5 years, according to the Centers for Disease Control and Prevention [2], perhaps indicating the effects of awareness and changes in eating and exercise habits or an effect in which the genetically and environmentally susceptible populations have reached an asymptote, or both.
Using BMI, population studies have established a link between obesity and several major human cancers (e.g., postmenopausal breast, colon, endometrium, esophagus, liver, and kidney cancers) and strong indications of a link with others. The role of obesity in cancer incidence and mortality is thought to be causal because of the consistency of large cohort studies, the relatively long latency period observed between the onset of obesity and a subsequent increase in cancer incidence, the evidence for a biologically plausible relationship in humans among the metabolic, endocrinologic, and inflammatory effects of obesity and their experimental links to cell proliferation and cell survival, and the early preliminary evidence for a lower incidence of cancer in women who have had sustained weight loss as a result of medical or surgical interventions [3, 4]. Here, I review the studies of obesity and the risk for hematological malignancies.
Risk for Leukemia, Non-Hodgkin's Lymphoma, and Myeloma Observed in General Studies of the Link of Obesity to Cancer
In a prospective study, the Cancer Prevention Study II, conducted by the American Cancer Society of 900,000 adults in the U.S. who had no evidence of cancer at enrollment in 1982, there were 57,145 deaths from cancer during a 16-year follow-up. The death rate from all cancers in members of the cohort with extreme obesity (BMI ≥40.0 kg/m2) was 52% higher for men and 62% higher for women than for individuals of normal weight. The relative risk (RR) for death from cancer in this group was 1.52 (CI, 1.13–1.87) in men and 1.62 (CI, 1.40–1.87) in women. The RR for cancer increased incrementally for both men and women in relationship to increasing BMI over the normal upper limit, a clearcut dose–response relationship for increasing risk for cancer mortality with increasing BMI [5]. These investigators used multivariate proportional hazard models to control for potentially confounding variables such as smoking, alcohol use, ethnicity, dietary variations, and others, and concluded that the excess death rates from cancer attributable to overweight (BMI, 25–29.9 kg/m2) or obesity (BMI ≥30.0 kg/m2) were approximately 9% in men and 17% in women. Of particular interest to this commentary, the RR for mortality from non-Hodgkin's lymphoma and myeloma was significantly greater with a significant trend with increasing BMI. In males, for example, the RR associated with a BMI of >29.9 but ≤39.9 kg/m2 for myeloma and non-Hodgkin's lymphoma was approximately 1.50; in females of the same BMI range, the RR was approximately 1.45. Leukemia deaths increased stepwise with increasing categories of BMI above the normal range, with each step statistically significant when compared with people with a normal BMI, for men. The RR for leukemia was 37% greater in men with a BMI of 30–34.9 kg/m2. No information was provided for major leukemia or lymphoma subtypes.
In a large cohort of male U.S. military veterans (3,668,486 whites, 832,214 blacks) hospitalized with a diagnosis of obesity in 1969–1996, the authors examined the risk for cancer at all major cancer [6]. Because of small numbers, women and ethnicities other than blacks and whites were excluded from the original number of 5,790,493 veterans discharged during that period. The specifications for the diagnosis of obesity in the Veterans Affairs hospital system was defined as receiving a discharge diagnosis coded as 277 according to the eighth revision of the International Classification of Diseases (ICD8) or 278 according to the ICD9. The authors calculated age- and calendar-year–adjusted RRs for cancer among white and black veterans, comparing obese men with nonobese men hospitalized for other reasons. For selected cancers, additional analyses were performed stratified by specific medical conditions related to both obesity and the risk for those cancers. Risk was significantly elevated for myeloma among obese white veterans (RR, 1.22; CI, 1.05–1.40) and among obese black veterans (RR, 1.26; CI, 1.02–1.56), but risk was not significantly elevated for Hodgkin's or other lymphomas. An elevated risk was also found for leukemia (aggregated) among obese white veterans (RR, 1.42; CI, 1.31–1.54) and among obese black veterans (RR, 1.77; CI, 1.45–2.15), for acute myelogenous leukemia among obese white veterans (RR, 1.59; CI, 1.33–1.90) and among obese black veterans (RR, 2.64; CI, 1.80–3.85), and for chronic lymphocytic leukemia among obese white veterans (RR, 1.30; CI, 1.13–1.49) and among obese black veterans (RR, 1.72; CI, 1.24–2.39). In the analysis of chronic myelogenous and acute lymphocytic leukemia, the RR was elevated, although not significantly, which may have been related to a much smaller number of cases, especially of adult acute lymphocytic leukemia (e.g., one case among obese black men).
Faculty at the Centre for Chronic Disease Prevention and Control in Ottawa, Canada did a case–control study of 21,022 incident cases of 19 types of cancers among people aged 20–76 years during 1994–1997 [7]. This group was compared with 5,039 control subjects. Individuals with a BMI ≥25 kg/m2 were compared with those with a BMI <25 kg/m2, using a multivariate adjusted OR to assess risk. Those scientists estimated that excess body mass accounted for 9.7% of cancers in males and 5.9% of cancers in women in Canada. The ORs for overweight men (1.32; CI, 1.07–1.60) and obese men (1.41; CI, 1.07–1,84) were significantly greater for the risk for leukemia (aggregate) than they were for overweight women (OR, 1.28; CI, 1.00–1.65) and obese women (OR, 2.01; CI, 1.49–2.71). The OR for the risk for non-Hodgkin's lymphoma was significantly greater in overweight men (OR, 1.25; CI, 1.05–1.48), obese men (OR, 1.42; CI, 1.12–1.80), and obese women (OR, 1.54; CI, 1.21–1.95). The OR for the risk for myeloma was significantly higher in overweight men (OR, 1.64; CI, 1.09–2.47), obese men (OR, 2.16; 1.25–3.75), and obese women (OR, 1.92; CI, 1.23–3.00). Although the risk for leukemia was elevated in both overweight and obese men and women, a breakdown among the four major types of leukemia was not reported. These investigators estimated that an elevated BMI accounted for about 18% of all leukemias.
Members of the Cancer Epidemiology Unit, University of Oxford, conducted a study on the relationship between BMI and incidence and mortality for 17 cancer sites among 1.3 million women, aged 50–64 years, or one in four women in the U.K. in that age group [8]. They adjusted the analysis for age, geographical region, socioeconomic status, age at first birth, parity, smoking, alcohol intake, physical activity, years since menopause, and use of hormone replacement therapy. The results of that study indicate that about 5% of the cancer incidence in postmenopausal women in the U.K. is attributable to overweight (BMI, 25.0–29.9 kg/m2) or obesity (BMI ≥30 kg/m2). A significant trend of an increasing RR for incidence for each 10-unit increase in BMI was noted for leukemia (aggregate) (RR, 1.50), non-Hodgkin's lymphoma (RR, 1.17), and myeloma (RR, 1.31).
Researchers at the Center of Health Promotion and Obesity Research and the National Health Insurance Corporation in South Korea assessed the relationship between BMI and cancer onset in a 10-year study of 781,283 Korean men who were cancer free at entry into the cohort [9]. They found a significant association between obesity and the risk for nine cancers, most of these found in other studies on this question, adding strength to the association. Of the hematological malignancies, a barely significant association was found between BMI and non-Hodgkin's lymphoma but not between BMI and leukemia or myeloma.
Investigators at the School of Cancer Studies, University of Manchester, U.K., did electronic searches of MEDLINE and Embase (1966 to November 2007) to identify prospective studies of incident cases of 20 cancer types [10]. They used these data to do a systematic review and meta-analysis to assess the strength of associations between BMI and different sites of cancer, between sex and ethnic groups, and between the risk for cancer and a 5 kg/m2 greater BMI. They examined 221 datasets (141 articles), including 282,137 incident cases. In men, a 5 kg/m2 higher BMI was associated with a modest but significantly higher risk for leukemia (aggregate) (RR, 1.08; CI, 1.02–1.14), myeloma (RR, 1.11; CI, 1.05–1.30), and non-Hodgkin's lymphoma (RR, 1.06; CI, 1.03–1.09). In women, the RR for leukemia with a 5 kg/m2 higher BMI was 1.17 (CI, 1.04–1.32); for myeloma it was 1.11 (CI, 1.07–1.15) and for non-Hodgkin's lymphoma it was 1.07 (CI, 1.00–1.14). The association between a higher BMI and the risk for a nonhematological malignancy was generally similar in studies from North America, Europe and Australia, and the Asia-Pacific region, but was not reported for hematological malignancies perhaps because of small numbers after stratification.
Investigators at the Institute of Environmental Medicine, Karolinska Institute, used data collected prospectively from two Swedish twin cohorts and a Finnish twin cohort (in total, 70,067 people) to study the effects of overweight (BMI, 25–29.9 kg/m2) and obesity (BMI ≥30 kg/m2) on the development of leukemia (aggregate), non-Hodgkin's lymphoma, Hodgkin's lymphoma, and myeloma [11]. The cohorts were followed from baseline through 2002 (Sweden) or through 2004 (Finland). These studies found a higher RR for myeloma in the older cohort of twins (RR, 2.1; CI, 1.1–3.7) and among men and women in both countries. A greater RR was found for chronic myeloid leukemia among obese people (RR, 2.5; CI, 1.0–6.2) and acute lymphoblastic leukemia in overweight people (RR, 2.7; CI, 0.8–9.6), when compared with those with a normal BMI. The latter CI was wide, however, and the RR for acute lymphoblastic leukemia, although relatively high, only approached significance.
A consortium of investigators from five institutions in three European countries, the U.K., The Netherlands, and Switzerland, determined the incident risk for cancer in relationship to excess BMI among individuals in 30 European countries [12]. The population attributable risk for cancer incidence in 2002 for a BMI ≥25 kg/m2 was 2.5% (CI, 1.5%–3.6%) for men and 4.1% (CI, 2.3%–5.9%) for women. This represented an excess of 70,288 (CI, 40,069–100,668) cases of cancer. When a scenario analysis of a contemporary population for the year 2008 was done, the population attributable risk for the incidence of cancer was 3.2% (CI, 2.1%–4.3%) for men and 8.6% (CI, 5.6%–11.5%) for women. This finding suggests a substantially greater number of cases of cancer attributable to overweight or obesity in 2008 than in 2002. The risk ratios for men were significantly higher for leukemia (1.08; CI, 1.00–1.58), non-Hodgkin's lymphoma (1.06; CI, 1.03–1.09), and myeloma (1.09; CI, 1.01–1.17). For women, the risk ratios were also significantly higher for leukemia (1.13; CI, 1.00–1.30), non-Hodgkin's lymphoma (1.10; CI, 1.00–1.24) and myeloma (1.11; CI, 1.07–1.15). The incident burden of the three major hematological malignancies in 2002 in these countries, as a result of overweight or obesity for men and women, was calculated to be 6,915 cases.
A cohort of 43,965 obese people was studied based on their discharge registrations from Danish hospitals, and the incidence of cancer in those individuals was compared with that of the Danish population [13]. An elevated risk for several sites of cancer, established to be greater in obese people, was found (e.g., esophagus, liver, uterus, kidney). An elevated risk for leukemia (aggregated) in men and women combined (RR, 1.3; CI, 1.0–1.7) was observed. The risk for non-Hodgkin's lymphoma was not significantly elevated (RR, 1.1; CI, 0.8–1.5). Myeloma risk was not reported.
A study by members of the Division of Epidemiology, Norwegian Institute of Public Health, explored this association for lymphohematopoietic diseases in a large Norwegian cohort [14]. Height and weight were measured in 2,000,611 Norwegian men and women aged 20–74 years during 1963–2001. Cox proportional hazards regression models with time since measurement of BMI as the time variable were used. During follow-up, 24,500 cases of a lymphohematopoietic malignancy, including lymphomas of all types, leukemia and myeloproliferative neoplasms of all types, and myeloma and plasmacytoma, were found. The RR for disease was estimated by Cox proportional hazards regression. The risk for these malignancies in the aggregate increased moderately with increasing BMI in both sexes. The RR for lymphohematopoietic malignancies (aggregate of lymphoma, leukemia, and myeloma) per five-unit increase in BMI was 1.11 (CI, 1.08–1.14) in men and 1.08 (CI, 1.05–1.11) in women. Separate analyses for lymphoproliferative and myeloproliferative malignancies showed a significant trend for risk when men were analyzed based on groups of increasing BMI—<18.5 kg/m2, 18.5–24.9 kg/m2, 25–29.9 kg/m2, and ≥30 kg/m2. The RR increased from approximately 0.83 to 1.24 over the four (increasing) BMI groups in men. In women, the BMI groups were broader—<18.5 kg/m2, 18.5–24.9 kg/m2, 25–29.9 kg/m2, 30–34.9 kg/m2, 35–35.9 kg/m2, and ≥40.0 kg/m2. Analyses of lymphoproliferative and myeloproliferative malignancies, individually, showed a significant test for trend of RR from approximately 0.97 to 1.48 over the six BMI groups.
Studies of the Relationship Between Overweight and Obesity and the Four Major Types of Leukemia
The general studies of cancer risk in the overweight and obese cited in the preceding section, with a few exceptions, have not examined the risk for developing the four major leukemia types: acute myelogenous and lymphocytic leukemia and chronic myelogenous and lymphocytic leukemia.
Epidemiologists who do not study leukemia specifically sometimes disregard the biological differences among the myelogenous and lymphocytic leukemia types and subtypes. In one study of male veterans, the incidences of acute myelogenous leukemia and chronic lymphocytic leukemia were significantly greater in male white and black veterans diagnosed as obese [6]. The numbers of cases for the other two major categories of leukemia were not presented, presumably because of relatively small numbers of cases.
A nationwide cohort study of 336,381 people was conducted in Sweden, in which the cohort had a mean age of 34.3 years and an age range of 14–82 years [15]. During the follow-up period, 372 cases of leukemia (acute lymphocytic leukemia, n = 47; acute myelogenous leukemia, n = 224; chronic myelogenous leukemia, n = 101) and 520 people with myeloma were diagnosed. Chronic lymphocytic leukemia was not studied. BMI in study subjects had no association with the risk for the types of leukemia studied or myeloma.
A prospective study was conducted of 40,909 Australians in the Melbourne Collaborative Cohort Study recruited in 1990–1994 and followed for an average of 8.4 years through December 2003 [16]. Cox proportional hazard regression models with age as the time axis were used to estimate hazard ratios (HRs). The risk, as judged by the HR, for myeloid leukemia (acute and chronic) was significantly associated with BMI in overweight (HR, 5.3; CI, 1.9–15.2) and obese (HR, 5.0; CI, 1.6–15.2) people, compared with individuals who had a normal BMI (<25 kg/m2). HRs were higher for the risk for chronic myelogenous leukemia than for the risk for acute myelogenous leukemia, but case numbers were too small to test homogeneity between the two myeloid leukemias. No association was found with lymphoproliferative diseases.
In an attempt to examine the issue of the risk for the four major leukemia types and obesity, faculty at the Division of Nutritional Epidemiology conducted a meta-analysis of cohort studies on the association between excess body weight and the incidence of leukemia (aggregated) and the four major subtypes [17]. The studies were published between 1966 and July 2007. A random-effects model was used to combine the results from individual studies. Nine cohort studies were found with data on BMI or obesity in relation to the incidence of leukemia. Compared with individuals who were not overweight (BMI <25 kg/m2), the RR for leukemia (aggregate) was 1.14 (CI, 1.03–1.25) for overweight individuals (BMI, 25.0–29.9 kg/m2) and 1.39 (CI, 1.25–1.54) for obese individuals (BMI ≥30 kg/m2). On a continuous scale, a 5-kg/m2 greater BMI was associated with a 13% higher risk for leukemia (RR, 1.13; CI, 1.07–1.19). In a meta-analysis of two to four cohort studies containing four to six separate populations (e.g., men or women, white or black men) reporting results on subtypes of leukemia, the RR associated with obesity (BMI ≥30 kg/m2) was significantly greater for acute lymphocytic leukemia (RR, 1.65; CI, 1.16–2.35), for acute myelogenous leukemia (RR, 1.52; CI, 1.19–1.95), for chronic myeloid leukemia (RR, 1.26; CI, 1.09–1.46), and for chronic lymphocytic leukemia (RR, 1.25; CI, 1.11–1.41).
Investigators in the Department of Social and Preventive Medicine, University of Laval, Québec, Canada, analyzed data obtained from a population-based, case–control study conducted in eight Canadian provinces in 1994–1997, using the Canadian National Enhanced Cancer Surveillance System [18]. Risk estimates were generated by applying multivariate logistic regression methods to 1,068 incident leukemia cases confirmed as to histological type and 5,039 controls, aged 20–74 years. They found a statistically significant higher risk among subjects with the highest BMI (≥30 kg/m2) for acute myelogenous leukemia (OR, 1.6; CI, 1.2–2.2), for chronic myelogenous leukemia (OR, 2.3; CI, 1.5–3.4), and for chronic lymphocytic leukemia (OR, 1.4; CI, 1.0–1.8), with a significant relationship between increasing BMI and RR (dose–response relationship). An elevated risk for AML associated with active smoking disappeared among obese subjects (BMI ≥30 kg/m2).
The Iowa Women's Health Study was used to determine whether a high BMI was associated with leukemia development [19]. Over 40,000 Iowa women (age, 55–69 years) completed a self-administered lifestyle and health questionnaire in 1986 that included their height and weight. One hundred ninety-four cases of leukemia during the period 1986–2001, including 72 cases of acute myelogenous leukemia and 84 cases of chronic lymphocytic leukemia, were used in the analysis. The risk for leukemia (aggregate) increased in relationship to increasing BMI, but that relationship seemed to be the result of a strong relationship for acute myelogenous leukemia. The risk for acute myelogenous leukemia was greater for women who reported being overweight (BMI, 25.0–29.9 kg/m2) (RR, 1.9; CI, 1.0–3.4) or obese (BMI ≥30 kg/m2) (RR, 2.4; CI, 1.3–4.5; ptrend = .006) than for women with a normal BMI (18.5–29.9 kg/m2). There was no significant positive association between BMI and chronic lymphocytic leukemia (ptrend = .6). The authors estimated that, given the prevalence of overweight and obesity in the U.S., the population attributable risk for acute myelogenous leukemia as a result of obesity could approach 30% based on these data.
Obesity and Acute Promyelocytic Leukemia
In most studies of the risk for acute myelogenous leukemia in overweight and obese people, the analyses have not examined the major subphenotypes of the disease. In a study of 1,245 newly diagnosed cases of acute myelogenous leukemia at M. D. Anderson Cancer Center in 1980–1995, of which 120 (9.6%) were cases of acute promyelocytic leukemia, a significant association between elevated BMI and the risk for acquiring acute promyelocytic leukemia was observed [20]. Five of the 120 patients with acute promyelocytic leukemia had a BMI >50 kg/m2, whereas none of the other cases of acute myelogenous leukemia did. Excluding these massively obese patients did not alter the conclusion that the patients with acute promyelocytic leukemia had a significantly higher BMI than patients with other subtypes of acute myelogenous leukemia.
The relationship between BMI and acute promyelocytic leukemia was supported by a study of acute myelogenous leukemia in 29 hospitals in the Shanghai district of China. The study population included 722 cases of acute myelogenous leukemia and 1,444 gender- and age-matched comparison patients at the same hospitals [21]. There were 124 cases of acute promyelocytic leukemia among the cases of acute myelogenous leukemia. Risk estimates (ORs) were calculated by conditional logistic regression. The risk (OR) of acute myelogenous leukemia was inversely related to BMI. However, there was a significant positive trend for a relationship between the OR of acute promyelocytic leukemia and increasing BMI. In addition, the OR for acute promyelocytic leukemia in obese subjects (BMI >28 kg/m2) was 2.15. (Note that the Working Group on Obesity in China uses a population value for normal, overweight, and obese that is approximately 2 kg/m2 less than in the U.S. and western Europe.) Acute promyelocytic leukemia was the only subtype of acute myelogenous leukemia for which the OR significantly increased in association with increasing BMI.
An elevated BMI may also increase the occurrence of the differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid (tretinoin) [22]. Among 39 acute promyelocytic leukemia patients treated with all-trans retinoic acid and idarubicin, 33 patients had a complete remission. Eleven of the 36 patients developed the differentiation syndrome within a median of 12 days (range, 3–23 days) of administration of all-trans retinoic acid. Six of the nine (66.6%) patients with a BMI ≥30 kg/m2 developed the syndrome, whereas only five of the 27 (18.5%) patients with a BMI <30 kg/m2 (p = 0.01) developed the syndrome. On multivariate analysis, BMI ≥30 kg/m2 remained an independent predictor of the differentiation syndrome in addition to baseline total leukocyte count.
A biological link between obesity and acute promyelocytic leukemia was provided by studies on the effect of leptin on these cells [23]. The primary role of leptin, encoded by the OB gene, is to control fat tissue mass. Leptin is principally secreted from fat cells and its serum levels are highly positively correlated with body fat volume [24]. Its regulation and role in regulation of the body mass of fat cells is, however, more complicated than a straightforward physiological setpoint relationship [24]. Leptin acts on the hypothalamus to decrease appetite, among other effects. The leptin receptor gene (OB-R) is a homologue of the interleukin (IL)-6–type, cytokine receptor gene. Leptin and its receptor play a role in hematopoiesis [25]. Leptin receptors are expressed on marrow mononuclear cells and the CD34+ cell population, specifically. Leptin acts synergistically with stem cell factor to stimulate the growth of granulocyte-monocyte progenitors in human marrow. That progenitor cell is thought to be the site of transformation of cases of acute promyelocytic leukemia. The long isoform variant of the leptin receptor is present on the CD34−CD33+ fraction of marrow cells from patients with acute promyelocytic leukemia but is not present on normal promyelocytes [23]. These findings have led to the postulation that the proliferation of leukemic promyelocytes may be driven in part by leptin levels, given that its receptor is on leukemic but not normal promyelocytes. In addition, leptin inhibits the programmed cell death of leukemic cells. Thus, its action on leukemic promyelocytes is to foster proliferation and inhibit cell death, the two major signals for promoting leukemia growth. These effects should be accentuated in overweight and obese. Leptin receptors were also found on the cells of a majority of cases of acute myelogenous leukemia, regardless of phenotypic subtype.
Obesity and Chronic Myelogenous Leukemia
In eight studies of the relation of obesity to chronic myelogenous leukemia, a significant association was found in six [11, 14, 16–18, 26], but not in two others [6, 15]. A case–control study (n = 253 cases and n = 270 controls), conducted at the University of Texas M. D. Anderson Cancer Center investigated the role of BMI and weight gain during adulthood in the risk for chronic myelogenous leukemia, using multivariate logistic regression for analysis [26]. Cases were more likely to be obese during adulthood than controls at age 25 (OR, 4.29; CI, 1.63–11.3), at age 40 (OR, 5.12; CI, 1.92–13.6), and at diagnosis (OR, 3.09; CI, 1.56–6.13). Obesity at all ages was found to be an independent risk factor for chronic myelogenous leukemia, with a significant dose–response effect. The OR increased strikingly from overweight to mildly obese to severely obese. Among participants ≥45 years old, patients gained significantly more weight each year during the ages of 25–40 than controls (0.78 versus 0.44 kg/year; p < .001), and the association with chronic myelogenous leukemia was strongest among those who gained >1 kg/year during the ages of 25–40 years (OR, 3.63; CI, 1.46–9.04). These investigators concluded that obesity and weight gain during adulthood play important roles in the risk for the onset of chronic myelogenous leukemia.
The Relationship Between Obesity and the Risk for Lymphoma
Hodgkin's Lymphoma
Investigators in the Epidemiology and Genetics Unit, University of York, U.K., studied the relationship between Hodgkin's lymphoma and obesity in a population-based case–control study that recruited incident cases of lymphoma in England during 1998–2003 [27]. Information on height and weight was collected from 216 cases with a pathologically confirmed diagnosis of Hodgkin's lymphoma and their age- and sex-matched controls. Obesity was defined as BMI ≥30 kg/m2 5 years prior to diagnosis. This weight status led to a higher risk for Hodgkin's lymphoma, by more than twofold compared with those in the normal BMI range (18.5–24.9 kg/m2). The OR for the risk for Hodgkin's lymphoma was 2.2 (CI, 1.1–4.3). The association was significant among men (OR, 2.8; CI, 1.2–6.5) but not among women (OR, 1.1; CI, 0.3–3.8). Elevated risk tended to be among older (aged >35 years) rather than younger (aged ≤35 years) individuals, and for Epstein Barr virus–negative rather than positive cases. Another study did not find a significantly greater risk for Hodgkin's lymphoma in white or black male military veterans related to BMI [6]. The calculated RRs were elevated modestly but the confidence limits extended below 1.0.
A very early study on the relationship of obesity to cancer, in 1978, using the medical records of 50,000 alumni of Harvard University and the University of Pennsylvania, found an association between a high ponderal index (weight/height [3]), a precursor of BMI, and the risk for Hodgkin's lymphoma [28]. That study, however, suffered from the use of histological diagnoses based on the 1957 ICD codes and the rudimentary experimental design and statistical methods of the day to deal with multivariate analysis.
Non-Hodgkin's Lymphoma
Staff at the Surveillance and Risk Assessment Division, Centre for Chronic Disease Prevention and Control, Public Health Agency of Canada, conducted a population-based case–control study of 1,030 cases of histologically confirmed non-Hodgkin's lymphoma and 3,106 controls to assess the impact of recreational physical activity, obesity, and energy intake on non-Hodgkin's lymphoma risk in Canada in 1994–1997 [29]. The study was part of the Canadian National Enhanced Cancer Surveillance System. Those investigators used unconditional logistic regression and distributed BMI into quartiles, based on its distribution in the control population. They also examined the relationship between obesity and subtypes of lymphoma. Obesity (BMI ≥30 kg/m2) was associated with a ORs for non-Hodgkin's lymphoma of 1.59 (CI, 1.18–2.12) for men and 1.36 (CI 1.00–1.84) for women. For men and women with a lifetime maximum BMI ≥30 kg/m2, the respective ORs were 1.55 (CI 1.16–2.06) and 1.10 (CI 0.83–1.46). The OR increased with each quartile of increasing BMI in both men (OR, 0.58, 1.00, 1.29, and 1.58) and women (OR, 0.68, 1.00, 1.16, and 1.36) after multivariable adjustment. This trend in ORs was highly significant among men (p < .001) but not quite significant for women (p < .068). Some differences were found among histologic subtypes of non-Hodgkin's lymphoma for the association with obesity. This study also indicated that recreational physical activity decreased non-Hodgkin's lymphoma risk, whereas obesity and excess calorie intake increased the risk. Cases were stratified by diffuse lymphoma (40.7% of total cases), follicular lymphoma (23.5%), small lymphocytic lymphoma (9.7%), and all other types of lymphoma (26.1%). The relationship with obesity was stronger in the diffuse lymphoma and all other types of lymphoma groups, but low case numbers after four-way stratification may have influenced this outcome.
In a collaboration between epidemiologists at the Karolinska Institute, Stockholm, and the U.S. National Cancer Institute, a population-based Swedish cohort of patients with a discharge diagnosis of obesity in 1965–1993 was studied and their incidence of cancer was obtained from the Swedish Cancer Registry [30]. The standardized incidence ratio (SIR) was used to assess cancer risk. Overall, a 33% excess cancer incidence was found. Among these was a higher incidence of lymphomas (SIR, 1.4; CI, 1.0–1.7). The risk for Hodgkin's lymphoma was elevated in men (SIR, 3.3; CI, 1.4–6.5) and the risk for non-Hodgkin's lymphoma was elevated in women (SIR, 1.6; CI, 1.2–2.1).
Investigators from five academic centers and the National Cancer Institute evaluated the role of obesity (and other variables) in a population-based, case–control study conducted in Detroit, Iowa, Los Angeles, and Seattle in 1998–2000 [31]. HIV− cases of non-Hodgkin's lymphoma, aged 20–74 years, were reported in each area (n = 1,321). Controls were identified through random digit dialing and Medicare files, and were matched to cases based on sex, age, race, and study site (n = 1,057). Risk factor data were collected by in-person interviews and self-administered questionnaires. Unconditional logistic regression was used to estimate the OR, adjusted for age, sex, race, and study center. High BMI (>35 kg/m2), compared with normal body size (<25 kg/m2), was positively associated with the risk for diffuse non-Hodgkin's lymphoma (OR, 1.73; CI, 1.15–2.59) but was not associated with the risk for follicular lymphoma or all non-Hodgkin's lymphomas combined. In a multivariate model to predict the risk for diffuse non-Hodgkin's lymphoma in patients with a high BMI (>35 kg/m2), compared with a normal BMI (<25 kg/m2), the OR was 2.15 (CI, 1.09–4.25). BMI was associated with the risk for this lymphoma subtype.
Members of the Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, evaluated the association of non-Hodgkin's lymphoma with BMI and postload plasma glucose level, which is positively associated with BMI. They analyzed data from a cohort study to investigate associations of interviewer-measured BMI and postload plasma glucose with risk for non-Hodgkin's lymphoma mortality and to explore associations with leukemia and myeloma [32]. Employees of 84 Chicago-area organizations, with an average age of 40 years at baseline, were screened in 1967–1973. Height and weight were measured by study nurses. A 50-g oral glucose load was administered to nondiabetic participants. Of the cohort at risk, 35,420 men and women, 129 died of non-Hodgkin's lymphoma, 151 died of leukemia, and 66 died of myeloma during an average of 31 years of follow-up. HRs and CIs were derived from Cox proportional hazards regression models. Among men, there was a positive dose–response relationship between BMI and mortality from non-Hodgkin's lymphoma (HR, 2.57; CI, 1.24–5.34) for the highest versus lowest quartile (ptrend = .01). Postload plasma glucose also was positively related to non-Hodgkin's lymphoma mortality (HR, 2.86; CI, 1.35–6.06) for the highest versus lowest category (ptrend = .004).
Researchers in the Scandinavian Lymphoma Etiology Study used telephone interviews of 3,055 patients with non-Hodgkin's lymphoma and 618 patients with Hodgkin's lymphoma diagnosed between October 1, 1999 and August 30, 2002 and 3,187 population-based control subjects [33]. The interviews assessed current height, adult weight, and other possible risk factors. Multivariable ORs for risk for lymphoma were estimated by unconditional logistic regression. In this telephone survey, BMI was not associated with the risk for overall non-Hodgkin's lymphoma or the risk for Hodgkin's lymphoma (e.g., comparing the highly obese group [BMI ≥35.0 kg/m2] with the normal-weight group [BMI, 18.5–24.9 kg/m2]. Although BMI was not associated with the risk for non-Hodgkin's lymphoma, there was evidence of a positive association with the risk for diffuse large B-cell lymphoma (OR, 1.5; CI, 0.9–2.4; ptrend = .05), comparing the highly obese group with the normal-weight group.
Two case–control studies of the relationship of BMI to lymphoma conducted in the province of Pordenone, greater Milan, and Naples, Italy in 1995–2002 included 671 patients with pathologically confirmed non-Hodgkin's lymphoma, aged 17–84 years (median, 58 years), and 220 cases of Hodgkin's lymphoma, aged 14–77 years (median, 37 years) [34]. They were compared with 1,799 control subjects. Trained interviewers questioned patients and controls as to their height and weight 1 year prior to diagnosis. Using a multivariate analysis of quintiles of BMI, they found no significant risk (OR) for non-Hodgkin's lymphoma or Hodgkin's lymphoma in any quintile of BMI when compared with the lowest BMI category (<22.3 kg/m2). They also looked at normal, overweight, and obese categories of BMI as defined by the World Health Organization and found no significant association of BMI with lymphoma. When BMI at age 30 and age 50 years was considered, there was no association of BMI with the risk for lymphoma [35].
Faculty at Northwestern University and the University of Nebraska examined the association between BMI and the risk for non-Hodgkin's lymphoma according to histologic subtypes and the BMI at different periods in a person's life. This population-based, case–control study of 387 patients with non-Hodgkin's lymphoma and 535 controls conducted in Nebraska in 1999–2002 recovered data on adult weight at age 20–29, 40–49, and 60–69 years, height, physical activity, and other lifestyle factors by telephone interview [36]. Risk was estimated by the OR, adjusting for age, total energy intake, physical activity, and other confounding factors. A higher adult BMI was associated with the risk for non-Hodgkin's lymphoma (OR, 1.4; CI, 0.9–2.0) when the obese group (BMI ≥30.0 kg/m2) was compared with the normal-weight group (BMI, 18.5–24.9 kg/m2). The risk was higher for those who were class 2 obese (BMI ≥35.0 kg/m2) (OR, 1.7; CI, 1.0–2.9). The positive association was similar among men and women. An excess risk for non-Hodgkin's lymphoma was associated with high BMI at ages 40–49 years (OR, 1.6; CI, 1.0–2.5), but although risk was elevated, it was not significant at ages 20–29 years (OR, 1.4; CI, 0.8–2.5). Obesity at ages 40–49 years was also associated with a higher risk for small lymphocytic lymphoma (OR, 4.5; CI, 1.5–13.3), diffuse large B-cell lymphoma (OR, 1.8; CI, 0.9–3.9), and follicular lymphoma (OR, 1.8; CI, 0.9–3.5).
Researchers at the School of Public Health, University of California, Berkeley, reviewed epidemiologic reports that have studied the relationship between obesity, physical activity, and diet and the risk for non-Hodgkin's lymphoma based on published case–control and prospective cohort studies. In 2005, they concluded that overweight and obesity probably increases the risk for non-Hodgkin's lymphoma, whereas moderate physical activity may reduce the risk [37].
Investigators at the Cancer Research Center in Honolulu, Hawaii explored the relation of non-Hodgkin's lymphoma to body size at different times in life within a multiethnic cohort that includes Americans of African ancestry, of European ancestry, of Japanese ancestry, of Hispanic ancestry, and Native Hawaiians from Hawaii and Los Angeles County. Participants were 45 to 75 years old at recruitment in 1993 to 1996. This analysis included 87,079 men and 105,972 women among whom there were 461 male and 378 female cases of non-Hodgkin's lymphoma [38]. Cox regression was used to model non-Hodgkin's lymphoma risk with age as the time metric, adjusting for age at entry into the cohort, ethnicity, education, alcohol intake, and age at first live birth. Body weight and BMI at age 21 were stronger predictors of non-Hodgkin's lymphoma risk than anthropometric characteristics at entry into the cohort. Men in the highest quartile of BMI and body weight at age 21 had a nonsignificant 86% and 41% higher risk for non-Hodgkin's lymphoma, respectively, whereas there was no association with their BMI at entry into the cohort. For women, the risk associated with the highest quartile of weight at age 21 was 1.6, Ptrend = 0.04, whereas women in the highest quartile of BMI at entry into the cohort had a nonsignificant risk of 27%. Despite the small numbers, there was some consistency for risk estimates across ethnic groups and weak evidence for an association with non-Hodgkin's lymphoma major subtypes. They concluded that weight at age 21 may represent lifetime effects of adiposity better than body weight at cohort entry. Weight at age 21 may be more relevant for the estimating risk for non-Hodgkin's lymphoma.
Investigators at eight academic institutions and the National Cancer Institute compared the risk for lymphoma subtype for several putative risk factors in a population-based case–control study, including diffuse large B-cell lymphoma (n = 416), follicular lymphoma (n = 318), marginal zone lymphoma (n = 106), and chronic lymphocytic leukemia/small lymphocytic lymphoma (n = 133) [39]. They required at least two of three analyses (polytomous logistic regression, homogeneity tests, or dichotomous logistic regression) to support differences in risk related to an anthropometric factor, such as BMI. Very high BMI (≥35 kg/m2) was associated with a higher risk for diffuse large B-cell lymphoma (OR, 1.7; CI, 1.1–2.5) but not follicular, marginal zone, or small lymphocytic lymphoma.
To investigate whether long-term over- or under-nutrition is associated with non-Hodgkin's lymphoma, self-reported anthropometric data on weight and height from >10,000 cases of non-Hodgkin's lymphoma and 16,000 controls were pooled across 18 case–control studies identified through the International Lymphoma Epidemiology Consortium [40]. Study-specific ORs were estimated using logistic regression and combined using a random-effects model. Severe obesity, defined as a BMI ≥40 kg/m2, was not associated with non-Hodgkin's lymphoma overall (pooled OR, 1.00; CI, 0.70–1.41) or the majority of non-Hodgkin's lymphoma subtypes examined. An excess risk was observed for diffuse large B-cell lymphoma (pooled OR, 1.80; CI, 1.24–2.62), although not all study-specific ORs were raised. Among the overweight (BMI, 25–29.9 kg/m2) and obese (BMI, 30–39.9 kg/m2), risk was elevated in some studies and not in others, whereas no association was observed among the underweight (BMI <18.5 kg/m2). There was little indication of an increasing OR for non-Hodgkin's lymphoma or its subtypes with every 5-kg/m2 rise in BMI above 18.5 kg/m2.
Members of the Department of Epidemiology, GROW-School for Oncology and Developmental Biology, Maastricht University, Maastricht, The Netherlands, examined the association between BMI and the risk for lymphocytic malignancies in the Netherlands Cohort Study [41]. The participants, 120,852 Dutch men and women aged 55–69 years, completed a self-administered questionnaire at entry into the cohort in 1986. After 13.3 years of follow-up, data on 1,042 lymphatic malignancy cases (including diffuse large-cell lymphoma, follicular lymphoma, chronic lymphocytic leukemia, myeloma, and macroglobulinemia) and 4,588 subcohort members were available. Incidence rate ratios were estimated using Cox regression models. BMI at baseline and BMI change since the age of 20 years were not associated with the risk for a lymphocytic malignancy. However, the rate ratio of lymphatic malignancies per four-unit increase in BMI at 20 years of age was 1.13 (CI, 1.01–1.25). BMI at 20 years of age and at entry into the study were self-reported by participants through a questionnaire.
Researchers in the Epidemiology and Genetics Unit, University of York, U.K., in a population-based case–control study of lymphomas in England collected height and weight details from 699 non-Hodgkin's lymphoma cases and 914 controls [42]. Obesity, defined as a BMI >30 kg/m2 5 years before diagnosis, was associated with an elevated risk for non-Hodgkin's lymphoma (OR, 1.5; CI 1.1–2.1). The excess risk was most pronounced for diffuse large B-cell lymphoma (OR, 1.9; CI 1.3–2.8).
Investigators in the Division of Nutritional Epidemiology, the National Institute of Environmental Medicine, Karolinska Institute, Stockholm, conducted a meta-analysis to summarize the epidemiologic evidence on the association between excess body weight and the risk for non-Hodgkin's lymphoma [43]. Relevant studies were identified by searching MEDLINE (1966 to February 2007) and the reference lists of retrieved publications. They included cohort and case–control studies that reported RR estimates for the association between BMI and non-Hodgkin's lymphoma incidence or mortality. A random-effects model was used to combine results from individual studies. Sixteen studies (10 cohorts and six case–control studies), with 21,720 cases, met the inclusion criteria. Compared with individuals of normal weight (BMI <25.0 kg/m2), the summary RR for non-Hodgkin's lymphoma was 1.07 (CI, 1.01–1.14) for overweight individuals (BMI, 25–29.9 kg/m2) and 1.20 (CI, 1.07–1.34) for those who were obese (BMI ≥30.0 kg/m2). A meta-analysis stratified by histologic subtypes showed that obesity was associated with a statistically significant greater risk for diffuse large B-cell lymphoma (RR, 1.40; CI, 1.18–1.66; n = 6 studies) but not follicular lymphoma (RR, 1.10; CI, 0.82–1.47; n = 6 studies) or small lymphocytic lymphoma/chronic lymphocytic leukemia (RR, 0.95; CI, 0.76–1.20; n = 3 studies). They concluded that excess body weight is associated with an elevated risk for non-Hodgkin's lymphoma, especially diffuse large B-cell lymphoma.
Investigators used the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial to evaluate the risk for non-Hodgkin's lymphoma and its subtypes in association with anthropometric factors, smoking, and alcohol consumption in a prospective cohort study. Lifestyle was assessed by a questionnaire completed by 142,982 male and female participants, aged 55–74 years, enrolled in the screening trial during 1993–2001 [44]. HRs were calculated using Cox proportional hazards regression. During 1,201,074 person-years of follow-up through 2006, 1,264 confirmed cases of non-Hodgkin's lymphoma and 243 cases of myeloma were identified. Higher BMI at ages 20 and 50 years and at enrollment (entry) was associated with a greater risk for non-Hodgkin's lymphoma (ptrend < .01 for all; e.g., at entry, BMI ≥30 versus 18.5–24.9 kg/m2; HR, 1.32; CI, 1.13–1.54) and for myeloma (ptrend < .01 to < .001 for all; at entry, BMI ≥30 versus 18.5–24.9 kg/m2; HR, 1.69; CI 1.18–2.41).
Obesity and the Risk for Myeloma
Staff at the Division of Research, Kaiser Permanente Medical Care Program, in Oakland, California, conducted a study of common clinical conditions as predictors of subsequent cancer in 143,574 outpatients of their health maintenance organization [45]. An association was noted between obesity, diagnosed in 14,388 patients, and the subsequent development of myeloma up to 21 years (33 cases observed, 21.3 expected based on the experience of the entire cohort; standardized morbidity ratio, 1.55; CI, 1.06–2.17). This association was evaluated further in a second cohort of 163,561 multiphasic-checkup examinees followed up for as many as 24 years. BMI at entry examination was associated positively with the incidence of myeloma in white men (e.g., RR, 1.07; CI, 1.01–1.15 per unit increase in BMI; RR, 1.68; CI, 0.75–3.78 comparing the highest with the lowest quartile). This association was absent in white women, partially confirmed in black men and women (BMI quartiles two, three, and four showed a higher risk than quartile one), and not explained by the presence of diabetes mellitus. The association with BMI was less or absent based on reported greatest adult weight, and in white women was inverse with BMI based on reported lowest adult weight. Among subjects with more than one checkup, greater risk was associated directly with weight loss among white men and associated inversely with weight gain among black women. These investigators concluded that body build or nutritional status may be involved in the development of myeloma.
A study of BMI and the risk for myeloma was conducted in 109,698 men and women in Japan, aged 40–79 years [46]. A Cox proportional hazard model was used to calculate the age- and sex-adjusted HR for myeloma. The ratio for men was significantly higher than for women (HR, 1.5; CI, 1.0–2.2). For men and women, a BMI >30 kg/m2 was associated with a significantly greater age- and sex-adjusted risk for myeloma (HR, 2.8; CI, 1.0–7.7).
Investigators from the Michigan Cancer Foundation in Detroit, the New Jersey Department of Health, Emory University, and the National Cancer Institute collaborated to explore the association of BMI with the risk for myeloma (OR) among black men and women compared with white men and women, age 30–79 years, in the U.S. in patients diagnosed in 1986–1989 [47]. The 345 white men and women and 191 black men and women with myeloma were compared with 1,082 white and 899 black controls who participated in a population-based, case–control study of myeloma in three areas of the U.S. (Michigan, Georgia, and New Jersey). The World Health Organization categories of underweight, normal, overweight, and obese, based on BMI, were used to categorize study subjects. Data were analyzed by unconditional logistic regression. An elevated risk for myeloma was associated with obesity (BMI ≥30 kg/m2) as compared with normal weight (BMI, 18.5–24.9 kg/m2)—OR, 1.9 (CI, 1.2–3.1) for white men and women and OR, 1.5 (CI, 0.9–2.4) for blacks. A significant trend in risk was present for white men and women and black women (but not black men) from normal to overweight to obese subjects. The frequency of obesity was greater for black than for white controls.
Investigators at the Mayo Clinic and University of Minnesota examined the association between anthropometric characteristics and the incidence of myeloma in a prospective, population-based sample of 37,083 postmenopausal women. In 1986, the women completed a mailed questionnaire that included self-report of height and weight and measurement of waist and hip circumferences [48]. During 16 years of follow-up, 95 cases of myeloma were identified through linkage to the Iowa Cancer Registry. In an age-adjusted model, women in the highest category of several anthropometric measurements, compared with the lowest category, had a higher risk for developing myeloma. For BMI (kg/m2), the rate ratio was 1.5 (CI, 0.92–2.6); for weight it was 1.9 (CI, 1.1–3.4), for waist circumference it was 2.0 (CI, 1.1–3.5), and for hip circumference it was 1.8 (CI, 1.0–3.0).
Researchers in the Department of Preventive Medicine, Keck School of Medicine, University of Southern California, the Medical University of South Carolina, and the National Cancer Institute evaluated IL-6 genotypes and BMI in a case–control study of myeloma and plasmacytoma [49]. DNA samples and questionnaires were obtained from 134 cases of myeloma and 16 cases of plasmacytoma from the Los Angeles County population-based cancer registry and from 112 siblings or cousins of cases (family controls) and 126 population controls. Genotypes evaluated included the IL-6 promoter gene single nucleotide polymorphisms at positions −174, −572, and −597; one variable number of tandem repeats (−373 A(n)T(n)); and one single nucleotide polymorphism in the IL-6 receptor α gene at position −358. The variant allele of the IL-6 promoter single nucleotide polymorphism −572 was associated with an approximately twofold greater risk for myeloma when cases were compared with family (OR, 1.8; CI, 0.7–4.7) or population controls (OR, 2.4; CI, 1.2–4.7). Obesity (BMI ≥30 kg/m2) was associated with a nonsignificant 40% and 80% higher risk when myeloma cases were compared with family controls or population controls, respectively, relative to persons with a BMI of <25 kg/m2. Increasing BMI was not significantly associated with an elevated risk for myeloma when cases were compared with the population control (ptrend < .08).
In a study of the relationship between BMI and myeloma incidence at the Harvard Medical School, Harvard School of Public Health, and Jerome Lipper Multiple Myeloma Center at the Dana-Farber Cancer Institute, 136,623 individuals were followed (>2.1 million person-years at risk) and 215 incident cases of myeloma occurred [50]. BMI was positively and significantly associated with myeloma incidence. The association was strongest in men with a BMI >30 kg/m2, when compared with those with a BMI <22 kg/m2, resulting in an RR two and one half times that of lean individuals (RR, 2.4; CI, 1.0–6.0). The RR was significantly elevated (RR, 1.6; CI, 1.0–2.7) for women in the overweight category (BMI, 25–29.9 kg/m2), but not in obese women (BMI ≥30 kg/m2) (RR, 1.2; CI, 0.7–2.2).
Epidemiologists at the Karolinsksa Institute, Stockholm, conducted a meta-analysis to quantitatively summarize the evidence from epidemiologic studies of the association of overweight and obesity with the risk for multiple myeloma. They searched the MEDLINE and EMBASE databases (1966 to May 2007) and the reference lists of retrieved articles. Cohort and case–control studies were included if they reported RR estimates for the relation between BMI and myeloma incidence or mortality. A random-effects model was used to combine study-specific results. In total, 11 cohort studies (involving 13,120 cases) and four case–control studies (1,166 cases and 8,247 controls) were included in the meta-analysis [51]. Compared with individuals of normal weight, the risk for myeloma was statistically significantly higher among those who were overweight (cohort studies: RR, 1.12; CI, 1.07–1.18; case–control studies: RR, 1.43; CI, 1.23–1.68) or obese (cohort studies: RR, 1.27; CI, 1.15–1.41; case–control studies: RR, 1.82; CI, 1.47–2.26).
Investigators at the Mayo Clinic and the National Cancer Institute screened 1,000 black and 996 white women (age 40–79 years) of similar socioeconomic status for essential monoclonal gammopathy in order to study the risk for acquiring essential monoclonal gammopathy in relation to obesity and ethnicity [52]. Thirty-nine (3.9%) Americans of African ancestry and 21 (2.1%) Americans of European ancestry had essential monoclonal gammopathy. On multivariate analysis, obesity (OR, 1.8; p = .04) and African ancestry (OR, 1.8; p = .04) were independently associated with an excess risk for this clonal precursor of myeloma.
Discussion
Leukemia Subtypes
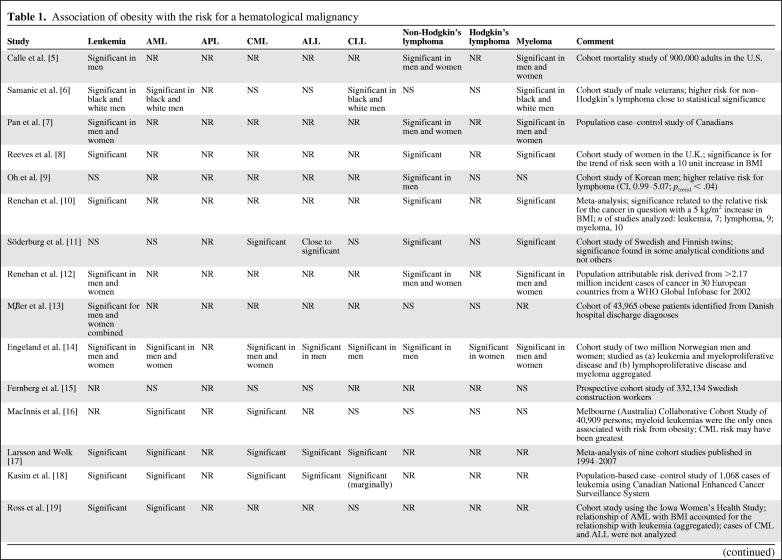
Table 1 summarizes the findings of the studies described in this paper. Some studies of obesity and leukemia have suffered from the failure to consider, at least, the four major categories of leukemia. There is a reluctance to stratify exhaustively because the sample size per analytical cell would be insufficient to minimize a type 2 statistical error. Nevertheless, showing the stratified data for subtypes in addition to the aggregate results in studies of leukemia and lymphoma would permit later meta-analyses to consider the question of subtypes. This problem is highlighted by a study indicating that a significant relationship between obesity and the risk for acute promyelocytic leukemia exists, but in that study there was no association with all cases of acute myelogenous leukemia [21]. The latter subtype represents about 8%–10% of all cases of acute myelogenous leukemia in the U.S., although in the study cited it represented about 17% of cases of acute myelogenous leukemia in the Shanghai district of China [21]. The putative role of elevated leptin levels in the higher RR for acute promyelocytic leukemia in obese persons was discussed above.
Table 1.
Association of obesity with the risk for a hematological malignancy
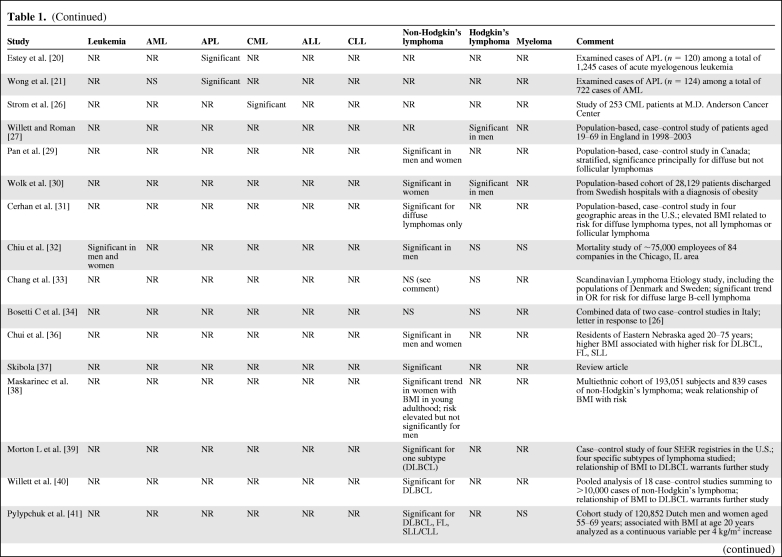
Table 1.
(Continued)
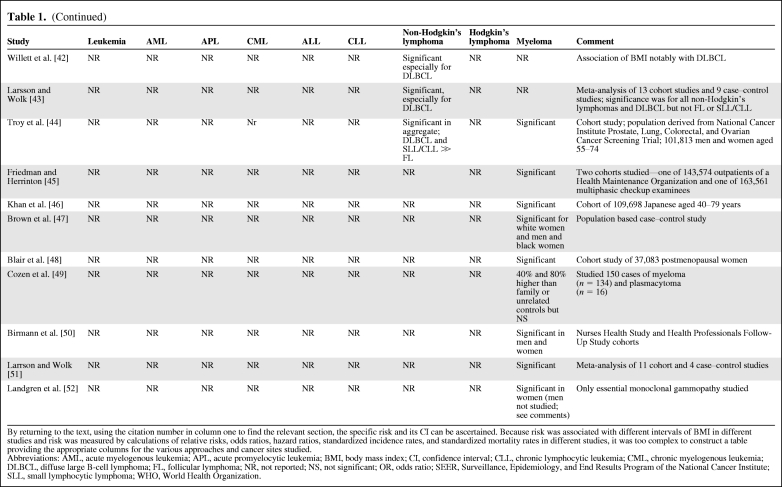
Table 1.
(Continued)
By returning to the text, using the citation number in column one to find the relevant section, the specific risk and its CI can be ascertained. Because risk was associated with different intervals of BMI in different studies and risk was measured by calculations of relative risks, odds ratios, hazard ratios, standardized incidence rates, and standardized mortality rates in different studies, it was too complex to construct a table providing the appropriate columns for the various approaches and cancer sites studied.
Abbreviations: AML, acute myelogenous leukemia; APL, acute promyelocytic leukemia; BMI, body mass index; CI, confidence interval; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; NR, not reported; NS, not significant; OR, odds ratio; SEER, Surveillance, Epidemiology, and End Results Program of the National Cancer Institute; SLL, small lymphocytic lymphoma; WHO, World Health Organization.
Considering the diagnostic category “leukemia” has the dual risk of missing the significance of an association with one subtype (e.g., acute myelogenous leukemia versus the other three types), because of the diluting effect of irrelevant cases on the one hand, and mistakenly concluding that all four types of leukemia have elevated risks, as a result of the strong effect of one or two relevant types, leading to a significant association with leukemia in the aggregate, on the other hand. In the findings reviewed in this paper, there was a significant association between obesity and the risk for leukemia in the aggregate [5–8, 10, 12–14, 17–19, 32], the risk for acute myelogenous leukemia [6, 14, 16–19], the risk for acute promyelocytic leukemia only among the acute myelogenous leukemias [20, 21], the risk for chronic myelogenous leukemia [11, 14, 16–18, 26], the risk for acute lymphocytic leukemia [14, 17, 18], and the risk for chronic lymphocytic leukemia [6, 11, 14, 17, 18].
Lymphoma Subtypes
The most carefully constructed and largest population studies concluded that obesity is a risk factor for lymphoma [5, 7–12, 14, 29, 30, 31, 32, 36–40, 41–44], but most studies have not examined the major subtypes. A few studies have not found an association between obesity and lymphoma [6, 13, 16, 33, 34]. Several studies have found a risk for diffuse lymphomas, or, more specifically, diffuse large B-cell lymphoma [31, 33, 36, 39, 40–44]. The association between obesity and the risk for follicular lymphoma was observed in three studies [32, 36, 44], but not in others [29, 31, 43]. Because diffuse large B-cell lymphoma makes up about 35%–40% of all cases of lymphoma in western societies, a strong association between that phenotype and obesity could account for some of the results studying all lymphomas in the aggregate. Although there have been ambiguous results for the association between obesity and the risk for follicular lymphoma, these two lymphomas constitute about 65%–75% of lymphoma cases in western countries and could result in a significant relationship with obesity when all lymphomas are aggregated.
Myeloma
The data linking obesity with myeloma may be the most compelling among the hematological malignancies, based on replication in a majority of studies [5–8, 10–12, 14, 24, 41–47, 50], but not in several others [9, 15, 16, 32, 41, 49]. The conclusion that a significant relationship exists between obesity and the risk for myeloma was supported by a meta-analysis of 11 cohort and four case–control studies [51]. The relationship between single nucleotide polymorphisms of the IL-6 promoter gene and obesity and between IL-6 and the growth-fostering and cell survival–promoting effects on myeloma cells is intriguing [49], but a definitive interrelationship has not been established.
Population Attributable Risk and Prevention
Although we do not have a certain value for the population attributable risk for hematological malignancies associated with obesity, even a small increase in risk is consequential because of the markedly shortened life span in persons with these diseases. The clinical importance of these finding is the possibility of decreasing the incidence of the hematological malignancies through prevention. Hematological malignancies in adults have onerous treatment protocols and a low cure rate [53]. An obstacle to invoking prevention is the inadequacy of methods for behavior modification that are consistent with a free society wrapped in abundance. The morbidity and mortality from cancer (let alone the cost) could be markedly reduced by a decrease in smoking, alcohol abuse, overeating, and underexercising. In the specific case of acute myelogenous leukemia, the incidence, and thereby the mortality rate, could be significantly decreased if tobacco smoking [54] was further curtailed and, apparently, if caloric intake could be reduced and exercise increased in relevant populations. In general, the prevention of hematological malignancies is not a focus of hematologists because the risk factors are either not yet established to a degree of medical certainty or, for those that are, seem beyond the ability of an individual physician to modify. Chemical exposure, notably to benzene, has been dealt with by government workplace regulation, making benzene-induced acute myelogenous leukemia or myelodysplasia vanishingly rare in developed countries. The association between acute myelogenous leukemia and tobacco smoking is being addresses by the American Cancer Society, the U.S. Public Health Service, state programs, and nongovernmental agencies through vigorous antismoking campaigns and programs of smoking cessation. Research that could lead to interventions that block central nervous system signals that mediate craving for tobacco or appetite could be useful.
Biological Plausibility
One of the important missing links in the putative relationship between overweight and obesity and the hematological malignancies is biological plausibility. How does obesity lead to cancer, in general, and leukemia, lymphoma, and myeloma, in particular? Because these diseases require a series of gene mutations, one could ask: “Are these mutations independent of obesity?” But the de novo genetic changes are more likely to lead to clonal dominance because of the metabolic and endocrinologic changes induced by obesity. The example given in acute promyelocytic leukemia links the greater concentration of leptin resulting from a greater mass of fat to a milieu that favors growth and impaired programmed cell death of leukemic promyelocytes. This explanation indicates that the transforming events occur at similar frequencies in lean and fat individuals but the latter have a higher probability of having a clone emerge. The latter can be thought of as the selection hypothesis. Alternatively, in some as yet undefined manner, obesity may accelerate the mutational rate of cells or interfere with cellular mechanisms of DNA repair resulting in mutational events, increasing the inherent rate of mutation in tissue cells or influencing the role of epigenetic factors in cell regulation. This represents an inductive effect. A dietary effect on microRNA dysregulation is another avenue being studied by biochemical nutritionists that could link obesity to carcinogenesis. The relationship between dietary patterns and food groups and the risk for non-Hodgkin's lymphoma is reviewed elsewhere [33].
Ideally, the relationship between a causal factor and a cancer is established by at least one, but preferably two, of the following approaches: carefully conducted epidemiological studies that, in the aggregate, provide compelling evidence for a causal association (e.g., smoking and lung cancer); animal studies showing that the causal factor under study can induce a reasonable facsimile of the human disease in experimental animals (e.g., experimental smoke inhalation in animals); and studies showing that the risk factor under study can cause genetic changes that replicate the causal genetic changes in the human disease (e.g., induction of the BCR-ABL oncogene with radiation of BCR-ABL− cells). The latter two requirements are often not unachievable, so we rely heavily on epidemiological studies, many of which are limited by (a) relatively small populations, (b) “convenience” sampling, (c) case–control approaches, making the selection of a comparison group critical, (d) questionnaires, (e) self-reported key variables, (f) multivariate complexities, (f) investigator bias, (g) attrition rates from cohort studies, and (h) other limiting factors. Meta-analysis can overcome some of these limitations, if the studies under consideration are well designed, if not conclusive.
Obesity, African Ancestry, and the Frequency of Myeloma
There are other questions that have arisen around the role of obesity and the risk for hematological malignancies. One is the greater prevalence of obesity in Americans of African descent and whether that explains, in part, the higher incidence of myeloma in that group (∼1.8-fold that of Americans of European ancestry in the U.S.). Differences in the normal regulation of the steady-state immunoglobulin level between persons of European and African descent [55] and the independent link between both obesity and African descent and the incidence of essential monoclonal gammopathy suggest a biological rather than environmental explanation for the higher rate of disease among Americans of African ancestry.
The Obesity Epidemic and the Lymphoma Epidemic
Another potential association is the, as yet unexplained, marked increase in the annual incidence of lymphoma tracked in the U.S. over the past 40 years, but probably initiated closer to 60 years ago based on European data. The increase in incidence of non-Hodgkin's lymphoma has been about 1.8-fold over the last 40 years. The Surveillance, Epidemiology, and End Results program of the U.S. National Cancer Institute has only been tracking cancer incidence and mortality for approximately 40 years, whereas several European countries have been doing so for a longer period. Does the increased incidence of obesity account for some of that increase? Current data do not permit a definitive conclusion, but obesity is probably not a principal explanation because the increase over the last 40 years has been found among all lymphoma subtypes and the data for obesity in that regard remain equivocal.
Weight Loss and the Prevention of Cancer
One of the important observations that adds more evidence to the obesity–cancer relationship is the data indicating a decrease in cancer incidence in persons who have had successful bariatric surgery [56] or who have undergone sustained weight loss from exercise and decreased caloric intake [57]. This effect has not been shown for hematological malignancies to my knowledge.
Possible Metabolic Effects of Obesity and Cancer Onset
The Fat Cell as an Endocrine Gland
The effects of obesity can be discriminated into two pathogenetic categories: the result of the greater mass of fat itself and the result of an expansion of the endocrine function of the enlarged and higher numbers of fat cells and the effects of these endocrine changes on target tissues (e.g., increased plasma leptin, insulin, insulin growth-factor [IGF]-1, androgens, estrogens, IL-6, and tumor necrosis factor-α) [58]. The former mechanism, for example, has been linked to a higher incidence of adenocarcinoma of the esophagus in the obese because of the greater frequency and severity of gastric reflux, whereas the higher incidence of carcinoma of the breast and uterus, for example, may be in part a reflection of elevated estrogen levels resulting from obesity.
Laboratory investigators have found that animals in which energy intake is reduced to about 60% of the intake of animals that are fed as much as they want have a considerably lower incidence of cancer than the animals in the comparison group without restricted intake. This predisposing factor apparently holds true for viral, chemical, and spontaneous carcinogenesis. Evidence from studies in humans has been largely compatible with these laboratory findings and indicates that obesity increases the risk for a wide spectrum of sites and types of tumors [3–10, 12, 13, 59].
The elevation of several hormones correlated with greater adiposity has been associated with cancer cell growth. IGF-1 enhances the growth of cell lines of several types cancers [3, 60]. The IGF-1 receptor is overexpressed in several tumor cell types and also can affect the expression of the p53 gene. IGF-1 is synthesized in the liver and is influenced by growth hormone and carbohydrate and protein intake. Fat cells are an important secondary source of IGF-1. Its plasma half-life and local availability in tissues are dependent on a family of binding proteins. IGF-1 fosters cell cycle progression from the G1 to S phase in normal and cancer cells. It is thought to mediate the anticancer effects of calorie restriction, whereas cells with high levels of IGF-1 receptor are susceptible to its antiapoptotic effect.
Hyperinsulinemia and insulin resistance increase cancer risk at some tissue sites and are induced by adiposity [3, 60, 61]. It is not known whether these effects are direct and mediated through the insulin receptor or whether they act by stimulation of IGF-1. The downstream targets in signaling pathways that control cell growth and survival include phosphoinositide 3-kinase/Akt, one of the most commonly aberrant pathways in epithelial tumors. Often, the activation of this signaling pathway is associated with an activation of the mammalian target of rapamycin signal, a potent effector of cell proliferation and resistance to apoptosis.
Leptin, a peptide hormone secreted principally by fat cells, regulates appetite through hypothalamic receptors and neuroendocrine pathways so as to maintain the amount of body fat tissue, albeit imperfectly. Leptin can stimulate neoplastic cells, but not normal cells, in culture and higher levels have been associated with colon and prostate carcinoma. In animal models, it fosters angiogenesis and tumor invasion [3, 60, 61]. It has been associated with enhanced growth of acute promyelocytic leukemia cells [23].
Adiponectin, a peptide hormone produced by adipocytes, is a regulator of insulin sensitivity and thereby carbohydrate and lipid metabolism. Paradoxically, adiponectin is decreased in extreme obesity. Its potential role in cancer causation is unclear [60, 61]. Lower adiponectin levels, controlling for age, gender, BMI, and leptin, have been associated with a lower risk for myeloma [62].
Adipose tissue is a principal site of estrogen synthesis in postmenopausal women and men. Aromatase in fat cells converts androgenic precursors (e.g., androstenedione) from the adrenal and gonads to estrone and estradiol. This process is a function of fat cell mass and can, for example, significantly increase estrogen levels in postmenopausal women and men [63]. This effect may play an important role in the relationship between obesity and endometrial and postmenopausal breast cancer [3, 60, 61, 63].
IL-6 has an essential role in the initial progression of myeloma cell tumors. IL-6 triggers proliferation of myeloma cells through the RAS–mitogen-activated protein kinase signaling pathway and it promotes myeloma cell survival through activation of the signal transducer and activator of transcription pathway's role in regulating the expression of BCL-2 antiapoptotic proteins. Fat cells are an additional source of IL-6 and may provide a link between adiposity and a higher risk for myeloma. In this case, we have a quantifiable proportion of persons with a nonprogressive clone (essential monoclonal gammopathy) with the potential to undergo clonal evolution into an aggressive plasma cell neoplasm. No data, however, exist to indicate that obesity plays a role in the 1% of patients per year with monoclonal gammopathy who progress to a lymphoma or a progressive plasma cell neoplasm.
Other Mechanisms Linking Obesity to Cancer
Other hypothetical links between obesity and the neoplastic transformation of tissue cells have included obesity-related hypoxia (e.g., melanocyte transformation), migrating adipose stromal cells (neovascularization, favoring tumor progression), shared genetic susceptibility (common genetic predisposition to obesity and cancer), obesity-related inflammation, obesity-related increase in oxidative stress, and nuclear-factor κ B as a mediator of insulin resistance [3]. Obesity has also been shown to impair immune responses, another possible link to non-Hodgkin's lymphoma [27].
Among the global questions that remain to be answered, assuming a role for obesity in the causation of hematological cancers, include whether the effect is inductive or selective, or perhaps both. That is, can the endocrinological, metabolic, and immunological changes incurred by obesity result in genetic or epigenetic changes in a tissue cell resulting in a malignant transformation or does the obese milieu permit the selection of transformed cells that had been in a state of dormancy?
References
- 1.Centers for Disease Control and Prevention. Overweight and Obesity. [accessed June 25, 2010]. Available at http://www.cdc.gov/obesity/index.html.
- 2.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: New perspectives. Annu Rev Med. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 4.Wolin KY, Carson K, Colditz GA. Obesity and cancer. The Oncologist. 2010;15:556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Samanic C, Gridley G, Chow WH, et al. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15:35–43. doi: 10.1023/B:CACO.0000016573.79453.ba. [DOI] [PubMed] [Google Scholar]
- 7.Pan SY, Johnson KC, Ugnat AM, et al. Canadian Cancer Registries Epidemiology Research Group. Association of obesity and cancer risk in Canada. Am J Epidemiol. 2004;159:259–268. doi: 10.1093/aje/kwh041. [DOI] [PubMed] [Google Scholar]
- 8.Reeves GK, Pirie K, Beral V, et al. Million Women Study Collaboration. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ. 2007;335:1134–1144. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23:4742–4754. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 10.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 11.Söderberg KC, Kaprio J, Verkasalo PK, et al. Overweight, obesity and risk of haematological malignancies: A cohort study of Swedish and Finnish twins. Eur J Cancer. 2009;45:1232–1238. doi: 10.1016/j.ejca.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Renehan AG, Soerjomataram I, Tyson M, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer. 2010;126:692–702. doi: 10.1002/ijc.24803. [DOI] [PubMed] [Google Scholar]
- 13.Ml̸ler H, Mellemgaard A, Lindvig K, et al. Obesity and cancer risk: A Danish record-linkage study. Eur J Cancer. 1994;30A:344–350. doi: 10.1016/0959-8049(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 14.Engeland A, Tretli S, Hansen S, et al. Height and body mass index and risk of lymphohematopoietic malignancies in two million Norwegian men and women. Am J Epidemiol. 2007;165:44–52. doi: 10.1093/aje/kwj353. [DOI] [PubMed] [Google Scholar]
- 15.Fernberg P, Odenbro A, Bellocco R, et al. Tobacco use, body mass index, and the risk of leukemia and multiple myeloma: A nationwide cohort study in Sweden. Cancer Res. 2007;67:5983–5986. doi: 10.1158/0008-5472.CAN-07-0274. [DOI] [PubMed] [Google Scholar]
- 16.MacInnis RJ, English DR, Hopper JL, et al. Body size and composition and the risk of lymphohematopoietic malignancies. J Natl Cancer Inst. 2005;97:1154–1157. doi: 10.1093/jnci/dji209. [DOI] [PubMed] [Google Scholar]
- 17.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: A meta-analysis of cohort studies. Int J Cancer. 2008;122:1418–1421. doi: 10.1002/ijc.23176. [DOI] [PubMed] [Google Scholar]
- 18.Kasim K, Levallois P, Abdous B, et al. Lifestyle factors and the risk of adult leukemia in Canada. Cancer Causes Control. 2005;16:489–500. doi: 10.1007/s10552-004-7115-1. [DOI] [PubMed] [Google Scholar]
- 19.Ross JA, Parker E, Blair CK, et al. Body mass index and risk of leukemia in older women. Cancer Epidemiol Biomarkers Prev. 2004;13:1810–1813. [PubMed] [Google Scholar]
- 20.Estey E, Thall P, Kantarjian H, et al. Association between increased body mass index and a diagnosis of acute promyelocytic leukemia in patients with acute myeloid leukemia. Leukemia. 1997;11:1661–1664. doi: 10.1038/sj.leu.2400783. [DOI] [PubMed] [Google Scholar]
- 21.Wong O, Harris F, Yiying W, et al. A hospital-based case-control study of acute myeloid leukemia in Shanghai: Analysis of personal characteristics, lifestyle and environmental risk factors by subtypes of the WHO classification. Regul Toxicol Pharmacol. 2009;55:340–352. doi: 10.1016/j.yrtph.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Jeddi R, Ghédira H, Mnif S, et al. High body mass index is an independent predictor of differentiation syndrome in patients with acute promyelocytic leukemia. Leuk Res. 2010;34:545–547. doi: 10.1016/j.leukres.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Konopleva M, Mikhail A, Estrov Z, et al. Expression and function of leptin receptor isoforms in myeloid leukemia and myelodysplastic syndromes: Proliferative and anti-apoptotic activities. Blood. 1999;93:1668–1676. [PubMed] [Google Scholar]
- 24.Considine RV, Caro JF. Leptin and the regulation of body weight. Int J Biochem Cell Biol. 1997;29:1255–1272. doi: 10.1016/s1357-2725(97)00050-2. [DOI] [PubMed] [Google Scholar]
- 25.Gainsford T, Willson TA, Metcalf D, et al. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci U S A. 1996;93:14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strom SS, Yamamura Y, Kantarijian HM, et al. Obesity, weight gain, and risk of chronic myeloid leukemia. Cancer Epidemiol Biomarkers Prev. 2009;18:1501–1506. doi: 10.1158/1055-9965.EPI-09-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willett EV, Roman E. Obesity and the risk of Hodgkin lymphoma (United Kingdom) Cancer Causes Control. 2006;17:1103–1106. doi: 10.1007/s10552-006-0042-6. [DOI] [PubMed] [Google Scholar]
- 28.Paffenbarger RS, Jr, Wing AL, Hyde RT. Characteristics in youth indicative of adult-onset Hodgkin's disease. J Natl Cancer Inst. 1977;58:1489–1491. doi: 10.1093/jnci/58.5.1489. [DOI] [PubMed] [Google Scholar]
- 29.Pan SY, Mao Y, Ugnat AM. Canadian Cancer Registries Epidemiology Research Group. Physical activity, obesity, energy intake, and the risk of non-Hodgkin's lymphoma: A population-based case-control study. Am J Epidemiol. 2005;162:1162–1173. doi: 10.1093/aje/kwi342. [DOI] [PubMed] [Google Scholar]
- 30.Wolk A, Gridley G, Svensson M, et al. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12:13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 31.Cerhan JR, Bernstein L, Severson RK, et al. Anthropometrics, physical activity, related medical conditions, and the risk of non-Hodgkin lymphoma. Cancer Causes Control. 2005;16:1203–1214. doi: 10.1007/s10552-005-0358-7. [DOI] [PubMed] [Google Scholar]
- 32.Chiu BC, Gapstur SM, Greenland P, et al. Body mass index, abnormal glucose metabolism, and mortality from hematopoietic cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2348–2354. doi: 10.1158/1055-9965.EPI-06-0007. [DOI] [PubMed] [Google Scholar]
- 33.Chang ET, Hjalgrim H, Smedby KE, et al. Body mass index and risk of malignant lymphoma in Scandinavian men and women. J Natl Cancer Inst. 2005;97:210–218. doi: 10.1093/jnci/dji012. [DOI] [PubMed] [Google Scholar]
- 34.Bosetti C, Dal Maso L, Negri E, et al. Re: Body mass index and risk of malignant lymphoma in Scandinavian men and women [letter] J Nat Cancer Inst. 2005;97:860–861. doi: 10.1093/jnci/dji150. [DOI] [PubMed] [Google Scholar]
- 35.Dal Maso L, Talamini R, Montella M, et al. Hepatitis B and C viruses and Hodgkin lymphoma: A case-control study from Northern and Southern Italy. Haematologica. 2004;89:ELT17. [PubMed] [Google Scholar]
- 36.Chiu BC, Soni L, Gapstur SM, et al. Obesity and risk of non-Hodgkin lymphoma (United States) Cancer Causes Control. 2007;18:677–685. doi: 10.1007/s10552-007-9013-9. [DOI] [PubMed] [Google Scholar]
- 37.Skibola CF. Obesity, diet and risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:392–395. doi: 10.1158/1055-9965.EPI-06-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maskarinec G, Erber E, Gill J, et al. Overweight and obesity at different times in life as risk factors for non-Hodgkin's lymphoma: The multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2008;17:196–203. doi: 10.1158/1055-9965.EPI-07-0716. [DOI] [PubMed] [Google Scholar]
- 39.Morton LM, Wang SS, Cozen W, et al. Etiological heterogeneity among non-Hodgkin lymphoma subtypes. Blood. 2008;112:5150–5160. doi: 10.1182/blood-2008-01-133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willett EV, Morton LM, Hartge P, et al. InterLymph Consortium. Non-Hodgkin lymphoma and obesity: A pooled analysis from the InterLymph Consortium. Int J Cancer. 2008;122:2062–2070. doi: 10.1002/ijc.23344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pylypchuk RD, Schouten LJ, Goldbohm RA, et al. Body mass index, height, and risk of lymphatic malignancies: A prospective cohort study. Am J Epidemiol. 2009;170:297–307. doi: 10.1093/aje/kwp123. [DOI] [PubMed] [Google Scholar]
- 42.Willett EV, Skibola CF, Adamson P, et al. Non-Hodgkin's lymphoma, obesity and energy homeostasis polymorphisms. Br J Cancer. 2005;93:811–816. doi: 10.1038/sj.bjc.6602762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larsson SC, Wolk A. Obesity and risk of non-Hodgkin's lymphoma: A meta-analysis. Int J Cancer. 2007;121:1564–1570. doi: 10.1002/ijc.22762. [DOI] [PubMed] [Google Scholar]
- 44.Troy JD, Hartge P, Weissfeld JL, et al. Associations between anthropometry, cigarette smoking, alcohol consumption, and non-Hodgkin lymphoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Epidemiol. 2010;171:1270–1281. doi: 10.1093/aje/kwq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman GD, Herrinton LJ. Obesity and multiple myeloma. Cancer Causes Control. 1994;5:479–483. doi: 10.1007/BF01694762. [DOI] [PubMed] [Google Scholar]
- 46.Khan MM, Mori M, Sakauchi F, et al. JACC Study Group. Risk factors for multiple myeloma: Evidence from the Japan Collaborative Cohort (JACC) study. Asian Pac J Cancer Prev. 2006;7:575–581. [PubMed] [Google Scholar]
- 47.Brown LM, Gridley G, Pottern LM, et al. Diet and nutrition as risk factors for multiple myeloma among blacks and whites in the United States. Cancer Causes Control. 2001;12:117–125. doi: 10.1023/a:1008937901586. [DOI] [PubMed] [Google Scholar]
- 48.Blair CK, Cerhan JR, Folsom AR, et al. Anthropometric characteristics and risk of multiple myeloma. Epidemiology. 2005;16:691–694. doi: 10.1097/01.ede.0000172135.61188.2d. [DOI] [PubMed] [Google Scholar]
- 49.Cozen W, Gebregziabher M, Conti DV, et al. Interleukin-6-related genotypes, body mass index, and risk of multiple myeloma and plasmacytoma. Cancer Epidemiol Biomarkers Prev. 2006;15:2285–2291. doi: 10.1158/1055-9965.EPI-06-0446. [DOI] [PubMed] [Google Scholar]
- 50.Birmann BM, Giovannucci E, Rosner B, et al. Body mass index, physical activity, and risk of multiple myeloma. Cancer Epidemiol Biomarkers Prev. 2007;16:1474–1478. doi: 10.1158/1055-9965.EPI-07-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsson SC, Wolk A. Body mass index and risk of multiple myeloma: A meta-analysis. Int J Cancer. 2007;121:2512–2516. doi: 10.1002/ijc.22968. [DOI] [PubMed] [Google Scholar]
- 52.Landgren O, Rajkumar SV, Pfeiffer RM, et al. Obesity is associated with an increased risk of monoclonal gammopathy of undetermined significance among black and white women. Blood. 2010;116:1056–1059. doi: 10.1182/blood-2010-01-262394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lichtman MA. Battling the hematological malignancies: The 200 years' war. The Oncologist. 2008;13:126–138. doi: 10.1634/theoncologist.2007-0228. [DOI] [PubMed] [Google Scholar]
- 54.Lichtman MA. Cigarette smoking, cytogenetic abnormalities, and acute myelogenous leukemia. Leukemia. 2007;21:1137–1140. doi: 10.1038/sj.leu.2404698. [DOI] [PubMed] [Google Scholar]
- 55.Lichtman MA, Vaughan JH, Hames CG. The distribution of serum immunoglobulins, anti-gamma-G globulins (“rheumatoid factors”) and antinuclear antibodies in White and Negro subjects in Evans County, Georgia. Arthritis Rheum. 1967;10:204–215. doi: 10.1002/art.1780100306. [DOI] [PubMed] [Google Scholar]
- 56.Sjöström L, Gummesson A, Sjöström CD, et al. Swedish Obese Subjects Study. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): A prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–662. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 57.Wolin KY, Colditz GA. Can weight loss prevent cancer? Br J Cancer. 2008;99:995–999. doi: 10.1038/sj.bjc.6604623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 59.Key TJ, Spencer EA, Reeves GK. Symposium 1. Overnutrition: Consequences and solutions. Obesity and cancer risk. Proc Nutr Soc. 2010;69:86–90. doi: 10.1017/S0029665109991698. [DOI] [PubMed] [Google Scholar]
- 60.Hursting SD, Lashinger LM, Wheatley KW, et al. Reducing the weight of cancer: Mechanistic targets for breaking the obesity-carcinogenesis link. Best Pract Res Clin Endocrinol Metab. 2008;22:659–669. doi: 10.1016/j.beem.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 61.Becker S, Dossus L, Kaaks R. Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Arch Physiol Biochem. 2009;115:86–96. doi: 10.1080/13813450902878054. [DOI] [PubMed] [Google Scholar]
- 62.Dalamaga M, Karmaniolas K, Panagiotou A, et al. Low circulating adiponectin and resistin, but not leptin, levels are associated with multiple myeloma risk: A case-control study. Cancer Causes Control. 2009;20:193–199. doi: 10.1007/s10552-008-9233-7. [DOI] [PubMed] [Google Scholar]
- 63.Bray GA. The underlying basis for obesity: Relationship to cancer. J Nutr. 2002;132(11 suppl):3451S–3455S. doi: 10.1093/jn/132.11.3451S. [DOI] [PubMed] [Google Scholar]