There is still much that needs to be understood about radiation recall, and it is not currently possible to predict which patients will be affected and to which drugs they will react. Furthermore, there are no clearly defined characteristics of drugs that cause radiation recall, and thus, it is a possibility that must be kept in mind with use of any drug after radiotherapy, including those from new drug classes. Although it is not yet possible to design treatment regimens to eliminate the risk of radiation recall, it seems likely that risks can be minimized by prolonging the interval between completion of radiotherapy and initiation of full-dose chemotherapy.
Keywords: Chemotherapy, Antineoplastic agents, Radiation recall, Radiation recall dermatitis
Abstract
Radiation recall is an acute inflammatory reaction confined to previously irradiated areas that can be triggered when chemotherapy agents are administered after radiotherapy. It remains a poorly understood phenomenon, but increased awareness may aid early diagnosis and appropriate management. A diverse range of drugs used in the treatment of cancer has been associated with radiation recall. As most data come from case reports, it is not possible to determine the true incidence, but to date the antineoplastic drugs for which radiation recall reactions have been most commonly reported include the anthracycline doxorubicin, the taxanes docetaxel and paclitaxel, and the antimetabolites gemcitabine and capecitabine. Radiation recall is drug-specific for any individual patient; it is not possible to predict which patients will react to which drugs, and rechallenge does not uniformly induce a reaction. There are no identifiable characteristics of drugs that cause radiation recall, and thus, it is a possibility that must be kept in mind with use of any drug after radiotherapy, including those from new drug classes. Although it is not yet possible to design treatment regimens to eliminate the risk of radiation recall, it seems likely that risks can be minimized by prolonging the interval between completion of radiotherapy and initiation of chemotherapy.
Introduction
Treatment of cancer involves the widespread use of radiotherapy in conjunction with chemotherapy. Both treatment paradigms are associated with well-described, but not always overlapping, profiles of tolerability. Although giving chemotherapy after radiotherapy can be valuable clinically, it can also induce the phenomenon of radiation recall.
Radiation recall is an uncommon and unpredictable phenomenon. It is characterized by an acute inflammatory reaction confined to previously irradiated areas that is triggered by the administration of precipitating systemic agents after radiation treatment [1–4]. The most commonly implicated drugs are anticancer agents, but other drugs can also cause radiation recall, including some antibiotics, antituberculosis drugs, and simvastatin [1, 3].
It is helpful to differentiate between radiation recall and radiosensitization. There is a period of enhanced sensitivity in the days after irradiation when reaction to systemically administered drugs is common, and if the time interval between end of radiation and chemotherapy is <7 days, such reactions can be considered radiation enhancement or sensitization. It has been suggested that radiation recall should be differentiated by a later occurrence after completion of radiotherapy (e.g., >7 days) [3]. Radiation recall is much less common clinically than radiosensitization. It can occur months or even many years after irradiation, suggesting that the mechanisms may be different from those of radiosensitization [3], although others have suggested that radiation recall is a form of delayed radiosensitization [4].
First described in 1959 [5], radiation recall remains a poorly understood phenomenon. The aim of this review is to provide an overview of the clinical presentation and treatment of radiation recall within the context of cancer management, including updated information on reactions reported with the newest anticancer agents. A detailed case study of the first reported case of radiation recall with ixabepilone (BMS-247550; Bristol-Myers Squibb, New York), the first of a new class of antineoplastic agents the epothilones, is included.
Incidence of Radiation Recall
Few systematic reports have examined the incidence of radiation recall, and most of the literature concerning this phenomenon is presented as case reports, limiting the opportunity to determine the actuarial risk of occurrence.
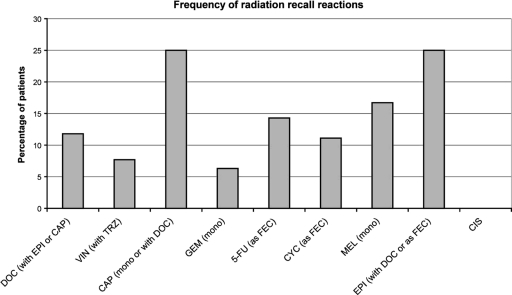
Radiation recall was reported in 8.8% of patients receiving a range of chemotherapeutic agents after completion of radiotherapy in an observational study of 91 patients undergoing palliative treatment for metastatic disease (Fig. 1) [6]. The time interval between the completion of radiation therapy and the administration of cytotoxic chemotherapy was between 6 and 37 days in patients in whom radiation recall developed [6]. In line with the expected incidence, no agent administered to less than five patients in this study elicited a radiation recall reaction. Cisplatin (n = 10) was the only drug taken by more than five patients that did not cause a radiation recall reaction. In the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial, 148 patients received chemotherapy within 90 days of accelerated partial breast irradiation (34 Gy) [7]. Chemotherapy included doxorubicin in 75% of cases. During a follow-up of 1 year, radiation recall occurred in 15 patients (11.5% of 131 evaluable patients).
Figure 1.
Frequency of radiation recall reactions in patients given chemotherapy within 6 months of radiotherapy in an observational study (91 patients). In total, 8 patients experienced radiation recall. Adapted from Kodym E, Kalinska R, Ehringfeld C et al. Frequency of radiation recall dermatitis in adult cancer patients. Onkologie 2005;28:18–21, with permission.
Abbreviations: 5-FU, 5-fluorouracil; CAP, capecitabine; CIS, cisplatin; CYC, cyclophosphamide; DOC, docetaxel; EPI, epirubicin; FEC, 5-fluorouracil, epirubicin, and cyclophosphamide; GEM, gemcitabine; MEL, melphalan; mono, monotherapy; TRZ, trastuzumab; VIN, vinorelbine.
Other studies have suggested incidences of radiation recall ≤6% among patients receiving systemic therapy after previous radiation exposure. One of 20 patients treated with capecitabine 4 weeks after previous combination therapy with capecitabine and radiotherapy developed radiation recall [8]. Among 32 patients treated with docetaxel (8 monotherapy and 24 combination therapy), 2 patients (6%) developed radiation recall reactions [4]. Finally, in a retrospective review of 171 patients who had previously received radiotherapy and subsequently received docetaxel, 1.8% developed radiation recall (3 patients were affected) [9].
Pathophysiology of Radiation Recall
The pathophysiological basis of cutaneous radiation recall is not known. Several hypotheses have been proposed, but are not yet supported by adequate evidence to be considered proven [1, 3, 4, 10, 11].
Given that reaction recall reactions are drug-specific and differ between individuals, it is unlikely that the cytotoxic or other pharmacological activities of anticancer drugs are solely responsible. Theories involving depletion and/or changes in function of stem cells in the irradiated area are difficult to reconcile with the unpredictable nature of radiation recall, the sometimes very rapid onset of action, and the fact that reactions are not uniformly elicited with rechallenge. It is possible that stem cells in the irradiated area have increased sensitivity, or display a “remembered” reaction, to subsequent chemotherapy [1, 3, 4].
Camidge and Price have proposed that cutaneous radiation recall reactions are caused by idiosyncratic drug hypersensitivity reactions that may be analogous to fixed drug eruptions [3]. This localized hypersensitivity likely involves direct activation of nonimmune inflammatory pathways; irradiation lowers the inflammatory response threshold. It has been suggested that this mechanism could be mediated by continued low-level secretion of the inflammation-mediating cytokines induced by radiation. The presence of a precipitating chemotherapy agent may then upregulate these cytokines, resulting in a radiation recall reaction [1].
Keratinocyte necrosis, related to cumulative direct DNA damage and oxidative stress, could play a major role in radiation recall dermatitis [11]. Specimens from eight patients with radiation recall dermatitis showed ballooning degeneration of epidermal keratinocytes with a mixed inflammatory infiltrate [11]. Underlying nutritional deficits were also identified, which may contribute to the process because of endogenous scavenger systems becoming depleted.
In the case of capecitabine, it has been suggested that radiation recall may result from upregulation of thymidine phosphorylase, which could induce angiogenesis in the radiated area and local prodrug activation. Capecitabine is a prodrug that is converted to the active agent fluorouracil [8, 12].
Presentation of Radiation Recall
One of the important features of radiation recall is that the reaction affects skin (or other organs) that was previously quiescent and apparently normal. The area affected clearly corresponds to an area previously irradiated, although in the case of skin reactions the local effect may occasionally spread or become generalized [3]. It should be differentiated from incomplete healing of ongoing skin reactions to the radiation itself. Patients who experience radiation recall reactions may or may not have experienced acute radiation reactions. Many patients who develop radiation recall have no reaction to radiation during or at the end of radiotherapy [1, 3, 13].
The time interval between end of radiation therapy and administration of the precipitating chemotherapy varies widely in cases of radiation recall (Table 1). Camidge and Price [3] advocate that the reaction should be considered to be radiation recall only if the time lag is >7 days. They calculated the median interval to be 40 days for radiation recall dermatitis linked to any systemic drugs [3]. However, there are several reports of radiation recall occurring years after completion of radiation treatment (Table 1). For example, one radiation recall reaction of ulcerative stomatitis was triggered by doxorubicin 15 years after radiotherapy [14]. In another patient, radiation recall dermatitis was triggered by pemetrexed when given 25 years after radiotherapy, and the reaction occurred again on rechallenge [15].
Table 1.
Summary of radiation recall cases associated with anticancer agents, where radiation recall is defined as occurring ≥1 wk after completion of radiation therapy unless otherwise stated
Table 1.
(Continued)
aBleeding did not recur when bevacizumab was discontinued, whereas gemcitabine was continued.
bInformation not provided on chemotherapy dosages, radiation dose, and/or time to onset in some reports.
cThe patient also received five cycles of vincristine, doxorubicin, and dexamethasone after radiotherapy and before administration of cyclophosphamide. The reaction occurred immediately after cyclophosphamide treatment and the latter was considered the causative drug.
dAlthough chemotherapy was initiated within 7 days of radiotherapy (outside the definition of radiation recall), this reaction was considered radiation recall.
ePemetrexed was administered with cisplatin in one case, but radiation recall was attributed to pemetrexed.
fChemotherapy was initiated within 7 days of radiotherapy in one patient (outside the definition of radiation recall), but the patient had no major skin reaction on completing radiotherapy and the reaction was considered radiation recall.
Abbreviations: bid, twice daily; mo, month; NR, not reported; wk, week; yr, year.
The onset of the symptoms of radiation recall usually occurs within days to a few weeks after exposure to the precipitating chemotherapy drug, frequently after the first dose and sometimes during or immediately after intravenous administration (Table 1). Less commonly, reactions become evident over a few months after oral administration [1, 3].
Radiation recall reactions most commonly present on the skin, with skin reactions comprising around two thirds of cases (Table 1). Radiation recall dermatitis is usually of mild or moderate intensity, but can be severe in some instances (<10%) [3]. Depending on severity, cutaneous radiation recall may be characterized by one or more dermatologic symptoms that can range from mild rash, dry desquamation and/or pruritus, to symptoms that are increasingly painful and may include swelling/edema, vesicles, maculopapular eruptions, and papules. In the most severe cases, ulceration and skin necrosis can occur [1, 3, 4]. The characteristic histopathological finding is a mixed nonspecific inflammatory infiltrate [1, 11].
U.S. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) can grade severity of radiation recall dermatitis. Radiation recall was previously listed separately in the CTCAE, but in the most recent version (v.3), it is merged into the category “Rash: dermatitis associated with radiation”. The five-stage classification is as follows:
Grade 1 (mild): Faint erythema or dry desquamation
Grade 2 (moderate): Moderate to brisk erythema; patchy moist desquamation, mostly confined to skin folds and creases; moderate edema
Grade 3 (severe): Moist desquamation other than skin folds and creases; bleeding induced by minor trauma or abrasion
Grade 4 (life-threatening/disabling): Skin necrosis or ulceration of full thickness dermis; spontaneous bleeding from involved site
Grade 5: Death
In about one third of cases, radiation recall reactions may arise in sites of prior radiation therapy other than the skin, including inflammatory reactions affecting the lungs, oral mucosa, gastrointestinal system, genitourinary tract, muscle layer, central nervous system, neck, and head, leading to possible functional impairment (Table 1). These effects may or may not occur in conjunction with cutaneous lesions. Interestingly, radiation recall reactions with gemcitabine appear to be predominantly noncutaneous, with about two thirds of cases involving internal tissues and organs [16] (Table 1).
Agents Associated with Radiation Recall
Although radiation recall is not common, many of the most widely used anticancer agents have been implicated in its development (Table 1).
Chemotherapeutic agents reported to precipitate a radiation recall reaction in patients who have previously received radiation therapy include agents from most classes, including alkylating agents, taxanes, anthracyclines, and other antitumor antibiotics, antimetabolites, vinca alkaloids, and taxanes (Table 1). The range of chemotherapeutic drugs associated with the phenomenon is diverse and no common features or characteristics of the drugs have been identified. It is not clear whether radiation recall reactions are a class effect or related to the drug dose or regimen. Reactions have occurred with a range of dosages of different drugs (Table 1).
The development of radiation recall is drug-specific for any individual patient, and it is not possible to predict which patients will react to which drugs. A drug that causes radiation recall in one patient may be able to be given safely to another patient that has previously had a radiation recall reaction to a different drug or vice versa. For instance, in a patient with a previous radiation recall reaction to doxorubicin, chemotherapy with vincristine, methotrexate, and cyclophosphamide was administered without reaction [17]. Radiation recall has been reported following treatment with pemetrexed, paclitaxel, or methotrexate in patients who have previously received other chemotherapy such as gemcitabine, cyclophosphamide, epirubicin, and/or doxorubicin without any reaction, although these agents have been associated with radiation recall in other patients [15, 18, 19].
To date, the drugs for which radiation recall reactions have been most commonly reported include the anthracycline doxorubicin, the taxanes docetaxel and paclitaxel, and the antimetabolites gemcitabine and capecitabine (Table 1). However, it must be kept in mind that because most data come from case reports, and not all identified cases of radiation recall will have been published, these trends may be influenced by reporting bias and will depend on how widely used the drug is. For instance, it is only over recent years that it has become evident that gemcitabine is one of the drugs most commonly reported to be associated with radiation recall reactions.
Radiation recall has also been reported with newer therapies used in the treatment of various cancers including pemetrexed [15, 20], gefitinib [21], trastuzumab in combination with vinorelbine [6], and bevacizumab in combination with gemcitabine [22]. It is notable that, in the case of bevacizumab, the reaction was considered possibly related to the new drug rather than gemcitabine; there was no recurrence when gemcitabine monotherapy was continued [22].
As an example of radiation recall with a new drug, we present in detail a case study of a radiation recall reaction induced by ixabepilone. This drug is the first of a new class of antineoplastic agent, the epothilones, and thus there has not previously been experience of radiation recall with this drug class. The epothilones stabilize microtubule dynamics leading to apoptotic cell death. The mechanism of action of the epothilones is similar to, but distinct from, that of the taxanes and the drug classes are not structurally related. Ixabepilone is approved for the treatment of metastatic breast cancer resistant to taxanes and anthracyclines. In this case, monotherapy with 40 mg/m2 ixabepilone given 1 week after radiotherapy was associated with a skin reaction and severe odynophagia in a 60-year-old woman with breast cancer (see section “Case Study”). The patient's radiation oncologist classified the recall reaction as much more severe (grade 3 as reported with second-degree burns and blisters) than the primary radiation effects. It should be noted that a patient completing a regimen of 30 Gy in 13 days could very well manifest pure radiation toxicity 7 days later. In this case, late radiation esophagitis/dermatitis may have been worsened because the patient was dosed with ixabepilone and the resulting hospitalization may have been a result of overlapping radiation and chemotherapy toxicities (palmar-plantar erythrodysesthesia).
Other drugs unrelated to cancer treatment have also been implicated in the development of radiation recall. For example, the antimicrobial/antibacterial agents gatifloxacin [23], cefotetan [24], cefazolin [25], trimetrexate [26, 27], and levofloxacin [28], the nonsteroidal anti-inflammatory agent, nimesulide [29], the lipid-lowering agent, simvastatin [30], antitubercular drugs [31], the anorexiant, phentermine [32], and the herbal medication, hypericin (St John's wort) [33]. Where stated, these events developed 8 months to 3 years after radiotherapy.
Case Study
A 60-year-old white female was diagnosed with stage IIA hormone receptor negative, HER-2 positive breast cancer in 2001. The patient underwent partial mastectomy and axilliary dissection followed by postoperative radiation therapy and received adjuvant chemotherapy with cyclophosphamide and doxorubicin, followed by single-agent paclitaxel. Treatment was completed in April 2002. In late 2006, the patient was found to have metastatic disease in the liver and lung. A liver biopsy confirmed adenocarcinoma and the patient was treated with vinflunine plus trastuzumab under an experimental protocol. However, scanning in March 2007 showed progressive liver disease. Vinflunine plus trastuzumab was discontinued and the patient started on capecitabine plus lapatinib. This regimen was tolerated well and the patient remained stable until September 2007 when a questionable new liver lesion was detected.
Computed tomography scan in January 2008 confirmed progressive disease and capecitabine plus lapatinib was withdrawn. A magnetic resonance imaging scan revealed multiple thoracic spine metastases. The patient received palliative radiotherapy totaling 30.25 Gy to T6 through T12 and 27.50 Gy to T1 through T3 over 13 and 14 days, concurrently.
One week later, she received chemotherapy with 40 mg/m2 ixabepilone. When ixabepilone was initiated, mild primary radiation effects were present (very slight nonpainful erythema and mild odynophagia). On days 3 to 4 after ixabepilone, the patient developed increasing erythema of her chest and increasing odynophagia. On day 7, the patient was admitted to hospital for severe abdominal pain, nausea, and vomiting, with associated symptoms of fatigue, musculoskeletal pain, evidence of palmar-plantar erythrodysesthesia, generalized oral tenderness but without overt mucositis/stomatitis, and chemotherapy-induced leucopenia and thrombocytopenia. Odynophagia had become severe.
Significant upon admission was the presence of second-degree burns to the patient's chest that framed the two recent radiation ports and was indicative of a radiation recall reaction. The patient also showed a first-degree burn on her back, which was more diffuse in pattern covering the thoracic spine. The abdominal pain in this patient was considered to be related to liver metastasis and/or cholelithiasis. Treatment consisted of supportive and preventive care consisting of IV fluids, antibiotics, pain control, promethazine, topical oral clotrimazole, and topical silver sulfadiazine cream and also nutritional and wound care. The patient recovered without infection and was discharged to home after 10 days. Abdominal pain was ongoing and controlled with narcotics.
Following the radiation recall reaction, the patient chose to continue treatment, receiving two more cycles of ixabepilone at a reduced dose of 20 mg/m2 every 3 weeks with no further radiation effect observed. There was a plan to increase her dose to 32 mg/m2, but blood counts did not safely allow for this. Unfortunately, after the additional two cycles of therapy, computed tomography scan demonstrated marked progression of metastatic disease to the liver, and her performance status was deteriorating. The patient entered hospice care and died after a short time.
Predicting and Preventing Radiation Recall
Radiation recall is unpredictable in nature. At present, it is not possible to identify which patients will develop radiation recall or design treatment regimens that will eliminate its occurrence.
Although often occurring shortly after the first dose of chemotherapy, radiation recall can also manifest after multiple courses of chemotherapy have been administered (Table 1). When reaction recall occurs after subsequent rather than the first dose, this may be indicative of a time lag for the onset of the reaction rather than presensitization [3]. Most reports of radiation recall are in response to administration of a single chemotherapeutic agent. However, there is no evidence to suggest that combination chemotherapy either increases or decreases the risk of radiation recall compared with monotherapy [3].
There does not appear to be a specific time window during which there is an increased susceptibility to radiation recall [6]. However, risks may be higher with a shorter time interval. In the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial, 9 of 50 (18%) patients with breast cancer receiving chemotherapy ≤3 weeks after radiotherapy developed radiation recall, compared with 6 of 81 (7.4%) patients receiving chemotherapy >3 weeks after radiotherapy (p = 0.09) [7]. Radiation recall occurred in 4 of 14 (28.6%) patients who received chemotherapy within 1 week of radiotherapy in this study; however, reactions in this time frame are difficult to differentiate from radiosensitization. It has also been reported that radiation reactions tend to be more severe when there is a shorter time between radiation and exposure to the precipitating agents, but this is not a definitive association [1, 3, 4, 5]. Ultimately, it seems likely that radiation recall occurs as a result of complex interplay between the radiation regimen, the chemotherapy dose and type, and timing.
It is not clear whether radiation recall reactions occur more commonly when high-dose radiotherapy with lower energy photon beams (≤6 MV) is applied [9]. In two cases where different radiation doses were applied to different areas of the body, radiation recall developed only in the areas that had received the higher radiation doses [34, 35]. However, such associations are not definitive, and no specific thresholds have yet been established. For instance, in one of the latter cases radiation recall occurred with doses ≥18.7 Gy, but in the other cases radiation recall did not occur with 20 Gy [34, 35]. Other reports indicate that radiation recall has occurred after radiation doses as low as 10 Gy (Table 1). The time elapsed since radiation exposure may be a related complicating factor; radiation recall reactions may be induced after a lower radiation dose if the period between chemotherapy and radiotherapy is relatively short [3].
Management of Radiation Recall
Radiation recall is usually diagnosed through evaluation of treatment history, symptoms, and physical examination. Where internal organs are affected, assessment may include radiologic studies. Biopsies are not normally necessary [2].
Treatment depends on the organ system affected and the severity of the reaction; however, no specific therapies are available. Most instances resolve with optimal symptom management. When the reaction is not severe, it may resolve spontaneously and an approach of close observation is adequate. Supportive medical care may be needed when internal organs are affected, and surgical intervention may be necessary for severe cases [1, 2].
The precipitating agent should be delayed or withdrawn to allow the skin to heal. It is very rare for radiation recall reactions to resolve whereas treatment with the implicated drug is continued [3]. Topical or systemic corticosteroids or nonsteroidal anti-inflammatory drugs are sometimes used to reduce inflammation. However, it is unclear whether administration of corticosteroids speeds resolution compared with the natural course of resolution after drug discontinuation [3]. Antihistamines can also be used for symptomatic relief.
The time over which radiation recall resolves appears to depend somewhat on the pharmacokinetics of the precipitating agent, and reactions may resolve more rapidly after discontinuation of intravenous treatment than oral treatment. Reactions often resolve within days or 1 to 2 weeks, although sometimes reactions to intravenous drugs may improve within hours, whereas resolution may take over a month for some oral drugs [3].
Rechallenge with a precipitating drug does not always elicit a reaction. Whether subsequent use of the precipitating agent is appropriate depends on individual circumstances, including patient preference and the extent, severity, and location of the reaction. The risk versus benefit balance and availability of alternative equally effective agents must be considered. When the radiation recall reaction is not severe, some patients may tolerate a reduced dose or even the same dose of the precipitating agent. Premedication with corticosteroids when rechallenging may help prevent the inflammatory response, although the value of this remains unproven [3, 36]. Although rechallenge has been possible without reaction in some cases, in other cases it has resulted in a similar or more severe reaction [1, 3, 37]. Camidge and Price reviewed 15 cases where rechallenge was attempted [3]. There was no recurrence of radiation recall in 5 patients who received a reduced dose of the precipitating drug and/or increased corticosteroid coverage. In 4 of the 10 patients with a recurring radiation recall, the reaction upon drug rechallenge was less severe than the initial reaction.
As with recently irritated skin, protection of the skin is important and patients should be advised to stay out of the sun, use sunscreens when exposure to the sun is unavoidable, avoid tanning beds, and wear loose, nonrestrictive clothing.
Conclusions
Radiation recall, although usually of mild intensity, can be severe and involve internal organs with possible functional consequences. As radiotherapy and chemotherapy are widely used in conjunction to treat cancer, familiarity with radiation recall reactions and their potential complications may aid early diagnosis and appropriate management.
There is still much that needs to be understood about radiation recall, and it is not currently possible to predict which patients will be affected and which drugs they will react to. Furthermore, there are no identifiable characteristics of drugs that cause radiation recall, and thus, it is a possibility that must be kept in mind with use of any drug after radiotherapy, including those from new drug classes. Although it is not yet possible to design treatment regimens to eliminate the risk of radiation recall, it seems likely that risks can be minimized by using the lowest possible dose of radiation and prolonging the interval between completion of radiotherapy and initiation of chemotherapy.
Footnotes
See accompanying editorial on page 1133
Author Contributions
Conception/design: Howard A. Burris III, Jane Hurtig
Provision of study materials or patients: Howard A. Burris III, Jane Hurtig
Collection and/or assembly of data: Howard A. Burris III, Jane Hurtig
Data analysis and interpretation: Howard A. Burris III, Jane Hurtig
Manuscript writing: Howard A. Burris III, Jane Hurtig
Final approval of manuscript: Howard A. Burris III, Jane Hurtig
The author takes full responsibility for the content of the paper but thanks Rebecca Goldstein, Ph.D., from StemScientific, for her assistance in organizing the published literature, preparing the initial draft of the manuscript following detailed discussions with the author, and collating author comments.
References
- 1.Azria D, Magne N, Zouhair A, et al. Radiation recall: a well recognized but neglected phenomenon. Cancer Treat Rev. 2005;31:555–570. doi: 10.1016/j.ctrv.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Caloglu M, Yurut-Caloglu V, Cosar-Alas R, et al. An ambiguous phenomenon of radiation and drugs: recall reactions. Onkologie. 2007;30:209–214. doi: 10.1159/000099632. [DOI] [PubMed] [Google Scholar]
- 3.Camidge R, Price A. Characterizing the phenomenon of radiation recall dermatitis. Radiother Oncol. 2001;59:237–245. doi: 10.1016/s0167-8140(01)00328-0. [DOI] [PubMed] [Google Scholar]
- 4.Yeo W, Johnson PJ. Radiation-recall skin disorders associated with the use of antineoplastic drugs. Pathogenesis, prevalence, and management. Am J Clin Dermatol. 2000;1:113–116. doi: 10.2165/00128071-200001020-00006. [DOI] [PubMed] [Google Scholar]
- 5.D'Angio GJ, Farber S, Maddock CL. Potentiation of x-ray effects by actinomycin D. Radiology. 1959;73:175–177. doi: 10.1148/73.2.175. [DOI] [PubMed] [Google Scholar]
- 6.Kodym E, Kalinska R, Ehringfeld C, et al. Frequency of radiation recall dermatitis in adult cancer patients. Onkologie. 2005;28:18–21. doi: 10.1159/000082175. [DOI] [PubMed] [Google Scholar]
- 7.Haffty BG, Vicini FA, Beitsch P, et al. Timing of Chemotherapy after MammoSite radiation therapy system breast brachytherapy: analysis of the American Society of Breast Surgeons MammoSite breast brachytherapy registry trial. Int J Radiat Oncol Biol Phys. 2008;72:1441–1448. doi: 10.1016/j.ijrobp.2008.02.070. [DOI] [PubMed] [Google Scholar]
- 8.Saif MW, Black G, Johnson M, et al. Radiation recall phenomenon secondary to capecitabine: possible role of thymidine phosphorylase. Cancer Chemother Pharmacol. 2006;58:771–775. doi: 10.1007/s00280-006-0223-8. [DOI] [PubMed] [Google Scholar]
- 9.Mizumoto M, Harada H, Asakura H, et al. Frequency and characteristics of docetaxel-induced radiation recall phenomenon. Int J Radiat Oncol Biol Phys. 2006;66:1187–1191. doi: 10.1016/j.ijrobp.2006.05.073. [DOI] [PubMed] [Google Scholar]
- 10.Kitani H, Kosaka T, Fujihara T, et al. The “recall effect” in radiotherapy: is subeffective, reparable damage involved? Int J Radiat Oncol Biol Phys. 1990;18:689–695. doi: 10.1016/0360-3016(90)90078-x. [DOI] [PubMed] [Google Scholar]
- 11.Smith KJ, Germain M, Skelton H. Histopathologic features seen with radiation recall or enhancement eruptions. J Cutan Med Surg. 2002;6:535–540. doi: 10.1007/s10227-001-0156-0. [DOI] [PubMed] [Google Scholar]
- 12.Ortmann E, Hohenberg G. Treatment side effects. Case 1. Radiation recall phenomenon after administration of capecitabine. J Clin Oncol. 2002;20:3029–3030. doi: 10.1200/JCO.2002.20.13.3029. [DOI] [PubMed] [Google Scholar]
- 13.Bronner AK, Hood AF. Cutaneous complications of chemotherapeutic agents. J Am Acad Dermatol. 1983;9:645–663. doi: 10.1016/s0190-9622(83)70177-5. [DOI] [PubMed] [Google Scholar]
- 14.Burdon J, Bell R, Sullivan J, et al. Adriamycin-induced recall phenomenon 15 years after radiotherapy. JAMA. 1978;239:931. [PubMed] [Google Scholar]
- 15.Barlési F, Tummino C, Tasei AM, et al. Unsuccessful rechallenge with pemetrexed after a previous radiation recall dermatitis. Lung Cancer. 2006;54:423–425. doi: 10.1016/j.lungcan.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Friedlander PA, Bansal R, Schwartz L, et al. Gemcitabine-related radiation recall preferentially involves internal tissue and organs. Cancer. 2004;100:1793–1799. doi: 10.1002/cncr.20229. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson SS, Glick JM, Wilbur JR. Letter: Adriamycin activating a recall phenomenon after radiation therapy. Ann Intern Med. 1974;81:407–408. doi: 10.7326/0003-4819-81-3-407. [DOI] [PubMed] [Google Scholar]
- 18.Bokemeyer C, Lampe C, Heneka M, et al. Paclitaxel-induced radiation recall dermatitis. Ann Oncol. 1996;7:755–756. doi: 10.1093/oxfordjournals.annonc.a010730. [DOI] [PubMed] [Google Scholar]
- 19.Rosen G, Tefft M, Martinez A, et al. Combination chemotherapy and radiation therapy in the treatment of metastatic osteogenic sarcoma. Cancer. 1975;35:622–630. doi: 10.1002/1097-0142(197503)35:3<622::aid-cncr2820350313>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Hureaux J, Le Guen Y, Tuchais C, et al. Radiation recall dermatitis with pemetrexed. Lung Cancer. 2005;50:255–258. doi: 10.1016/j.lungcan.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Miya T, Ono Y, Tanaka H, et al. [Radiation recall pneumonitis induced by Gefitinib (Iressa): a case report] Nihon Kokyuki Gakkai Zasshi. 2003;41:565–568. [PubMed] [Google Scholar]
- 22.Saif MW, Ramos J, Knisely J. Radiation recall phenomenon secondary to bevacizumab in a patient with pancreatic cancer. JOP. 2008;9:744–747. [PubMed] [Google Scholar]
- 23.Jain S, Agarwal J, Laskar S, et al. Radiation recall dermatitis with gatifloxacin: a review of literature. J Med Imaging Radiat Oncol. 2008;52:191–193. doi: 10.1111/j.1440-1673.2008.01942.x. [DOI] [PubMed] [Google Scholar]
- 24.Ayoola A, Lee YJ. Radiation recall dermatitis with cefotetan: a case study. The Oncologist. 2006;11:1118–1120. doi: 10.1634/theoncologist.11-10-1118. [DOI] [PubMed] [Google Scholar]
- 25.Garza LA, Yoo EK, Junkins-Hopkins JM, et al. Photo recall effect in association with cefazolin. Cutis. 2004;73:79–80. 85. [PubMed] [Google Scholar]
- 26.Jolivet J, Landry L, Pinard MF, et al. A phase I study of trimetrexate, an analog of methotrexate, administered monthly in the form of nine consecutive daily bolus injections. Cancer Chemother Pharmacol. 1987;20:169–172. doi: 10.1007/BF00253973. [DOI] [PubMed] [Google Scholar]
- 27.Weiss RB, James WD, Major WB, et al. Skin reactions induced by trimetrexate, an analog of methotrexate. Invest New Drugs. 1986;4:159–163. doi: 10.1007/BF00194596. [DOI] [PubMed] [Google Scholar]
- 28.Cho S, Breedlove JJ, Gunning ST. Radiation recall reaction induced by levofloxacin. J Drugs Dermatol. 2008;7:64–67. [PubMed] [Google Scholar]
- 29.Ng AW, Wong FC, Tung SY, et al. Nimesulide–a new trigger of radiation recall reaction. Clin Oncol (R Coll Radiol) 2007;19:364–365. doi: 10.1016/j.clon.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Abadir R, Liebmann J. Radiation reaction recall following simvastatin therapy: a new observation. Clin Oncol (R Coll Radiol) 1995;7:325–326. doi: 10.1016/s0936-6555(05)80545-x. [DOI] [PubMed] [Google Scholar]
- 31.Extermann M, Vogt N, Forni M, et al. Radiation recall in a patient with breast cancer treated for tuberculosis. Eur J Clin Pharmacol. 1995;48:77–78. doi: 10.1007/BF00202177. [DOI] [PubMed] [Google Scholar]
- 32.Ash RB, Videtic GM. Radiation recall dermatitis after the use of the anorexiant phentermine in a patient with breast cancer. Breast J. 2006;12:186–187. doi: 10.1111/j.1075-122X.2006.00235.x. [DOI] [PubMed] [Google Scholar]
- 33.Putnik K, Stadler P, Schafer C, et al. Enhanced radiation sensitivity and radiation recall dermatitis (RRD) after hypericin therapy–case report and review of literature. Radiat Oncol. 2006;1:32. doi: 10.1186/1748-717X-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeo W, Leung SF, Johnson PJ. Radiation-recall dermatitis with docetaxel: establishment of a requisite radiation threshold. Eur J Cancer. 1997;33:698–699. doi: 10.1016/s0959-8049(96)00461-3. [DOI] [PubMed] [Google Scholar]
- 35.Stelzer KJ, Griffin TW, Koh WJ. Radiation recall skin toxicity with bleomycin in a patient with Kaposi sarcoma related to acquired immune deficiency syndrome. Cancer. 1993;71:1322–1325. doi: 10.1002/1097-0142(19930215)71:4<1322::aid-cncr2820710425>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 36.Borgia F, Guarneri C, Guarneri F, et al. Radiation recall dermatitis after docetaxel administration: absolute indication to replace the drug? Br J Dermatol. 2005;153:674–675. doi: 10.1111/j.1365-2133.2005.06801.x. [DOI] [PubMed] [Google Scholar]
- 37.Hird AE, Wilson J, Symons S, et al. Radiation recall dermatitis: case report and review of the literature. Curr Oncol. 2008;15:53–62. doi: 10.3747/co.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.George J, Saif MW, Truss C. Radiation recall phenomenon, manifested as diffuse gastritis and upper gastrointestinal bleed after capecitabine administration. J Appl Res. 2004;4:495–498. [Google Scholar]
- 39.Borroni G, Vassallo C, Brazzelli V, et al. Radiation recall dermatitis, panniculitis, and myositis following cyclophosphamide therapy: histopathologic findings of a patient affected by multiple myeloma. Am J Dermatopathol. 2004;26:213–216. doi: 10.1097/00000372-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Wallenborn PA, III, Postma DS. Radiation recall supraglottitis. A hazard in head and neck chemotherapy. Arch Otolaryngol. 1984;110:614–617. doi: 10.1001/archotol.1984.00800350056015. [DOI] [PubMed] [Google Scholar]
- 41.Kennedy RD, McAleer JJ. Radiation recall dermatitis in a patient treated with dacarbazine. Clin Oncol (R Coll Radiol) 2001;13:470–472. doi: 10.1053/clon.2001.9316. [DOI] [PubMed] [Google Scholar]
- 42.D'Angio GJ. Clinical and biologic studies of actinomycin D and roentgen irradiation. Am J Roentgenol Radium Ther Nucl Med. 1962;87:106–109. [PubMed] [Google Scholar]
- 43.Stein RS. Radiation-recall enteritis after actinomycin-D and adriamycin therapy. South Med J. 1978;71:960–961. doi: 10.1097/00007611-197808000-00024. [DOI] [PubMed] [Google Scholar]
- 44.Tan CT, Dargeon HW, Burchenal JH. The effect of actinomycin D on cancer in childhood. Pediatrics. 1959;24:544–561. [PubMed] [Google Scholar]
- 45.Wiatrak BJ, Myer CM., III Radiation recall supraglottitis in a child. Am J Otolaryngol. 1991;12:227–229. doi: 10.1016/0196-0709(91)90122-v. [DOI] [PubMed] [Google Scholar]
- 46.Camidge DR, Kunkler IH. Docetaxel-induced radiation recall dermatitis and successful rechallenge without recurrence. Clin Oncol (R Coll Radiol) 2000;12:272–273. [PubMed] [Google Scholar]
- 47.Culp LR, Pou AM, Jones DV, et al. A case of radiation recall mucositis associated with docetaxel. Head Neck. 2004;26:197–200. doi: 10.1002/hed.10352. [DOI] [PubMed] [Google Scholar]
- 48.Kandemir EG, Karabudak O, Maydagli A. Docetaxel-induced radiation recall dermatitis. Swiss Med Wkly. 2005;135:34–35. doi: 10.4414/smw.2005.10896. [DOI] [PubMed] [Google Scholar]
- 49.Morkas M, Fleming D, Hahl M. Challenges in oncology. Case 2. Radiation recall associated with docetaxel. J Clin Oncol. 2002;20:867–869. doi: 10.1200/JCO.2002.20.3.867. [DOI] [PubMed] [Google Scholar]
- 50.Piroth MD, Krempien R, Wannenmacher M, et al. Radiation recall dermatitis from docetaxel. Onkologie. 2002;25:438–440. doi: 10.1159/000067438. [DOI] [PubMed] [Google Scholar]
- 51.Zulian GB, Aapro MS. Docetaxel and radiation-recall severe mucositis. Ann Oncol. 1994;5:964. doi: 10.1093/oxfordjournals.annonc.a058742. [DOI] [PubMed] [Google Scholar]
- 52.Cassady JR, Richter MP, Piro AJ, et al. Radiation-adriamycin interactions: preliminary clinical observations. Cancer. 1975;36:946–949. doi: 10.1002/1097-0142(197509)36:3<946::aid-cncr2820360316>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 53.Eifel PJ, McClure S. Severe chemotherapy-induced recall of radiation mucositis in a patient with non-Hodgkin's lymphoma of Waldeyer's ring. Int J Radiat Oncol Biol Phys. 1989;17:907–908. doi: 10.1016/0360-3016(89)90086-2. [DOI] [PubMed] [Google Scholar]
- 54.Etcubanas E, Wilbur JR. Letter: Uncommon side effects of adriamycin (NSC-123127) Cancer Chemother Rep. 1974;58:757–758. [PubMed] [Google Scholar]
- 55.Hill AB, Tattersall SF. Recall of radiation pneumonitis after intrapleural administration of doxorubicin. Med J Aust. 1983;1:39–40. doi: 10.5694/j.1326-5377.1983.tb136023.x. [DOI] [PubMed] [Google Scholar]
- 56.Ma LD, Taylor GA, Wharam MD, et al. “Recall” pneumonitis: adriamycin potentiation of radiation pneumonitis in two children. Radiology. 1993;187:465–467. doi: 10.1148/radiology.187.2.8475291. [DOI] [PubMed] [Google Scholar]
- 57.Mayer EG, Poulter CA, Aristizabal SA. Complications of irradiation related to apparent drug potentiation by adriamycin. Int J Radiat Oncol Biol Phys. 1976;1:1179–1188. doi: 10.1016/0360-3016(76)90091-2. [DOI] [PubMed] [Google Scholar]
- 58.McInerney DP, Bullimore J. Reactivation of radiation pneumonitis by adriamycin. Br J Radiol. 1977;50:224–227. doi: 10.1259/0007-1285-50-591-224. [DOI] [PubMed] [Google Scholar]
- 59.Jimeno A, Ciruelos EM, Castellano D, et al. Radiation recall dermatitis induced by pegylated liposomal doxorubicin. Anticancer Drugs. 2003;14:575–576. doi: 10.1097/00001813-200308000-00011. [DOI] [PubMed] [Google Scholar]
- 60.Perez EA, Campbell DL, Ryu JK. Radiation recall dermatitis induced by edatrexate in a patient with breast cancer. Cancer Invest. 1995;13:604–607. doi: 10.3109/07357909509024929. [DOI] [PubMed] [Google Scholar]
- 61.Fontana JA. Radiation recall associated with VP-16–213 therapy. Cancer Treat Rep. 1979;63:224–225. [PubMed] [Google Scholar]
- 62.von Essen CF, Kligerman MM, Calabresi P. Radiation and 5-fluorouracil: A controlled clinical study. Radiology. 1963;81:1018–1026. doi: 10.1148/81.6.1018. [DOI] [PubMed] [Google Scholar]
- 63.Sroa N, Bartholomew DA, Magro CM. Lipodermatosclerosis as a form of vascular compromise-associated radiation recall dermatitis: case report and a review of literature. J Cutan Pathol. 2006;33(suppl 2):55–59. doi: 10.1111/j.1600-0560.2006.00496.x. [DOI] [PubMed] [Google Scholar]
- 64.Bar-Sela G, Beny A, Bergman R, et al. Gemcitabine-induced radiation recall dermatitis: case report. Tumori. 2001;87:428–430. doi: 10.1177/030089160108700614. [DOI] [PubMed] [Google Scholar]
- 65.Castellano D, Hitt R, Cortes-Funes H, et al. Side effects of chemotherapy. Case 2. Radiation recall reaction induced by gemcitabine. J Clin Oncol. 2000;18:695–696. doi: 10.1200/JCO.2000.18.3.695. [DOI] [PubMed] [Google Scholar]
- 66.Fakih MG. Gemcitabine-induced rectus abdominus radiation recall. JOP. 2006;7:306–310. [PubMed] [Google Scholar]
- 67.Fogarty G, Ball D, Rischin D. Radiation recall reaction following gemcitabine. Lung Cancer. 2001;33:299–302. doi: 10.1016/s0169-5002(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 68.Ganem G, Solal-Celigny P, Joffroy A, et al. Radiation myositis: the possible role of gemcitabine. Ann Oncol. 2000;11:1615–1616. doi: 10.1023/a:1008353224251. [DOI] [PubMed] [Google Scholar]
- 69.Jeter MD, Janne PA, Brooks S, et al. Gemcitabine-induced radiation recall. Int J Radiat Oncol Biol Phys. 2002;53:394–400. doi: 10.1016/s0360-3016(02)02773-6. [DOI] [PubMed] [Google Scholar]
- 70.Marisavljević D, Ristic B, Hajder J. Gemcitabine-induced radiation recall dermatitis in a patient with resistant Hodgkin lymphoma. Am J Hematol. 2005;80:91. doi: 10.1002/ajh.20379. [DOI] [PubMed] [Google Scholar]
- 71.Miura G, Matsumoto T, Tanaka N, et al. [Two cases of radiation myositis probably induced by recall phenomenon] Nippon Igaku Hoshasen Gakkai Zasshi. 2003;63:420–422. [PubMed] [Google Scholar]
- 72.Saif MW, Sellers S, Russo S. Gemcitabine-related radiation recall in a patient with pancreatic cancer. Anticancer Drugs. 2006;17:107–111. doi: 10.1097/01.cad.0000181590.85476.e3. [DOI] [PubMed] [Google Scholar]
- 73.Schwartz BM, Khuntia D, Kennedy AW, et al. Gemcitabine-induced radiation recall dermatitis following whole pelvic radiation therapy. Gynecol Oncol. 2003;91:421–422. doi: 10.1016/s0090-8258(03)00404-9. [DOI] [PubMed] [Google Scholar]
- 74.Tan DHS, Bunce PE, Liles WC, et al. Gemcitabine-related “pseudocellulitis”: report of 2 cases and review of the literature. Clin Infect Dis. 2007;45:e72–e76. doi: 10.1086/520684. [DOI] [PubMed] [Google Scholar]
- 75.Welsh JS, Torre TG, DeWeese TL, et al. Radiation myositis. Ann Oncol. 1999;10:1105–1108. doi: 10.1023/a:1008365221440. [DOI] [PubMed] [Google Scholar]
- 76.Schwarte S, Wagner K, Karstens JH, et al. Radiation recall pneumonitis induced by gemcitabine. Strahlenther Onkol. 2007;183:215–217. doi: 10.1007/s00066-007-1688-z. [DOI] [PubMed] [Google Scholar]
- 77.Castellano D, Hitt R, Ciruelos E, et al. Biweekly vinorelbine and gemcitabine: a phase I dose-finding study in patients with advanced solid tumors. Ann Oncol. 2003;14:783–787. doi: 10.1093/annonc/mdg196. [DOI] [PubMed] [Google Scholar]
- 78.Burstein HJ. Side effects of chemotherapy. Case 1. Radiation recall dermatitis from gemcitabine. J Clin Oncol. 2000;18:693–694. doi: 10.1200/JCO.2000.18.3.693. [DOI] [PubMed] [Google Scholar]
- 79.Sears ME. Erythema in areas of previous irradiation in patients treated with hydroxyurea (NSC-32065) Cancer Chemother Rep. 1964;40:31–32. [PubMed] [Google Scholar]
- 80.Gabel C, Eifel PJ, Tornos C, et al. Radiation recall reaction to idarubicin resulting in vaginal necrosis. Gynecol Oncol. 1995;57:266–269. doi: 10.1006/gyno.1995.1139. [DOI] [PubMed] [Google Scholar]
- 81.Thomas R, Stea B. Radiation recall dermatitis from high-dose interferon alfa-2b. J Clin Oncol. 2002;20:355–357. doi: 10.1200/JCO.2002.20.1.355. [DOI] [PubMed] [Google Scholar]
- 82.Kellie SJ, Plowman PN, Malpas JS. Radiation recall and radiosensitization with alkylating agents. Lancet. 1987;1:1149–1150. doi: 10.1016/s0140-6736(87)91711-9. [DOI] [PubMed] [Google Scholar]
- 83.Kharfan Dabaja MA, Morgensztern D, Markoe AM, et al. Radiation recall dermatitis induced by methotrexate in a patient with Hodgkin's disease. Am J Clin Oncol. 2000;23:531–533. doi: 10.1097/00000421-200010000-00020. [DOI] [PubMed] [Google Scholar]
- 84.Chan RT, Au GK, Ho JW, et al. Radiation recall with oxaliplatin: report of a case and a review of the literature. Clin Oncol (R Coll Radiol) 2001;13:55–57. doi: 10.1053/clon.2001.9216. [DOI] [PubMed] [Google Scholar]
- 85.McCarty MJ, Peake MF, Lillis P, et al. Paclitaxel-induced radiation recall dermatitis. Med Pediatr Oncol. 1996;27:185–186. doi: 10.1002/(SICI)1096-911X(199609)27:3<185::AID-MPO9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 86.Phillips KA, Urch M, Bishop JF. Radiation-recall dermatitis in a patient treated with paclitaxel. J Clin Oncol. 1995;13:305. doi: 10.1200/JCO.1995.13.1.305. [DOI] [PubMed] [Google Scholar]
- 87.Schweitzer VG, Juillard GJ, Bajada CL, et al. Radiation recall dermatitis and pneumonitis in a patient treated with paclitaxel. Cancer. 1995;76:1069–1072. doi: 10.1002/1097-0142(19950915)76:6<1069::aid-cncr2820760623>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 88.Shenkier T, Gelmon K. Paclitaxel and radiation-recall dermatitis. J Clin Oncol. 1994;12:439. doi: 10.1200/JCO.1994.12.2.439. [DOI] [PubMed] [Google Scholar]
- 89.Kundak I, Oztop I, Soyturk M, et al. Paclitaxel-carboplatin induced radiation recall colitis. Tumori. 2004;90:256–258. doi: 10.1177/030089160409000219. [DOI] [PubMed] [Google Scholar]
- 90.Boström A, Sjolin-Forsberg G, Wilking N, et al. Radiation recall–another call with tamoxifen. Acta Oncol. 1999;38:955–959. doi: 10.1080/028418699432653. [DOI] [PubMed] [Google Scholar]
- 91.Kundranda MN, Daw HA. Tamoxifen-induced radiation recall dermatitis. Am J Clin Oncol. 2006;29:637–638. doi: 10.1097/01.coc.0000189693.23157.8d. [DOI] [PubMed] [Google Scholar]
- 92.Parry BR. Radiation recall induced by tamoxifen. Lancet. 1992;340:49. doi: 10.1016/0140-6736(92)92460-w. [DOI] [PubMed] [Google Scholar]
- 93.Singer EA, Warren RD, Pennanen MF, et al. Tamoxifen-induced radiation recall dermatitis. Breast J. 2004;10:170–171. doi: 10.1111/j.1075-122x.2004.21222.x. [DOI] [PubMed] [Google Scholar]
- 94.Lampkin BC. Skin reaction to vinblastine. Lancet. 1969;1:891. doi: 10.1016/s0140-6736(69)91941-2. [DOI] [PubMed] [Google Scholar]
- 95.Nemechek PM, Corder MC. Radiation recall associated with vinblastine in a patient treated for Kaposi sarcoma related to acquired immune deficiency syndrome. Cancer. 1992;70:1605–1606. doi: 10.1002/1097-0142(19920915)70:6<1605::aid-cncr2820700627>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]