Dexrazoxane's impact on the antitumor effect and toxicity profile of the anthracyclines and the role of dexrazoxane in the development of secondary malignant neoplasms in pediatric patients who received dexrazoxane are reviewed. Based on the available data, dexrazoxane appears to be a safe and effective cardioprotectant in children, and it does not appear to alter overall survival times in children with cancer.
Keywords: Doxorubicin, Daunorubicin, Cardiomyopathy, Childhood cancer
Learning Objectives
After completing this course, the reader will be able to:
Identify children who may be at relatively higher risk of developing cardiotoxicity as a result of treatment with anthracyclines.
Cite considerations for administering dexrazoxane prior to anthracycline treatment to mitigate anthracycline-related cardiotoxicity in children.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Abstract
Anthracyclines play a critical role in the treatment of a variety of childhood cancers. However, the cumulative cardiotoxic effects of anthracyclines limit the use of these agents in many treatment regimens. Dexrazoxane is a cardioprotectant that significantly reduces the incidence of adverse cardiac events in women with advanced breast cancer treated with doxorubicin-containing regimens. Clinical evidence for the efficacy of dexrazoxane as a cardioprotectant in children, especially from randomized clinical trials, is limited, but the available data support a short-term cardioprotective effect. Long-term follow-up in children treated with dexrazoxane has not been reported. Dexrazoxane's impact on the antitumor effect and toxicity profile of the anthracyclines and the role of dexrazoxane in the development of secondary malignant neoplasms in patients who received dexrazoxane are reviewed. Based on the available data, dexrazoxane appears to be a safe and effective cardioprotectant in children, and it does not appear to alter overall survival times in children with cancer. Continued follow-up from previous trials is needed to determine the long-term effect of dexrazoxane on cardiac outcomes and quality of life.
Introduction
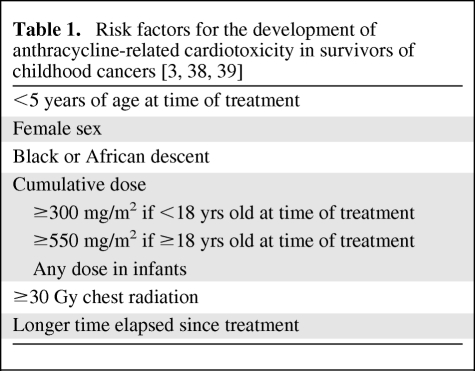
The anthracyclines doxorubicin, daunorubicin, and idarubicin and the related anthracenedione mitoxantrone are important components of frontline treatment in current Children's Oncology Group clinical trials for acute lymphoblastic leukemia (ALL), acute myeloblastic leukemia, Hodgkin's disease (HD), non-Hodgkin lymphoma, osteosarcoma, Ewing's sarcoma, neuroblastoma, and Wilms' tumor. As a result, half of childhood cancer survivors have received anthracyclines, which are known to cause cumulative cardiotoxicity. Approximately 5% of children who were previously treated with anthracyclines will develop congestive heart failure (CHF) and 40% will develop arrhythmias [1]. These cardiotoxic effects may be manifest soon after treatment or may not become apparent until decades after completion of therapy [2]. Risk factors for the development of anthracycline-induced cardiotoxicity in survivors of childhood cancer are listed in Table 1. Cardiopulmonary diseases, which include anthracycline-related cardiac toxicity, are the third leading cause of early mortality in survivors of childhood cancer behind cancer recurrence and second malignancies [3].
Table 1.
Limiting the cumulative lifetime dose of anthracyclines is the primary approach used to reduce the risk for cardiotoxicity, but alternate dosing schedules that lower the peak plasma concentration of doxorubicin and other anthracyclines have also been studied. Administration of smaller, divided, bolus doses of doxorubicin on consecutive days in children receiving a mean cumulative anthracycline dose of 341 mg/m2 for the treatment of osteosarcoma and rhabdomyosarcoma did not result in a significantly lower occurrence of cardiotoxicity than with the administration of a single bolus dose when patients were followed for a period of 4–180 months [4]. In adults, continuous i.v. infusion of doxorubicin over 24–48 hours is cardioprotective [5, 6], but this has not been confirmed in pediatric studies. Continuous infusion of doxorubicin over 48 hours in children receiving a mean cumulative doxorubicin dose of 360 mg/m2 for high-risk ALL was not cardioprotective at a median of 1.5 years after completion of therapy [7], and infusion of anthracyclines over >24 hours in children with a variety of tumor types treated with a median cumulative anthracycline dose of 365 mg/m2 did not lead to a lower incidence of anthracycline-related cardiac toxicity at a mean follow-up time of 7 years after the end of therapy, when compared with bolus dosing [8]. Lastly, in children receiving a cumulative daunorubicin dose of 180 mg/m2 as a 6-hour infusion for ALL, cardiac function was similar to that of historical controls who received the same cumulative dose of daunorubicin as a bolus. Despite the fact that this is a low cumulative anthracycline dose, 180 mg/m2 of daunorubicin was associated with significant changes in left ventricular dimensions and functional indices in both groups. The duration of follow-up of these patients was almost 5.5 years [9]. Alternative approaches to prevent anthracycline-related cardiac damage are needed for children.
The mechanism by which anthracyclines exert their cardiotoxic effects differs from the mechanism thought to be primarily responsible for the anticancer effect of these drugs. Most evidence suggests that myocardial damage is mediated through the formation of iron-dependent oxygen free radicals and subsequent peroxidation of lipids in the membranes of myocardial mitochondria [10]. Other suggested mechanisms of anthracycline-induced cardiac damage include reduced myocardial ATP production, lower expression of mRNA coding for Ca2+-ATPase in the sarcoplasmic reticulum leading to decreased contractility, lower cardiac glutathione peroxidase activity, which detoxifies oxygen free radicals, and mitochondrial DNA damage leading to respiratory chain defects and increased production of free radicals [11]. Myocytes exposed to anthracyclines also undergo apoptosis [12]. The cytotoxic anticancer effect appears to come primarily from inhibition of topoisomerase II. This difference may allow for selective rescue from anthracycline cardiotoxicity.
Dexrazoxane (ICRF-187), which was originally developed as an anticancer drug, is a U.S. Food and Drug Administration–approved cardioprotective agent that chelates intracellular free iron and iron bound to anthracycline and reduces the formation of oxygen free radicals [13]. Dexrazoxane also appears to protect myocytes from anthracycline-induced apoptosis [12]. Dexrazoxane is administered as a short i.v. infusion immediately prior to a bolus dose of doxorubicin at a recommended dose of 10 mg per 1 mg of doxorubicin. Despite its efficacy in preclinical models and demonstrated cardioprotective effects in adults, the use of dexrazoxane as a cardioprotectant has been limited, primarily by concerns about its acute and long-term toxic effects and the potential for rescuing tumor cells from the cytotoxic effects of anthracyclines.
The efficacy of dexrazoxane as a cardioprotectant was demonstrated using laboratory and clinical measures of cardiac function, including endomyocardial biopsies, multigated acquisition (MUGA) scans, and electrocardiograms, in women with breast cancer treated with high doses of doxorubicin and in adults with small cell lung carcinoma and soft tissue sarcomas treated with doxorubicin or epirubicin [10, 14–17]. Dexrazoxane preserved cardiac function and allowed for escalation of anthracyclines doses to >1,000 mg/m2. Evaluation of the long-term cardioprotective effects of dexrazoxane in these trials was limited by tumor progression (the median overall survival [OS] time was 15–18 months across all disease types).
A meta-analysis of nine clinical studies evaluating dexrazoxane as a cardioprotective agent in adults and children concluded that the relative risk for developing heart failure when dexrazoxane was administered with doxorubicin was 0.29 (95% confidence interval [CI], 0.2–0.41). Additionally, there was no difference in the response rate to therapy of the cancers or the OS time in patients receiving dexrazoxane compared with controls [18].
Cardioprotective Effects of Dexrazoxane in Children
Cardioprotective effects from dexrazoxane have been observed in small studies of children with cancer receiving anthracyclines [19, 20]. In one of those studies, 15 patients with newly diagnosed solid tumors received cumulative anthracycline doses in the range of 270–340 mg/m2 and received dexrazoxane prior to administration of either doxorubicin, daunorubicin, or epirubicin. Those patients were compared with a historical control group that did not receive dexrazoxane. Echocardiograms were performed prior to and following administration of anthracyclines in both groups. In the group treated with dexrazoxane, there was no change in cardiac function after receiving anthracyclines. However, in the control group, two of the 15 patients (13.3%) had a decrease in the shortening fraction to <28% and one of those patients (6.6%) developed dilated cardiomyopathy [20].
In a study by Bu'Lock et al. [19], five children who had relapsed cancers received dexrazoxane prior to an anthracycline, and their cardiac function was compared with that of five retrospectively selected controls who had not received dexrazoxane. The patients receiving dexrazoxane and the controls were treated with mean cumulative anthracycline doses of 925 mg/m2 and 831 mg/m2, respectively. Cardiac function was evaluated by a physical examination performed by a cardiologist and an echocardiogram performed prior to the initiation of anthracycline therapy for relapse and 1 month after completion of therapy. None of the five patients who received dexrazoxane developed cardiac dysfunction. However, two children in the control group developed CHF and another patient experienced a decrease in the shortening fraction [19].
Overall, the patients who received dexrazoxane in those two studies had better cardiac outcomes than patients in the control groups. Additionally, the patients who received dexrazoxane did not experience more severe treatment-related toxicities. However, those studies were limited by their small size and heterogeneity in the cancer diagnoses and chemotherapeutic regimens used.
Wexler et al. [21] conducted an open-label, randomized trial of dexrazoxane in 38 children and young adults receiving frontline therapy with a doxorubicin-containing regimen for high-risk sarcomas. Patients were randomized to receive doxorubicin administered as an i.v. bolus with or without dexrazoxane. The dexrazoxane dose was 20 mg per 1 mg of doxorubicin. The resting left ventricular ejection fraction (LVEF) was serially measured with a MUGA scan at baseline and 6–12 weeks following doses of doxorubicin. Results revealed that dexrazoxane-treated patients were less likely to develop subclinical cardiotoxicity (22% versus 67%; p < .01), had a smaller decrease in LVEF for every 100 mg/m2 of doxorubicin (1.0% versus 2.7%; p = .02), and tolerated a higher cumulative dose of doxorubicin (410 mg/m2 versus 310 mg/m2, p<0.05). In the patients who received a cumulative doxorubicin dose of 410 mg/m2, the mean LVEF was higher in those who received dexrazoxane than in controls (54% versus 44%; p = .03). The authors concluded that dexrazoxane reduces the risk for short-term cardiotoxicity in young sarcoma patients who received up to 410 mg/m2 of doxorubicin. The small sample size and high tumor recurrence rate in this high-risk population did not allow for a comparison of long-term functional cardiac outcome in the two groups.
A randomized trial in children with ALL evaluated the effect of dexrazoxane on doxorubicin-induced myocardial injury, which was assessed using serial serum cardiac troponin T levels [22]. High post-treatment cardiac troponin T levels are predictive of subsequent subclinical and clinical cardiac morbidity and mortality [23]. Two hundred five patients were randomized to receive doxorubicin alone (30 mg/m2 per dose; cumulative dose, 300 mg/m2) or dexrazoxane immediately followed by doxorubicin. Patients treated with doxorubicin alone were more likely than those treated with dexrazoxane to have elevated troponin T levels (50% versus 21%; p < .001), extremely elevated troponin T levels (32% versus 10%; p < .001), or multiple elevated troponin T levels (37% versus 12%; p < .001). However, fractional shortening measured on echocardiogram in a subgroup of children was similar in the randomized groups during and after doxorubicin therapy. Patients had been followed for a median of 2.7 years at the time that the data were published. The investigators concluded that dexrazoxane prevents or reduces doxorubicin-induced cardiac injury in children receiving doxorubicin for high-risk ALL.
Children and adolescents with HD treated in one of two frontline clinical trials using doxorubicin-containing regimens were randomly assigned to receive dexrazoxane as a cardiopulmonary protectant prior to doxorubicin and bleomycin [24]. Bleomycin-induced pulmonary fibrosis also appears to be related to iron-dependent production of reactive oxygen species [25], and dexrazoxane was administered prior to bleomycin to prevent bleomycin-induced pulmonary fibrosis. The effect of dexrazoxane on cardiac outcome from these two trials will be combined and reported later.
A series of three pilot studies evaluating new treatment regimens for nonmetastatic osteosarcoma included dexrazoxane as a cardioprotectant. In two regimens, doxorubicin cumulative doses were escalated to 600 mg/m2. There was no significant increase in left ventricular dysfunction with this dose escalation [26], illustrating that dexrazoxane may allow a higher cumulative dose of doxorubicin to be safely administered without acute cardiotoxicity. However, the impact of dexrazoxane on late cardiotoxicity at high cumulative doses of doxorubicin remains unknown.
Impact of Dexrazoxane on the Antitumor Effect of Doxorubicin
Although dexrazoxane was initially developed as an anticancer drug based on its ability to chelate and deplete iron in tumor cells, it has subsequently been shown to be a topoisomerase II inhibitor [27], which is a mechanism common to a number of cytotoxic anticancer drugs, including the anthracyclines. Despite this, the potential for a negative impact of dexrazoxane on the antitumor effect of the concomitantly administered chemotherapy and on survival outcomes remains a major concern. Although most of the trials conducted with dexrazoxane are too small to ensure true equivalency and long-term follow-up is limited, dexrazoxane does not appear to have a negative impact on disease outcome in childhood cancers.
The randomized trial in children with ALL described above included 206 patients, and the 5-year event-free survival (EFS) rates with a median follow-up duration of 5.7 years were 76% in the doxorubicin alone group and 77% in the group randomized to receive dexrazoxane and doxorubicin (p = .99) [28]. In the high-risk sarcoma study reported by Wexler et al. [21], the median potential follow-up time was 39 months for all patients. The median EFS duration in both the doxorubicin and doxorubicin plus dexrazoxane groups was 17 months. The median OS time in the doxorubicin alone group was 24 months, versus 43 months for patients who received doxorubicin and dexrazoxane. Overall, 44% of patients in the control group and 61% of patients in the dexrazoxane group were alive at the last follow-up point [21]. These differences in EFS and OS were not statistically significantly different. The findings of these studies do not indicate that dexrazoxane compromises the antitumor effect of doxorubicin.
The impact of dexrazoxane on disease outcome was assessed in a pediatric advanced stage HD trial. The proportion of patients with a rapid early response to chemotherapy in the dexrazoxane arm was 56%, versus 69% for the no dexrazoxane arm (p = .07). There was no difference in the proportions of patients who achieved a complete response after chemotherapy (60% versus 70%) or after radiation therapy (86% versus 94%) in the dexrazoxane versus nondexrazoxane arms. Furthermore, the use of dexrazoxane did not affect the 5-year EFS or OS rate (83% versus 86% and 89% versus 84%, respectively) [24].
The histologic response to doxorubicin-containing neoadjuvant chemotherapy of osteosarcoma patients receiving dexrazoxane was compared with that of a historical control group that had not received dexrazoxane [26]. A good histological response at the time of resection after 12 weeks of chemotherapy was defined as >98% tumor necrosis. A good response was seen in 61% of patients and the 2-year EFS rate was 69%, both of which were consistent with historical data.
Dexrazoxane Toxicity
In a pediatric phase I trial of dexrazoxane administered as a single agent (a 2-hour infusion daily for 3 days) to children with refractory solid tumors, the dose-limiting toxicity was hepatic toxicity, and the maximum-tolerated dose was 3,500 mg/m2 per day. Myelosuppression was the dose-limiting toxicity in the dose-finding study in adults, and the maximum-tolerated dose was 1,000–1,250 mg/m2 per day for 3 days when administered as a short infusion [29]. Myelosuppression was more prominent in the pediatric phase II trials in patients with solid tumors and acute leukemia. The single-agent maximum-tolerated dose of dexrazoxane in children is significantly higher than the dosage range used in the protocols studying its use as a cardioprotective agent [30]. However, dexrazoxane, even at lower doses, could enhance the toxicity of the chemotherapy with which it is combined.
Schwartz et al. [24] evaluated noncardiac toxicity in 214 pediatric patients with advanced stage HD and found higher grade hematologic toxicity with associated infection and sepsis in the patients randomized to the dexrazoxane arm. Acute pulmonary toxicity (grade 3 or 4) also occurred more often in the dexrazoxane group (p = .005). Additionally, there was a trend toward a higher incidence of grade 3 or 4 typhilitis in patients receiving dexrazoxane. However, these results were not statistically significant (p = .06), and once the bleomycin and prednisone doses were modified in the treatment protocol, no difference in the incidence of typhilitis was seen between the two groups [24]. No mention was made regarding the effect of dexrazoxane on acute pulmonary toxicity after the treatment protocol was modified.
In the study by Wexler et al. [21], 41 patients were assessable for noncardiac toxicity evaluation following at least one cycle of doxorubicin-containing chemotherapy. Grade 1 aspartate aminotransferase elevation occurred more frequently in the dexrazoxane group than in the control group (p < .001) following the first three cycles of vincristine, doxorubicin, and cyclophosphamide (VAdriaC). Similarly, there was a higher frequency of alanine aminotransferase elevation in the dexrazoxane group after cycle 2 of VAdriaC. There was a statistically significantly higher incidence of grade 3 thrombocytopenia, lower platelet nadirs, higher incidence of grade ≥3 anemia, and longer median time to recovery of absolute neutrophil count to >1,000 u/l in the dexrazoxane group in various treatment cycles. However, these differences were not clinically significant, because there was no significant difference between the groups in the incidence of dose modifications for hepatotoxicity or hematologic toxicity or the incidence of significant infections [21].
Risk for Secondary Malignancy
Topoisomerase inhibitors, such as etoposide, are associated with a higher risk for a secondary malignant neoplasm (SMN), raising a concern that dexrazoxane use may enhance the risk for SMNs. Investigators involved in the HD trials that incorporated dexrazoxane conducted a secondary analysis evaluating the incidence of and risk factors for acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS) and SMNs [31]. Two hundred sixty-two eligible pediatric patients were enrolled in the low-risk HD study and 216 eligible pediatric patients were enrolled in the advanced stage HD study. Low-risk patients were treated with doxorubicin, bleomycin, vincristine, and etoposide (ABVE) and advanced stage patients received dose-intensified ABVE with prednisone and cyclophosphamide (ABVE-PC). Two hundred thirty-nine patients were randomly assigned to receive dexrazoxane prior to doxorubicin. The 4-year cumulative incidence rate of AML/MDS was 2.55% with dexrazoxane, versus 0.85% without dexrazoxane (p = .16). The risk for developing AML/MDS as measured by the standardized incidence ratio (SIR) was higher in the group receiving dexrazoxane (SIR, 613.6; 95% CI, 225.2–1335.6) than in the control group (SIR, 202.37; 95% CI, 24.5–731; p = .099). The 4-year cumulative incidences of any SMN were 3.43% in the dexrazoxane group and 0.85% in the control group (p = .06). These differences were not statistically significant at the p = .05 level. When AML/MDS and SMN were analyzed as first events, the 4-year cumulative incidence of AML/MDS was 2.1% in patients treated with dexrazoxane and 0.42% in patients treated without dexrazoxane (p = .1052), and the cumulative incidences of SMNs were 2.98% and 0.42%, respectively (p = .0355). Only when SMN was analyzed as a first event did the investigators find a statistically significant difference between the treatment and control groups. The investigators conceded that these analyses were not designed for comparison of AML/MDS and SMN between the two groups and that the tests were underpowered.
A higher risk for AML/MDS and SMN was not confirmed in the ALL population receiving dexrazoxane [32]. Only two of the 491 patients developed an SMN, both of which were malignant melanomas. The 5-year cumulative incidences of SMN were 0.2% for all patients and 0.5% for high-risk patients. No SMNs were reported in standard-risk ALL patients and no SMNs were reported in patients assigned to receive dexrazoxane. No cases of secondary AML/MDS were reported. Therefore, the authors concluded that there was no association between the use of dexrazoxane and development of SMNs in this patient population. As in the HD studies, the sample size was small and the power to detect differences between the groups was limited.
Dexrazoxane was evaluated as a cardioprotectant in a randomized trial in children with T-cell ALL and advanced T-cell lymphoblastic lymphoma treated with a cumulative doxorubicin dose of 360 mg/m2. Cardiac outcome was not reported, but with 10 years of follow-up, there was no difference in the EFS rate between the dexrazoxane and no dexrazoxane arms (71% versus 73%; p = .85). The 10-year cumulative incidences of SMNs were 1.3% in the arm without dexrazoxane and 4.2% in the dexrazoxane arm (p = .15). This incidence of SMNs was similar to the 4% rate observed in the preceding T-cell ALL study, in which no dexrazoxane was given [33].
The conflicting findings from these studies probably reflect differences in the patient populations, chemotherapy regimens, and radiation exposures.
Role of Dexrazoxane as a Cardioprotectant in Children Receiving Anthracyclines
Anthracycline-induced cardiotoxicity adversely impacts the health and quality of life of childhood cancer survivors and limits the cumulative dose of anthracyclines that can be safely administered. The potentially life-threatening cardiac toxicity associated with anthracyclines can be a major barrier to using these agents in the most clinically effective manner.
Based on clinical data from adults and children, dexrazoxane appears to be an effective cardioprotectant for patients receiving anthracyclines. However, definitive clinical trials to assess the long-term effects of dexrazoxane on functional cardiac outcome in the pediatric population have not been conducted. Longer follow-up time is needed in children to determine whether dexrazoxane protects against the long-term cardiac toxicity that can manifest >10 years after the completion of therapy and whether this translates into longer survival and better quality of life for childhood cancer survivors.
Ideally, cardioprotectants and other rescue agents should not compromise the doses of the anticancer drugs administered because of greater toxicity, or mitigate their clinical efficacy. At the current recommended dose of 10 mg per 1 mg of doxorubicin, dexrazoxane does not increase the regimen toxicity significantly. Although there was more myelosuppression and transaminitis when dexrazoxane was added to doxorubicin-containing regimens, these differences did not compromise the dose or timing of the chemotherapy. Although the published clinical experience with dexrazoxane in pediatric cancer patients is limited, there is no evidence to indicate that the drug negatively impacts the antitumor effects of the concurrent chemotherapy. Response, EFS, and OS rates in the dexrazoxane and control groups were similar in the various studies. The clinical data also fail to show a survival advantage in patients receiving dexrazoxane.
Recommendations
Based on the substantial risk for the development of anthracycline-induced clinical and subclinical cardiac toxicity in children and on the preliminary evidence of the efficacy of dexrazoxane in preventing early-onset cardiotoxicity in adults and children, dexrazoxane use prior to the administration of anthracyclines should be considered in children who will receive one of these agents, especially those who are at highest risk for cardiotoxicity (Table 1). Subclinical cardiotoxicity may develop in children at anthracycline doses ≤300 mg/m2 [34–36]. Therefore, dexrazoxane use should begin prior to the first dose of anthracycline and should not be delayed until after the patient has received 300 mg/m2, as has been recommended in adult breast cancer patients [16].
The ages of the patients in the pediatric trials that have evaluated dexrazoxane as a cardioprotectant have been skewed toward older children and adolescents because high-risk ALL, HD, and sarcomas are cancers that tend to occur in older children. However, younger age at the time of treatment is a risk factor for anthracycline-induced cardiac toxicity. Therefore, dexrazoxane's cardioprotectant effects should be further evaluated in younger children. Additionally, there are limited data describing dexrazoxane pharmacokinetics in younger children. The dexrazoxane doses used in pediatric trials have either been 10× or 20× the doxorubicin dose, with the current recommended dose of dexrazoxane in the U.S. set at 10× that of the doxorubicin dose [37]. An important area for future research is further delineating the pharmacokinetics of anthracyclines and dexrazoxane in pediatric patients in order to better understand the optimal dose and administration schedule of dexrazoxane in children. Also, defining a more precise timeline of anthracycline-induced cardiac toxicity may prove to be crucial in more effectively implementing dexrazoxane use as an effective cardioprotectant in children.
Author Contributions
Conception/Design: Dana M. Sepe, Frank M. Balis
Collection and/or assembly of data: Dana M. Sepe
Data analysis and interpretation: Dana M. Sepe
Manuscript writing: Dana M. Sepe, Frank M. Balis, Jill P. Ginsberg
Final approval of manuscript: Dana M. Sepe, Frank M. Balis, Jill P. Ginsberg
References
- 1.Ginsberg JP, Cnaan A, Zhao H, et al. Using health-related quality of life measures to predict cardiac function in survivors exposed to anthracyclines. J Clin Oncol. 2004;22:3149–3155. doi: 10.1200/JCO.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 3.Shankar SM, Marina N, Hudson MM, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: Report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics. 2008;121:e387–e396. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- 4.Ewer MS, Jaffe N, Ried H, et al. Doxorubicin cardiotoxicity in children: Comparison of a consecutive divided daily dose administration schedule with single dose (rapid) infusion administration. Med Pediatr Oncol. 1998;31:512–515. doi: 10.1002/(sici)1096-911x(199812)31:6<512::aid-mpo8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Legha SS, Benjamin RS, Mackay B, et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med. 1982;96:133–139. doi: 10.7326/0003-4819-96-2-133. [DOI] [PubMed] [Google Scholar]
- 6.Hortobagyi GN, Frye D, Buzdar AU, et al. Decreased cardiac toxicity of doxorubicin administered by continuous intravenous infusion in combination chemotherapy for metastatic breast carcinoma. Cancer. 1989;63:37–45. doi: 10.1002/1097-0142(19890101)63:1<37::aid-cncr2820630106>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Lipshultz SE, Giantris AL, Lipsitz SR, et al. Doxorubicin administration by continuous infusion is not cardioprotective: The Dana-Farber 91–09 Acute Lymphoblastic Leukemia protocol. J Clin Oncol. 2002;20:1677–1682. doi: 10.1200/JCO.2002.20.6.1677. [DOI] [PubMed] [Google Scholar]
- 8.Gupta M, Steinherz PG, Cheung NK, et al. Late cardiotoxicity after bolus versus infusion anthracycline therapy for childhood cancers. Med Pediatr Oncol. 2003;40:343–347. doi: 10.1002/mpo.10298. [DOI] [PubMed] [Google Scholar]
- 9.Levitt GA, Dorup I, Sorensen K, et al. Does anthracycline administration by infusion in children affect late cardiotoxicity? Br J Haematol. 2004;124:463–468. doi: 10.1111/j.1365-2141.2004.04803.x. [DOI] [PubMed] [Google Scholar]
- 10.Cvetkovic RS, Scott LJ. Dexrazoxane: A review of its use for cardioprotection during anthracycline chemotherapy. Drugs. 2005;68:1005–1024. doi: 10.2165/00003495-200565070-00008. [DOI] [PubMed] [Google Scholar]
- 11.Wouters KA, Kremer LCM, Miller TL, et al. Protecting against anthracycline-induced myocardial damage: A review of the most promising strategies. Br J Haematol. 2005;131:561–578. doi: 10.1111/j.1365-2141.2005.05759.x. [DOI] [PubMed] [Google Scholar]
- 12.Popelová O, Sterba M, Hasková P, et al. Dexrazoxane-afforded protection against chronic anthracycline cardiotoxicity in vivo: Effective rescue of cardiomyocytes from apoptotic cell death. Br J Cancer. 2009;101:792–802. doi: 10.1038/sj.bjc.6605192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasinoff BB. The interaction of the cardioprotective agent ICRF-187 ((+)-1,2-bis(3,5-dioxopiperazinyl-1-yL)propane); its hydrolysis product (ICRF-198); and other chelating agents with the Fe(III) and Cu(II) complexes of adriamycin. Agents Actions. 1989;26:378–385. doi: 10.1007/BF01967305. [DOI] [PubMed] [Google Scholar]
- 14.Speyer JL, Green MD, Kramer E, et al. Protective effect of the bispiperazinedione ICRF-187 against doxorubicin-induced cardiac toxicity in women with advanced breast cancer. N Engl J Med. 1988;319:745–752. doi: 10.1056/NEJM198809223191203. [DOI] [PubMed] [Google Scholar]
- 15.Swain SM, Whaley FS, Gerber MC, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol. 1997;15:1318–1332. doi: 10.1200/JCO.1997.15.4.1318. [DOI] [PubMed] [Google Scholar]
- 16.Swain SM, Whaley FS, Gerber MC, et al. Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin-containing therapy. J Clin Oncol. 1997;15:1333–1340. doi: 10.1200/JCO.1997.15.4.1333. [DOI] [PubMed] [Google Scholar]
- 17.Lopez M, Vici P, Di Lauro K, et al. Randomized prospective clinical trial of high-dose epirubicin and dexrazoxane in patients with advanced breast cancer and soft tissue sarcomas. J Clin Oncol. 1998;16:86–92. doi: 10.1200/JCO.1998.16.1.86. [DOI] [PubMed] [Google Scholar]
- 18.van Dalen EC, Caron HN, Dickinson HO, et al. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2008;(2):CD003917. doi: 10.1002/14651858.CD003917.pub3. [DOI] [PubMed] [Google Scholar]
- 19.Bu'Lock FA, Gabriel HM, Oakhill A, et al. Cardioprotection by ICRF187 against high dose anthracycline toxicity in children with malignant disease. Br Heart J. 1993;70:185–188. doi: 10.1136/hrt.70.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiavetti A, Castello MA, Versacci P, et al. Use of ICRF-187 for prevention of anthracycline cardiotoxicity in children: Preliminary results. Pediatr Hematol Oncol. 1997;14:213–222. doi: 10.3109/08880019709009491. [DOI] [PubMed] [Google Scholar]
- 21.Wexler LH, Andrich MP, Venzon D, et al. Randomized trial of the cardioprotective agent ICRF-187 in pediatric sarcoma patients treated with doxorubicin. J Clin Oncol. 1996;14:362–372. doi: 10.1200/JCO.1996.14.2.362. [DOI] [PubMed] [Google Scholar]
- 22.Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145–153. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- 23.Lipshultz SE, Rifai N, Sallan SE, et al. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation. 1997;96:2641–2648. doi: 10.1161/01.cir.96.8.2641. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: The results of P9425. Blood. 2009;114:2051–2059. doi: 10.1182/blood-2008-10-184143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oury TD, Thakker K, Menache M, et al. Attenuation of bleomycin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am J Respir Cell Mol Biol. 2001;25:164–169. doi: 10.1165/ajrcmb.25.2.4235. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz CL, Wexler LH, Devidas M, et al. P9754 therapeutic intensification in non-metastatic osteosarcoma: A COG trial. J Clin Oncol. 2004;22(suppl 14):8514. [Google Scholar]
- 27.Classen S, Olland S, Berger JM. Structure of the topoisomerase II ATPase region and its mechanism of inhibition by the chemotherapeutic agent ICRF-187. Proc Natl Acad Sci U S A. 2003;100:10629–10634. doi: 10.1073/pnas.1832879100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95–01 for children with acute lymphoblastic leukemia. Blood. 2007;109:896–904. doi: 10.1182/blood-2006-06-027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holcenberg JS, Tutsch KD, Earhart RH, et al. Phase I study of ICRF-187 in pediatric cancer patients and comparison of its pharmacokinetics in children and adults. Cancer Treat Rep. 1986;70:703–709. [PubMed] [Google Scholar]
- 30.Vats T, Kamen B, Krischer JP. Phase II trial of ICRF-187 in children with solid tumors and acute leukemia. Investig New Drugs. 1991;9:333–337. doi: 10.1007/BF00183575. [DOI] [PubMed] [Google Scholar]
- 31.Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol. 2007;25:493–500. doi: 10.1200/JCO.2005.02.3879. [DOI] [PubMed] [Google Scholar]
- 32.Barry EV, Vrooman LM, Dahlberg SE, et al. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J Clin Oncol. 2008;26:1106–1111. doi: 10.1200/JCO.2007.12.2481. [DOI] [PubMed] [Google Scholar]
- 33.Salzer WL, Devidas M, Carroll WL, et al. Long-term results of the Pediatric Oncology Group studies for childhood acute lymphoblastic leukemia 1984–2001: A report from the Children's Oncology Group. Leukemia. 2010;24:355–370. doi: 10.1038/leu.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rathe M, Carlsen NL, Oxhøj H, et al. Long-term cardiac follow-up of children treated with anthracycline doses of 300 mg/m2 or less for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;54:444–448. doi: 10.1002/pbc.22302. [DOI] [PubMed] [Google Scholar]
- 35.Kremer LC, van Dalen EC, Offringa M, et al. Frequency and risk factors of anthracycline-induced clinical heart failure in children: A systematic review. Ann Oncol. 2002;13:503–512. doi: 10.1093/annonc/mdf118. [DOI] [PubMed] [Google Scholar]
- 36.Hudson MM, Rai SN, Nunez C, et al. Noninvasive evaluation of late anthracycline cardiac toxicity in childhood cancer survivors. J Clin Oncol. 2007;25:3635–3643. doi: 10.1200/JCO.2006.09.7451. [DOI] [PubMed] [Google Scholar]
- 37.Kalamazoo, MI: Pharmacia and Upjohn Company; 2003. Zinecard® (dexrazoxane for injection) [U.S. prescribing information] [Google Scholar]
- 38.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children's Oncology Group long-term follow-up guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 39.Silber JH, Jakacki RI, Larsen RL, et al. Increased risk of cardiac dysfunction after anthracyclines in girls. Med Pediatr Oncol. 1993;21:477–479. doi: 10.1002/mpo.2950210704. [DOI] [PubMed] [Google Scholar]