Breast surgery, radiotherapy, chemotherapy, hormonotherapy, and targeted agents are all being used together concomitantly or sequentially with the aim to achieve local and distant control and improve survival in breast cancer patients. With this goal being reached more and more often nowadays, quality of life emerges as another issue of pivotal importance. Existing data on the maximum acceptable delay of radiotherapy when given as sole adjuvant treatment after surgery and the optimal sequence of all these modalities with respect to each other are reviewed.
Keywords: Radiotherapy, Chemotherapy, Hormone therapy, Trastuzumab, Sequence, Delay, Breast cancer, AROME
Abstract
The adjuvant setting of early breast cancer treatment is an evolving field where different modalities must be combined to improve outcomes; moreover, quality of life of breast cancer survivors emerges as a new important parameter to consider, thus implying a better understanding of toxicities of these modalities. We have conducted a review focusing on the latest literature of the past 3 years, trying to evaluate the existing data on the maximum acceptable delay of radiotherapy when given as sole adjuvant treatment after surgery and the optimal sequence of all these modalities with respect to each other. It becomes evident radiotherapy should be given as soon as possible and within a time frame of 6–20 weeks. Chemotherapy is given before radiotherapy and hormone therapy. However, radiotherapy should be started within 7 months after surgery in these cases. Hormone therapy with tamoxifen might be given safely concomitantly or sequentially with radiotherapy although solid data are still lacking. The concurrent administration of letrozole and radiotherapy seems to be safe, whereas data on trastuzumab can imply only that it is safe to use concurrently with radiotherapy. Randomized comparisons of hormone therapy and trastuzumab administration with radiotherapy need to be performed.
Introduction
The adjuvant setting of early breast cancer treatment is an exciting field where one has to combine many various emerging modalities. These have contributed to a decline in breast cancer mortality over the last decades, despite the excess breast cancer incidence [1]. Breast surgery, radiotherapy (RT), chemotherapy (chemoT), hormonotherapy (HT), and targeted agents are all being used together concomitantly or sequentially with the aim to achieve local and distant control and improve survival. With this goal being reached more and more often nowadays, quality of life emerges as another issue of pivotal importance considering their use and their impact on the patient's other-than breast-cancer–related mortality [2].
The clinical issue that has recently been addressed by five reviews [3–7], including one by our group and one retrospective, but not breast cancer-restricted combined analysis [8], is how best to combine these modalities. This arises as a question posed in everyday clinical practice, such as when and how to administer each one of them. Namely, patients are anxious to know whether after their breast-conserving surgery (BCS) or mastectomy (M) they should get RT or chemoT (sequence), how long they can wait before they get RT if they are put on the waiting list of the RT department because of machine shortage (maximum acceptable RT delay after surgery), if they can receive tamoxifen or an aromatase inhibitor (if they host hormone receptor–positive tumors) while they are being irradiated (concomitant vs sequential use), and if they can receive trastuzumab concomitantly with their chemoT and RT (sequence) in case of a HER-2–positive tumor.
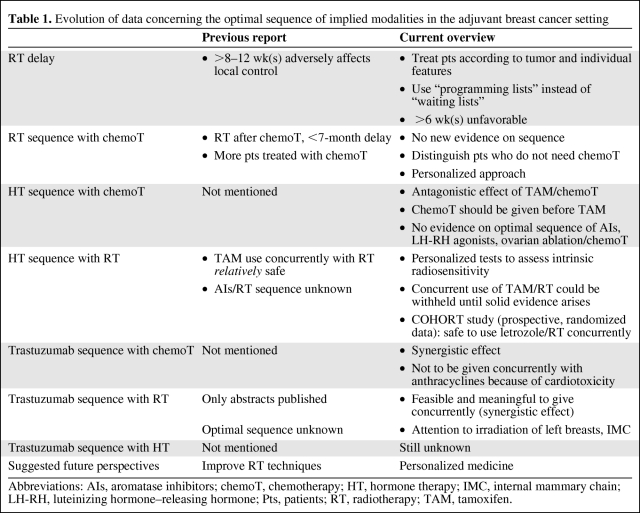
Existing answers to these questions have been given in our previously published review [5] and therefore literature until 2007 is not going to be discussed extensively in this present work. Evolving data that have been published after this review was written are going to be presented, the focus being on the clinical interpretation of this new information. As breast cancer is perhaps the most active field of evolution in oncology, new information is becoming available quickly, thus rendering our understanding of breast disease more solid; therefore, it was felt by our group that an update on the previous review was necessary to incorporate and translate clinically the new data published. All this emerging information put together was pointing toward a more personalized approach in the timing and sequence of the involved modalities in the adjuvant early breast cancer setting; to explore this idea, the most recent papers are being discussed. Table 1 summarizes the evolution of data in the time between our previous publication and this report.
Table 1.
Evolution of data concerning the optimal sequence of implied modalities in the adjuvant breast cancer setting
Abbreviations: AIs, aromatase inhibitors; chemoT, chemotherapy; HT, hormone therapy; IMC, internal mammary chain; LH-RH, luteinizing hormone–releasing hormone; Pts, patients; RT, radiotherapy; TAM, tamoxifen.
Nevertheless, it cannot be ignored that many countries globally have limited resources, translating in more pronounced delays in optimum treatment delivery [9]. It has also been recognized that patients might get undertreated due to geographical reasons (distance from an oncology unit) and that disparities in cancer treatment exist even within developed, Western countries such as the United States or the United Kingdom [10, 11], with the socioeconomic status of the patient being an almost independent prognostic factor [12]. Moreover, this time the focus will be in underlying the questions that have not been clarified yet and in pointing out what clinical studies need to be undertaken to give definitive answers to the issue of how best to combine the various treatment modalities in the adjuvant setting of early breast cancer.
Methodology
A literature search according to our previous methodology [5] was done through PubMed and major papers were searched for their references and citations. Editorials, letters to the editor, and any relevant publications were taken into account to evaluate other investigators' opinions. Emphasis was given on articles published after September 2007, when our previous literature search was completed and this new evidence was critically evaluated.
Rt Delay When Given as Sole Modality after BCS or M
As already discussed, it is well known that more than half of the patients receiving RT after BCS or M are delayed because of lack of equipment and personnel, even in developed countries [13]; the percentage can be even more substantial for countries with limited resources [9]. For the latter, question even arises as to whether there is enough availability of RT machinery for every early breast cancer patient in need. From all the previous data combined, we were able to reach the conclusion in our previous report that a delay of RT of more than 8–12 weeks after surgery adversely affects local control [5].
Although data on survival are inconclusive and not definitive, given the well-established conclusion of the Early Breast Cancer Trialists' Collaborative Group (EBCTCG), (being that for any four local recurrences avoided, one breast cancer death is avoided), we cannot assume that any impact of a delay on RT administration on local recurrence does not affect survival [15]. Moreover, since this impact on survival is seen on data with long (15 years) follow-up and given the characteristics of the relevant studies (small studies, with short follow-up), it is evident why a detriment on survival could not be detected [15–17]. Indeed, the only direct evidence, until our previous report, existing on the impact of a delay in RT administration after BCS on survival, comes from the large evaluation of the Surveillance, Epidemiology, and End Results (SEER)—Medicare data involving almost 14,000 women [18]. In the 3% of them who received RT >3 months after surgery, survival was severely impaired (hazard ratio, 3.84).
More recent studies have been published in the last 3 years: The Florence experience study, involving almost 5,000 women and evaluating the relationship between RT delay and local recurrence, was unable to detect a surgery-RT interval that resulted in increased local recurrence [19]. Authors of the study argue that local recurrence does not depend on the delay of RT administration as much as it does depend on tumors' and patients' characteristics. They only found a statistically significant relevance between local recurrence and RT delay in patients receiving chemoT (hazard ratio, 1.59) and conclude that “waiting lists” should become “programming lists” with patients being scheduled depending on their individual and tumor characteristics and their prognostic significance.
The study by Olivotto et al. [20] has evaluated the BCS-RT interval that adversely affects local control and survival. With a total of almost 6,500 women, they were able to detect a statistically significant interval of >20 weeks after surgery that resulted in impaired local recurrence–free, distant recurrence–free, and breast cancer–specific survival. Therefore, authors of the study suggest the 20-week interval should not be overlooked, while patients should be given the time for their breast to heal and their informed decisions to be made within that interval.
Finally, the most recently published study by Punglia et al., involving >18,000 women treated with BCS and receiving no chemoT, has shown that delays of >6 weeks can be detrimental for patients in terms of a local recurrence, while there exists a continuous relationship between BCS-RT intervals and local recurrence in older women with early stage breast cancer [10].
Therefore, more recent and reliable evidence, coming within the last 3 years, pertains to the maximum acceptable surgery (mainly BCS)-RT intervals that do not jeopardize patients' local and distant control and survival. This is robust, well-conducted, and comes from large centers' data. However, results from these studies are not uniform: the 6-week to 20-week interval is far from coinciding, whereas the Florence experience does not even detect a concrete detrimental delay. However, in our mind, two messages arise from all of these data.
First, with the nonuniformity of breast cancer disease being more widely recognized, it becomes evident that studies will never coincide as long as they involve patients with various prognostic and predictive factors of the disease because, obviously, the impact of RT delay on their risk of local recurrence is not the same for every early breast cancer patient. Therefore, before we rush to convince a patient that their delay in receiving their adjuvant RT poses no risks for their health, we might as well consider the individual's and tumor's unique probability for relapse. In that direction, the sense of prognostic factors attains additional importance for departmental and health policy design, implementation, and scheduling.
Second, it is obvious from existing studies that, although a long delay of >6, 8, 12, or 20 weeks should be avoided, a minimum interval of up to 6 weeks is not harmful and, moreover, might be essential for the breast to heal and the patient to be psychologically and physically prepared for another step in her breast cancer odyssey. Therefore, with proper resource planning, timing can be an advantage instead of being a drawback; moreover, present data suggest that a multidisciplinary upfront approach is essential and that RT appointments should not be given by secretaries but instead by radiation oncologists.
ChemoT Sequence with RT
It is now common practice after the 1996 publication by Recht et al. that chemoT is administered before RT in the adjuvant setting of breast cancer treatment [21]. Various associations' guidelines hold to this concept, or propose a “sandwich approach” or concomitant use [22–24]. As discussed in our earlier work, most existing studies are inconclusive. The three major studies included in the Cochrane meta analysis have led to the conclusion that delivery of chemoT before RT does not compromise local control as long as RT is delivered within 7 months after surgery [25]. The Citron et al. study has also shown that more effective dose dense or taxane-containing chemoT results in improved survival, despite the longer surgery-RT interval produced by the addition of taxanes to the regimen [26]. Therefore, data collected until 2007 have pointed out that when effective chemoT is administered, a longer surgery-RT interval is not detrimental. At this point, the contribution of chemoT to local control must also be underlined, as shown by the EBCTG meta analysis [27].
The usually small and contradictory studies that have retrospectively treated the issue have shown a trend toward increased local recurrence rates when RT was delayed for chemoT to be given first and toward increased distant failure when the contrary was done [28–38]. The largest retrospective study among them failed to show an impact of RT delay on local recurrence or survival when chemoT was administered first [35]. The historical study by Recht has claimed that it is preferable to give a 3-month chemoT regimen before RT for patients at substantial risk for distant metastases [21]. The updated results by Bellon et al. have failed to show any superiority of the one modality given before the other in terms of local or distant control and overall survival [38]. However, patients with close margins had an increased rate of locoregional recurrence when chemoT was administered first and even when RT was administered first. Patients with positive or close margins should be treated with re-excision, although a certain debate exists on “how close is close” [39].
Although no recent relevant literature has emerged since our last review on studies directly addressing the issue of RT/chemoT sequence, the study by Yock et al. has shown that an interval of > 7 months after surgery to administer chemoT is not appropriate; furthermore, they have shown that patients with positive margins consist of a subgroup that warrants further attention, as already discussed; for them re-excision or, when this is not possible, early RT might be imperative [33].
It must be emphasized that no study to date has been able to show an impact of sequence of administration on survival, probably because of the different priorities on control (local, distant) given depending on the modality chosen to be administered first. Studies on the maximum acceptable delay of chemoT administration have shown that it should be offered within 12 weeks [40], whereas others have not established a maximum acceptable chemoT-RT interval after surgery that adversely affects survival [41, 42]. This information taken together underlines the importance of a well-designed multidisciplinary plan made before any treatment start that avoids long delays and leaves the patient time to consider her options and make informed choices [41].
Finally, the option of delivering concomitantly chemoT/RT has been tested in several studies that have shown the efficacy and acceptable, albeit slightly increased, toxicity of the modality [43–45]. Only one retrospective study has emerged recently that has shown acceptable toxicities and equal local and distant control rates for both sequences [46]. Therefore, although this concomitant regimen has not gained universal acceptance and is not routinely used, it does exist as an interesting option that is feasible and effective; moreover, it shortens treatment times, which can be important for the patients' comfort.
More effective chemoT is expected to further improve these outcomes. It should be taken into account that most of these studies are small and refer to older chemoT regimens, whereas emerging knowledge through the last few years is reconsidering the proper regimen for distinct patients' and tumors' characteristics and is expected to be more appropriate and effective. Nevertheless, although there has been a tendency to administer chemoT to every patient with a tumor larger than 1 cm, recent knowledge has rendered the chemoT use more eclectic and concrete [47–49].
This has been the result of the development of gene arrays technology (Oncotype®, Mammaprint®, etc.) that not only has led to a better understanding of the heterogeneity of breast cancer disease but also has, at the clinical level, led to a more individualized and tailored use of chemotherapy [50]. As a result, fewer patients with favorable characteristics are being submitted to chemoT these days compared to those in the last decade, thus leaving more patients the choice of RT as the sole adjuvant modality or RT with hormone therapy with or without trastuzumab, the latter being the more frequent options.
HT Given Concomitantly or Sequentially with ChemoT
Another issue to consider is the feasibility and the point of administering HT concomitantly with chemoT. This regards patients with hormone-positive tumors that still have enough aggressive disease that requires also chemoT administration. One can barely see the point of concomitant use, from a practical point of view, since HT is a chronic treatment prolonged for at least 5 years from the start of administration. Therefore, a delay of some months should not be an issue.
However, it could be an issue if we expect these two modalities to work in a synergistic way or if we anticipate increased toxicity by their concurrent use. Data considering these two questions are emerging: In the recent Breast Cancer Intergroup of North America randomized trial involving >1,500 women, it has been shown that CAF (anthracycline-containing chemoT) followed by tamoxifen is superior to concomitant HT-chemoT or tamoxifen alone for estrogen receptor–positive and node–positive patients [51].
Although different hypotheses have been suggested, regarding the combination of chemoT with tamoxifen (antagonistic effect due to the tamoxifen cytostatic action versus starting chemoT as soon as feasibly possible to block highly estrogen receptor–positive expressing tumors), clinical data were unable to provide concrete proof of which the hypothesis holds true. The rationale behind the anticipated antagonistic actions of chemotherapy and hormone therapy lie in the fact that chemotherapy kills cells that are actively cycling, whereas hormone therapy acts in a cytostatic way, that is, by preventing cells from cycling [52]. In most of the main trials of tamoxifen, it was given concurrently with chemoT, whereas there have been trials when it was administered after chemoT completion [52].
Therefore, data considering the concomitant use of tamoxifen and chemotherapy have to date implied that there is an antagonistic effect between them and moreover concomitant use of the two modalities might produce enhanced toxicities; thus, it would be better to avoid using them in combination [53–56]. It is now widely accepted that adjuvant chemotherapy should be completed before beginning tamoxifen. No trials examining concurrent versus sequential treatment have been performed with HT and chemoT in the premenopausal setting or with aromatase inhibitors and chemotherapy in postmenopausal women [52].
Furthermore, as far as tamoxifen is concerned, we must take into account that there exists a different tamoxifen sensitivity in various tumors and that it can have possible yet unexplored interactions with other medications and irradiation [57]. Moreover, CYP2D6 variants are associated with a worse disease-free survival and with a higher frequency of severe and mild toxicities [58]. Therefore, the action of tamoxifen on the tumor cell is complex and not fully understood.
When considering HT, three different drug categories are involved: tamoxifen, aromatase inhibitors, and LHRH agonists. Data on the appropriate use of the latter, concerning their optimum duration and sequence with other modalities are, as yet, inconclusive. However, existing evidence supports their effectiveness in estrogen receptor–positive women, their interactions with chemoT and tamoxifen and even more with aromatase inhibitors still remaining unclarified [52]. This regards the whole spectrum of hormone therapy, including aromatase inhibitors and ovarian ablation. Therefore, these questions still need to be addressed in a randomized way and formal evidence needs to be produced.
Furthermore, in light of emerging new evidence suggesting a role for zoledronic acid in the adjuvant setting of breast cancer in terms of local control improvement, its optimal sequence and combination with chemoT and RT needs to be evaluated [59–62]. Although the most recent meta analysis has not shown a distinct impact of zoledronic acid on the natural history of the disease, there is a trend toward better local control and better clinical outcomes, which leaves in this area an exciting field for research, yet not a routinely recommended practice [63]. However, we do not currently have any data on the optimal sequence of zoledronic acid and chemoT when they are to be combined in clinical trials.
HT Given Concomitantly or after RT
Preclinical data on tamoxifen and irradiation show that a possible antagonistic effect on tumor cell death might exist [57]. This has been shown in vitro but has not been further demonstrated in clinical studies. It is important here to underline that this notion regards the use of tamoxifen and RT. As already discussed in our previous review, data regarding outcomes coming from three retrospective studies published in 2005 [64–66] are equal; furthermore, data regarding toxicity suggest a somehow increased toxicity such as pulmonary fibrosis and impaired breast cosmesis that has not been properly validated yet [67–69].
It can be concluded from preclinical studies that the action of tamoxifen on a breast that has harbored cancer is far more complex than adhering to a simple straightforward pathway. What we know from clinical trials, however, is quite direct: The three retrospective studies published to date have shown that tamoxifen use concomitantly or after RT is equally effective in terms of local and distant control and overall survival [64–66]. In terms of toxicity, none of the previous studies have reported excess toxicities with concurrent use; however, there have been reports of excess pulmonary or skin toxicity with the concurrent use of tamoxifen and RT [67, 68]. The recent overview of trials on the combination of tamoxifen and RT has concluded that since no robust data exist, sequential use of tamoxifen after completion of RT should be advised [57].
It is true that since we do not anticipate an advantage from concurrent use (and the three independent, albeit small, studies agree on that), one can barely see why a patient should be submitted to a poorly evaluated risk of excess toxicity. It has been shown now that personalized tests, such as the lymphocyte apoptosis test proposed by Azria et al., exist and that they can accurately predict patients with intrinsic radiosensitivity who are going to develop greater than grade II toxicities; these patients express even more toxicity with the concurrent combination of tamoxifen and RT [70]. Therefore, it should at least be advised that patients with high radiosensitivity profiles should not be submitted to concurrent tamoxifen-RT use when the previously mentioned test is available.
Alternatively, as aromatase inhibitors have become the mainstay of treatment for postmenopausal women, the issue of their possibility to be combined concurrently with RT arises as well. What is known from preclinical data is that letrozole is a radiosensitizer [71]. It has even been proposed that its use start within 3 weeks before start of RT, to enhance the radiosensitizing potential [5]. However, the possible radiosensitizing effect on the intact breast should not be underestimated.
Clinical data have emerged that have directly compared concurrent versus sequential use of letrozole and RT. The only randomized study published today is the COHORT study which, with a short follow-up of 26 months, has shown that it is safe to use letrozole and RT concurrently [72]. Moreover, investigators of the study were able to show that the radiosensitivity lymphocyte apoptosis test accurately predicted patients who were going to develop grade II or more toxicities in the whole population, as well as in the concurrent and sequential groups. It has even been suggested by a respective comment that aromatase inhibitors, such as letrozole, might actually have a radioprotective role [73].
Data coming from a retrospective study are also in agreement with these conclusions [74]. Therefore, the issue of sequence of hormone therapy with RT starts to be clarified. Tamoxifen use has not been evaluated in a randomized comparison. Until then, its concurrent use with RT is relatively safe, however, not necessarily meaningful. Letrozole seems to be safe to combine concurrently with RT. Further follow-up will clarify whether this also provides an advantage on outcome. Personalized medicine will probably become a routine in the decade to come and therefore might directly indicate patients at increased risk for radiation-induced toxicity due to their intrinsic radiosensitivity. However, a definitive answer on whether concurrent use improves outcomes needs to be given by a randomized trial on both hormone therapy drug categories.
Trastuzumab Administration with ChemoT, RT, and HT
What is even more interesting and imperative to answer is the issue of trastuzumab sequence with RT in HER-2–positive patients. Trastuzumab given concurrently with chemoT (anthracyclines and/or taxanes) is known to possess a synergistic effect [75–82]. However, it is clear that trastuzumab should not be administered concurrently with anthracyclines because of an anticipated increased cardiac toxicity [79].
In this setting, there are clear hints suggesting that concurrent trastuzumab with RT might enhance trastuzumab efficacy, as already seen with trastuzumab and chemoT. It has been shown in preclinical studies that HER-2 overexpression confers radioresistance; moreover, it is believed that blocking HER-2 receptors with trastuzumab will render the tumor cell more radiosensitive [83]. Therefore, improved outcomes are anticipated with concurrent use.
Conversely, trastuzumab and RT have both been associated with cardiac dysfunction [84–88]. As seen in the four large adjuvant trials in which trastuzumab was not given concomitantly with doxorubicin and cyclophosphamide, cardiac toxicity incidence was 0.4%–3.8%, while remaining 0.1%–0.9% in the nontrastuzumab arms; RT was given after chemoT in all of these studies [88–91]. This cardiac toxicity was in most cases medically managed and basically reversible [92]. This is even more relevant when left breasts and internal mammary chains (IMC) are being irradiated.
Evidence to date comes only from retrospective studies or retrospective evaluation from data of already completed studies. These analyses have shown that concurrent combination of trastuzumab with RT is feasible and cardiac toxicity is slightly increased when IMC is irradiated [93–96]. The larger study among them, including >1,500 patients and with a follow-up of >3.5 years (the longer published to date) has shown that concurrent administration of RT and trastuzumab does not increase toxicities except leukopenia. IMC irradiation with limited cardiac exposure also seemed feasible, although the study “prohibited intentional internal mammary nodes (IMN) RT but not incidental cardiac irradiation” [95]. A randomized study in the neoadjuvant setting has also shown acceptable toxicities and enhanced local control [96]. The IMC was also irradiated in most (71%) of the patients, and despite this fact, increased cardiotoxicity was not observed, nor were any other radiation-related toxicities. Consequently, limited data to date suggest it might be safe and meaningful to administer trastuzumab concurrently with selected chemoT regimens followed by concurrent trastuzumab-RT.
Questions That Still Need To Be Addressed
It becomes obvious from this updated review that issues on the optimal sequence of the various modalities that have improved survival in the adjuvant setting of early breast cancer treatment still need to be clarified. These include a randomized comparison of hormone therapies—in all drug categories given concomitantly with RT as well as randomized comparisons of trastuzumab given concurrently with non-anthracycline–containing chemoT and the respective ones for concurrent use of trastuzumab with RT. The emerging role of bisphosphonates and other targeted therapies such as lapatinib will also ask for an answer on their optimal sequence with each other; however, the road is still long ahead before we define their proper place in the adjuvant setting.
Conclusions
Recent evidence has clarified that a RT delay when given as sole modality in the adjuvant setting after BCS should be avoided; RT should be administered within a time frame of 6–20 weeks; therefore, departmental planning should focus on the shortening of waiting/“programming” lists. As far as the sequence of RT with chemoT is concerned, although data do not prove the superiority of any modality given first, it is now common practice that chemoT is administered before RT; it is however important in this context not to administer RT in a delay longer than 7 months after surgery. As far as the sequence of HT with chemoT is concerned, their combination should be avoided to avoid unnecessary toxicity; currently, the only available data have shown it is safer to administer tamoxifen after chemoT completion. The combination of hormone agents with RT seems to be safe and possibly even more effective for letrozole, whereas there is a trend of data showing a slightly increased toxicity of concurrent tamoxifen and RT.
Finally, the issue of the optimal combination of trastuzumab with chemoT and RT and even HT needs to be addressed in a randomized way, since there are hints suggesting a possible synergistic role, albeit with possibly increased toxicity. As data show it might be safe to use trastuzumab concurrently with RT, a definitive answer must be given soon in order to not miss the possible clinical benefit for reasons of prudence.
Author Contributions
Conception/Design: Pelagia G. Tsoutsou, Yazid Belkacemi, Joseph Gligorov, Michael I. Koukourakis, Nuran Bese
Provision of study material or patients: Pelagia G. Tsoutsou, Yazid Belkacemi, Joseph Gligorov, Nuran Bese, Michael I. Koukourakis, Abraham Kuten, Hamouda Boussen
Collection and/or assembly of data: Pelagia G. Tsoutsou, Michael I. Koukourakis
Data analysis and interpretation: Pelagia G. Tsoutsou, Michael I. Koukourakis
Manuscript writing: Pelagia G. Tsoutsou, Yazid Belkacemi, Joseph Gligorov, Michael I. Koukourakis, Nuran Bese, Abraham Kuten, Hamouda Boussen
Final approval of manuscript: Pelagia G. Tsoutsou, Yazid Belkacemi, Joseph Gligorov, Michael I. Koukourakis, Nuran Bese, Abraham Kuten, Hamouda Boussen
References
- 1.Berry DA, Cronin KA, Plevritis SK, et al. Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators. Effect of screening and adjuvant therapy. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 2.Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;27:32. doi: 10.1186/1756-9966-27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balduzzi A, Leonardi MC, Cardillo A, et al. Timing of adjuvant systemic therapy and radiotherapy after breast-conserving surgery and mastectomy. Cancer Treat Rev. 2010;36:443–450. doi: 10.1016/j.ctrv.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Adamowicz K, Marczewska M, Jassem J. Combining systemic therapies with radiation in breast cancer. Cancer Treat Rev. 2009;35:409–416. doi: 10.1016/j.ctrv.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Tsoutsou PG, Koukourakis MI, Azria D, et al. Optimal timing for adjuvant radiation therapy in breast cancer: a comprehensive review and perspectives. Crit Rev Oncol Hematol. 2009;71:102–116. doi: 10.1016/j.critrevonc.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Hébert-Croteau N, Freeman CR, Latreille J, et al. A population-based study of the impact of delaying radiotherapy after conservative surgery for breast cancer. Breast Cancer Res Treat. 2004;88:187–196. doi: 10.1007/s10549-004-0594-7. [DOI] [PubMed] [Google Scholar]
- 7.Ruo Redda MG, Verna R, Guarneri A, et al. Timing of radiotherapy in breast cancer conserving treatment. Cancer Treat Rev. 2002;28:5–10. doi: 10.1053/ctrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol. 2003;21:555–563. doi: 10.1200/JCO.2003.04.171. [DOI] [PubMed] [Google Scholar]
- 9.Bese NS, Munshi A, Budrukkar A, et al. Breast Health Global Initiative Radiation Therapy Focus Group. Breast radiation therapy guideline implementation in low- and middle-income countries. Cancer. 2008;113(8 Suppl):2305–2314. doi: 10.1002/cncr.23838. [DOI] [PubMed] [Google Scholar]
- 10.Punglia RS, Saito AM, Neville BA, et al. Impact of interval from breast conserving surgery to radiotherapy on local recurrence in older women with breast cancer: retrospective cohort analysis. BMJ. 2010;340:c845. doi: 10.1136/bmj.c845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wyatt RM, Jones BJ, Dale RG. Radiotherapy treatment delays and their influence on tumour control achieved by various fractionation schedules. Br J Radiol. 2008;81:549–563. doi: 10.1259/bjr/94471640. [DOI] [PubMed] [Google Scholar]
- 12.Dunn BK, Agurs-Collins T, Browne D, et al. Health disparities in breast cancer: biology meets socioeconomic status. Breast Cancer Res Treat. 2010;121:281–292. doi: 10.1007/s10549-010-0827-x. [DOI] [PubMed] [Google Scholar]
- 13.Benk V, Levinton C, Fortin PR, et al. Effect of delay in initiating radiotherapy for patients with early stage breast cancer: results of a natural experiment. Int J Radiat Oncol Biol Phys. 1999;45:305–306. [Google Scholar]
- 14.Clarke M, Collins R, Darby S, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–20106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 15.Vujovic O, Yu E, Cherian A, et al. Eleven-year follow-up results in the delay of breast irradiation after conservative breast surgery in node-negative breast cancer patients. Int J Radiat Oncol Biol Phys. 2006;64:760–764. doi: 10.1016/j.ijrobp.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Nixon AJ, Recht A, Neuberg D, et al. The relation between the surgery-radiotherapy interval and treatment outcome in patients treated with breast-conserving surgery and radiation therapy without systemic therapy. Int J Radiat Oncol Biol Phys. 1994;30:17–21. doi: 10.1016/0360-3016(94)90514-2. [DOI] [PubMed] [Google Scholar]
- 17.Slotman BJ, Meyer OW, Njo KH, et al. Importance of timing of radiotherapy in breast conserving treatment for early stage breast cancer. Radiother Oncol. 1994;30:206–212. doi: 10.1016/0167-8140(94)90459-6. [DOI] [PubMed] [Google Scholar]
- 18.Hershman DL, Wang X, McBride R, et al. Delay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survival. Int J Radiat Oncol Biol Phys. 2006;65:1353–1360. doi: 10.1016/j.ijrobp.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 19.Livi L, Borghesi S, Saieva C, et al. Radiotherapy timing in 4,820 patients with breast cancer: university of florence experience. Int J Radiat Oncol Biol Phys. 2009;73:365–369. doi: 10.1016/j.ijrobp.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 20.Olivotto IA, Lesperance ML, Truong PT, et al. Intervals longer than 20 weeks from breast-conserving surgery to radiation therapy are associated with inferior outcome for women with early-stage breast cancer who are not receiving chemotherapy. J Clin Oncol. 2009;27:16–23. doi: 10.1200/JCO.2008.18.1891. [DOI] [PubMed] [Google Scholar]
- 21.Recht A, Come SE, Henderson IC, et al. The sequencing of chemotherapy and radiation therapy after conservative surgery for early-stage breast cancer. N Engl J Med. 1996;334:1356–1361. doi: 10.1056/NEJM199605233342102. [DOI] [PubMed] [Google Scholar]
- 22.Kaufmann M, Morrow M, von Minckwitz G, et al. Biedenkopf Expert Panel Members. Locoregional treatment of primary breast cancer: consensus recommendations from an International Expert Panel. Cancer. 2010;116:1184–1191. doi: 10.1002/cncr.24874. [DOI] [PubMed] [Google Scholar]
- 23.Goldhirsch A, Ingle JN, Gelber RD, et al. Panel members. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldhirsch A, Glick JH, Gelber RD, et al. Panel members. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol. 2005;16:1569–1583. doi: 10.1093/annonc/mdi326. [DOI] [PubMed] [Google Scholar]
- 25.Hickey BE, Francis D, Lehman MH. Sequencing of chemotherapy and radiation therapy for early breast cancer. Cochrane Database Syst Rev. 2006;4:CD005212. doi: 10.1002/14651858.CD005212.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 27.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 28.Hartsell WF, Recine DC, Griem DM, et al. Delaying the initiation of intact breast irradiation for patients with lymph node positive breast cancer increases the risk of local recurrence. Cancer. 1995;76:2497–2503. doi: 10.1002/1097-0142(19951215)76:12<2497::aid-cncr2820761214>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Buchholz TA, Austin-Seymour MM, Moe RE, et al. Effect of delay in radiation in the combined modality treatment of breast cancer. Int J Radiat Oncol Biol Phys. 1993;26:23–35. doi: 10.1016/0360-3016(93)90169-v. [DOI] [PubMed] [Google Scholar]
- 30.Recht A, Come SE, Gelman RS, et al. Integration of conservative surgery, radiotherapy, and chemotherapy for the treatment of early-stage, node-positive breast cancer: sequencing, timing, and outcome. J Clin Oncol. 1991;9:1662–1667. doi: 10.1200/JCO.1991.9.9.1662. [DOI] [PubMed] [Google Scholar]
- 31.Leonard CE, Wood ME, Zhen B, et al. Does administration of chemotherapy before radiotherapy in breast cancer patients treated with conservative surgery negatively impact local control? J Clin Oncol. 1995;13:2906–2915. doi: 10.1200/JCO.1995.13.12.2906. [DOI] [PubMed] [Google Scholar]
- 32.Meek AG, Park TL, Weiss TA, et al. Effect of delayed radiation therapy on local control in breast conservation therapy. Radiology. 1996;200:615–619. doi: 10.1148/radiology.200.3.8756905. [DOI] [PubMed] [Google Scholar]
- 33.Yock I, Taghian AG, Kachnic LA, et al. The effect of delaying radiation therapy for systemic chemotherapy on local-regional control in breast cancer. Breast Cancer Res Treat. 2004;84:161–171. doi: 10.1023/B:BREA.0000018414.36274.72. [DOI] [PubMed] [Google Scholar]
- 34.Buzdar AU, Kau SW, Smith TL, et al. The order of administration of chemotherapy and radiation and its effect on the local control of operable breast cancer. Cancer. 1993;71:3680–3684. doi: 10.1002/1097-0142(19930601)71:11<3680::aid-cncr2820711134>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 35.Benchalal M, Le Prise E, de Lafontan E, et al. Influence of the time between surgery and radiotherapy on local recurrence in patients with lymph node-positive, early-stage, invasive breast carcinoma undergoing breast-conserving surgery: results of the French Adjuvant Study Group. Cancer. 2005;104:240–250. doi: 10.1002/cncr.21161. [DOI] [PubMed] [Google Scholar]
- 36.Metz JM, Schultz DJ, Fox K, et al. Analysis of outcomes for high-risk breast cancer based on interval from surgery to postmastectomy radiation therapy. Cancer J. 2000;6:324–330. [PubMed] [Google Scholar]
- 37.Hickey BE, Francis D, Lehman MH. Sequencing of chemotherapy and radiation therapy for early breast cancer. Cochrane Database Syst Rev. 2006;4:CD005212. doi: 10.1002/14651858.CD005212.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Bellon JR, Come SE, Gelman RS, et al. Sequencing of chemotherapy and radiation therapy in early-stage breast cancer: updated results of a prospective randomized trial. J Clin Oncol. 2005;23:1934–1940. doi: 10.1200/JCO.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 39.Povoski SP, Jimenez RE, Wang WP, et al. Standardized and reproducible methodology for the comprehensive and systematic assessment of surgical resection margins during breast-conserving surgery for invasive breast cancer. BMC Cancer. 2009;9:254. doi: 10.1186/1471-2407-9-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lohrisch C, Paltiel C, Gelmon K, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2006;24:4888–4894. doi: 10.1200/JCO.2005.01.6089. [DOI] [PubMed] [Google Scholar]
- 41.Shannon C, Ashley S, Smith IE. Does timing of adjuvant chemotherapy for early breast cancer influence survival? J Clin Oncol. 2003;21:3792–3797. doi: 10.1200/JCO.2003.01.073. [DOI] [PubMed] [Google Scholar]
- 42.Cold S, Düring M, Ewertz M, et al. Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Results of the Danish Breast Cancer Cooperative Group (DBCG) Br J Cancer. 2005;93:627–632. doi: 10.1038/sj.bjc.6602734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rouëssé J, de la Lande B, Bertheault-Cvitkovic F, et al. A phase III randomized trial comparing adjuvant concomitant chemoradiotherapy versus standard adjuvant chemotherapy followed by radiotherapy in operable node-positive breast cancer: final results. Int J Radiat Oncol Biol Phys. 2006;64:1072–1080. doi: 10.1016/j.ijrobp.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Haffty BG, Kim JH, Yang Q, et al. Concurrent chemoradiation in the conservative management of breast cancer. Int J Radiat Oncol Biol Phys. 2006;66:1306–1312. doi: 10.1016/j.ijrobp.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 45.Toledano A, Azria D, Garaud P, et al. Phase III trial of concurrent or sequential adjuvant chemoradiotherapy after conservative surgery for early-stage breast cancer: final results of the ARCOSEIN trial. J Clin Oncol. 2007;25:405–410. doi: 10.1200/JCO.2006.07.8576. [DOI] [PubMed] [Google Scholar]
- 46.Ismaili N, Mellas N, Masbah O, et al. Concurrent chemoradiotherapy in adjuvant treatment of breast cancer. Radiat Oncol. 2009;4:12. doi: 10.1186/1748-717X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gelber RD, Gelber S International Breast Cancer Study Group. Breast International Group. Facilitating consensus by examining patterns of treatment effects. Breast. 2009;18(suppl 3):S2–S8. doi: 10.1016/S0960-9776(09)70265-6. [DOI] [PubMed] [Google Scholar]
- 48.Ng W, Delaney GP, Jacob S, et al. Estimation of an optimal chemotherapy utilisation rate for breast cancer: setting an evidence-based benchmark for the best-quality cancer care. Eur J Cancer. 2010;46:703–712. doi: 10.1016/j.ejca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Bickell NA, Weidmann J, Fei K, et al. Underuse of breast cancer adjuvant treatment: patient knowledge, beliefs, and medical mistrust. J Clin Oncol. 2009;27:5160–5167. doi: 10.1200/JCO.2009.22.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 51.Albain KS, Barlow WE, Ravdin PM, et al. Breast Cancer Intergroup of North America. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:2055–2063. doi: 10.1016/S0140-6736(09)61523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pritchard KI. Combining endocrine agents with chemotherapy: which patients and what sequence? Cancer. 2008;112(3 suppl):718–722. doi: 10.1002/cncr.23189. [DOI] [PubMed] [Google Scholar]
- 53.Fisher B, Redmond C, Brown A, et al. Adjuvant chemotherapy with and without tamoxifen in the treatment of primary breast cancer: 5 year results from the National Surgical Adjuvant Breast and Bowel Project Trial. J Clin Oncol. 1986;4:459–471. doi: 10.1200/JCO.1986.4.4.459. [DOI] [PubMed] [Google Scholar]
- 54.Pritchard KI, Paterson AH, Fine S, et al. Randomized trial of cyclophosphamide, methotrexate, and fluorouracil chemotherapy added to tamoxifen as adjuvant therapy in postmenopausal women with node-positive estrogen and/or progesterone receptor-positive breast cancer: a report of the National Cancer Institute of Canada Clinical Trials Group. Breast Cancer Site Group. J Clin Oncol. 1997;15:2302–2311. doi: 10.1200/JCO.1997.15.6.2302. [DOI] [PubMed] [Google Scholar]
- 55.Sertoli MR, Pronzato P, Ventorini M, et al. A randomized study of concurrent versus sequential adjuvant chemotherapy and tamoxifen in stage II breast cancer [abstract] Proc Am Soc Clin Oncol. 2002;21:46a. [Google Scholar]
- 56.Pico C, Martin M, Jara C, et al. Epirubicin-cyclophosphamide adjuvant chemotherapy plus tamoxifen administered concurrently versus sequentially: randomized phase III trial in postmenopausal node-positive breast cancer patients. A GEICAM 9401 study. Ann Oncol. 2004;15:79–87. doi: 10.1093/annonc/mdh016. [DOI] [PubMed] [Google Scholar]
- 57.Chargari C, Toillon RA, Macdermed D, et al. Concurrent hormone and radiation therapy in patients with breast cancer: what is the rationale? Lancet Oncol. 2009;10:53–60. doi: 10.1016/S1470-2045(08)70333-4. [DOI] [PubMed] [Google Scholar]
- 58.Ramón y Cajal T, Altés A, Paré L, et al. Impact of CYP2D6 polymorphisms in tamoxifen adjuvant breast cancer treatment. Breast Cancer Res Treat. 2010;119:33–38. doi: 10.1007/s10549-009-0328-y. [DOI] [PubMed] [Google Scholar]
- 59.Gnant M. Bisphosphonates in the prevention of disease recurrence: current results and ongoing trials. Curr Cancer Drug Targets. 2009;9:824–833. doi: 10.2174/156800909789760267. [DOI] [PubMed] [Google Scholar]
- 60.Coleman RE. Adjuvant bisphosphonates in breast cancer: are we witnessing the emergence of a new therapeutic strategy? Eur J Cancer. 2009;45:1909–1915. doi: 10.1016/j.ejca.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 61.Rossi E, Morabito A, Di Rella F, et al. Endocrine effects of adjuvant letrozole compared with tamoxifen in hormone-responsive postmenopausal patients with early breast cancer: the HOBOE trial. J Clin Oncol. 2009;27:3192–3197. doi: 10.1200/JCO.2008.18.6213. [DOI] [PubMed] [Google Scholar]
- 62.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 63.Mauri D, Valachis A, Polyzos NP, et al. Does adjuvant bisphosphonate in early breast cancer modify the natural course of the disease? A meta-analysis of randomized controlled trials. J Natl Compr Canc Netw. 2010;8:279–286. doi: 10.6004/jnccn.2010.0020. [DOI] [PubMed] [Google Scholar]
- 64.Ahn PH, Vu HT, Lannin D, et al. Sequence of radiotherapy with tamoxifen in conservatively managed breast cancer does not affect local relapse rates. J Clin Oncol. 2005;23:17–23. doi: 10.1200/JCO.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 65.Harris EE, Christensen VJ, Hwang WT, et al. Impact of concurrent versus sequential tamoxifen with radiation therapy in early-stage breast cancer patients undergoing breast conservation treatment. J Clin Oncol. 2005;23:11–16. doi: 10.1200/JCO.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 66.Pierce LJ, Hutchins LF, Green SR, et al. Sequencing of tamoxifen and radiotherapy after breast-conserving surgery in early-stage breast cancer. J Clin Oncol. 2005;23:24–29. doi: 10.1200/JCO.2005.01.198. [DOI] [PubMed] [Google Scholar]
- 67.Bentzen S, Skoczylas J, Overgaard M, et al. Radiotherapy-related lung fibrosis enhanced by tamoxifen. J Natl Cancer Inst. 1996;88:918–922. doi: 10.1093/jnci/88.13.918. [DOI] [PubMed] [Google Scholar]
- 68.Fowble B, Fein DA, Hanlon AL, et al. The impact of tamoxifen on breast recurrence, cosmesis, complications, and survival in estrogen receptor-positive early-stage breast cancer. Int J Radiat Oncol Biol Phys. 1996;35:669–677. doi: 10.1016/0360-3016(96)00185-x. [DOI] [PubMed] [Google Scholar]
- 69.Wazer D, DiPetrillo T, Schmidt-Ulrich R, et al. Factors influencing cosmetic outcome and complication risk after conservative surgery and radiotherapy for early-stage breast carcinoma. J Clin Oncol. 1992;10:356–363. doi: 10.1200/JCO.1992.10.3.356. [DOI] [PubMed] [Google Scholar]
- 70.Azria D, Gourgou S, Sozzi WJ, et al. Concomitant use of tamoxifen with radiotherapy enhances subcutaneous breast fibrosis in hypersensitive patients. Br J Cancer. 2004;91:1251–1260. doi: 10.1038/sj.bjc.6602146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Azria D, Larbouret C, Cunat S, et al. Letrozole sensitizes breast cancer cells to ionizing radiation. Breast Cancer Res. 2005;7:R156–R163. doi: 10.1186/bcr969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Azria D, Belkacemi Y, Romieu G, et al. Concurrent or sequential adjuvant letrozole and radiotherapy after conservative surgery for early-stage breast cancer (CO-HO-RT): a phase 2 randomised trial. Lancet Oncol. 2010;11:258–265. doi: 10.1016/S1470-2045(10)70013-9. [DOI] [PubMed] [Google Scholar]
- 73.Recht A. Radiotherapy, antihormonal therapy, and personalised medicine. Lancet Oncol. 2010;11:215–216. doi: 10.1016/S1470-2045(10)70037-1. [DOI] [PubMed] [Google Scholar]
- 74.Ishitobi M, Komoike Y, Motomura K, et al. Retrospective analysis of concurrent vs. sequential administration of radiotherapy and hormone therapy using aromatase inhibitor for hormone receptor-positive postmenopausal breast cancer. Anticancer Res. 2009;29:4791–4794. [PubMed] [Google Scholar]
- 75.Boone JJ, Bhosle J, Tilby MJ, et al. Involvement of the HER2 pathway in repair of DNA damage produced by chemotherapeutic agents. Mol Cancer Ther. 2009;8:3015–3023. doi: 10.1158/1535-7163.MCT-09-0219. [DOI] [PubMed] [Google Scholar]
- 76.Végran F, Boidot R, Coudert B, et al. Gene expression profile and response to trastuzumab-docetaxel-based treatment in breast carcinoma. Br J Cancer. 2009;101:1357–1364. doi: 10.1038/sj.bjc.6605310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Longo R, D'Andrea M, Sarmiento R, et al. Pharmacogenetics in breast cancer: focus on hormone therapy, taxanes, trastuzumab and bevacizumab. Expert Opin Investig Drugs. 2010;19:S41–S50. doi: 10.1517/13543781003732701. [DOI] [PubMed] [Google Scholar]
- 78.Straehle C, Cardoso F, Azambuja E, et al. Better translation from bench to bedside: breakthroughs in the individualized treatment of cancer. Crit Care Med. 2009;37:S22–S29. doi: 10.1097/CCM.0b013e3181921598. [DOI] [PubMed] [Google Scholar]
- 79.Belkacémi Y, Laharie-Mineur H, Gligorov J, et al. Potential risk and benefit of the combination of trastuzumab to chemotherapy and radiation therapy in non-metastatic breast cancer. Cancer Radiother. 2007;11:266–275. doi: 10.1016/j.canrad.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 80.Colozza M, de Azambuja E, Cardoso F, et al. Breast cancer: achievements in adjuvant systemic therapies in the pre-genomic era. The Oncologist. 2006;11:111–125. doi: 10.1634/theoncologist.11-2-111. [DOI] [PubMed] [Google Scholar]
- 81.Slamon D, Pegram M. Rationale for trastuzumab (Herceptin) in adjuvant breast cancer trials. Semin Oncol. 2001;28(1 Suppl 3):13–19. doi: 10.1016/s0093-7754(01)90188-5. [DOI] [PubMed] [Google Scholar]
- 82.Nabholtz JM, Slamon D. New adjuvant strategies for breast cancer: meeting the challenge of integrating chemotherapy and trastuzumab (Herceptin) Semin Oncol. 2001;28(1 Suppl 3):1–12. doi: 10.1016/s0093-7754(01)90187-3. [DOI] [PubMed] [Google Scholar]
- 83.Pietras RJ, Poen JC, Gallardo D, et al. Monoclonal antibody to HER-2/neu receptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of breast cancer cells overexpressing this oncogene. Cancer Res. 1999;59:1347–1355. [PubMed] [Google Scholar]
- 84.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 85.Harris EE, Correa C, Hwang WT, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24:4100–4106. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 86.Cuzick J, Stewart H, Rutqvist L, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447–453. doi: 10.1200/JCO.1994.12.3.447. [DOI] [PubMed] [Google Scholar]
- 87.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 88.Rastogi P, Jeong J, Geyer CE, et al. Five year update of cardiac dysfunction on NSABP B-31: A randomized trial of AC→paclitaxel vs. AC→paclitaxel with trastuzumab in HER2 positive, node positive operable breast cancer [Abstract] Proc Am Soc Clin Oncol. 2007;25:6s. [Google Scholar]
- 89.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 90.Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 Adjuvant Breast Cancer Trial. J Clin Oncol. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Slamon DJ, Eiermann W, Robert N, et al. Phase III trial comparing AC-T with AC-TH and with TCH in the adjuvant treatment of HER2-positive early breast cancer patients: Second interim efficacy analysis [Abstract] Breast Cancer Res Treat. 2006;100(52a):S63. [Google Scholar]
- 92.Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 93.Belkacémi Y, Gligorov J, Ozsahin M, et al. Concurrent trastuzumab with adjuvant radiotherapy in HER2-positive breast cancer patients: acute toxicity analyses from the French multicentric study. Ann Oncol. 2008;19:1110–1116. doi: 10.1093/annonc/mdn029. [DOI] [PubMed] [Google Scholar]
- 94.Kirova YM, Caussa L, Granger B, et al. [Monocentric evaluation of the skin and cardiac toxicities of the concomitant administration of trastuzumab and radiotherapy] Cancer Radiother. 2009;13:276–280. doi: 10.1016/j.canrad.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 95.Halyard MY, Pisansky TM, Dueck AC, et al. Radiotherapy and adjuvant trastuzumab in operable breast cancer: tolerability and adverse event data from the NCCTG Phase III Trial N9831. J Clin Oncol. 2009;27:2638–2644. doi: 10.1200/JCO.2008.17.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shaffer R, Tyldesley S, Rolles M, et al. Acute cardiotoxicity with concurrent trastuzumab and radiotherapy including internal mammary chain nodes: a retrospective single-institution study. Radiother Oncol. 2009;90:122–126. doi: 10.1016/j.radonc.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 97.Horton JK, Halle J, Ferraro M, et al. Radiosensitization of chemotherapy-refractory, locally advanced or locally recurrent breast cancer with trastuzumab: a phase II trial. Int J Radiat Oncol Biol Phys. 2010;76:998–1004. doi: 10.1016/j.ijrobp.2009.03.027. [DOI] [PubMed] [Google Scholar]