A meta-analysis of adverse events with bevacizumab in patients with advanced cancer is reported.
Keywords: Bevacizumab, Vascular endothelial growth factor, VEGF, Anti-VEGF antibody, Cancer, Meta-analysis, Adverse events, Safety
Abstract
Objective.
We performed a meta-analysis on adverse events seen with bevacizumab to combine the existing evidence about its safety in patients with advanced cancer.
Methods.
A systematic literature search was conducted to identify published, randomized controlled trials of bevacizumab in cancer patients with data on adverse events available. The primary endpoint was “severe adverse event,” a composite of grade 3 and 4 adverse events. Secondary endpoints for the exploratory analysis were individual adverse events. We used random-effects meta-analysis to combine data.
Results.
Thirteen eligible publications were identified and eight trials reported the primary endpoint. Compared with the control group, the bevacizumab group had a slightly higher risk for any severe adverse event (pooled relative risk, 1.10; 95% confidence interval [95% CI], 1.01–1.19). The pooled risk difference was 7% (95% CI, 1%–13%), with a number needed to harm of 14 treated patients. Exploratory analyses showed a statistically significant higher risk for eight of the 15 evaluated secondary endpoints: bevacizumab was associated with a fourfold higher risk for hypertension, epistaxis, and gastrointestinal hemorrhage/perforation; a threefold higher risk for any bleeding events; and a lower, but elevated risk for proteinuria, leukopenia, diarrhea, and asthenia. No statistically significant differences were found for any thrombotic event (arterial or venous), hemoptysis, cardiac event, thrombocytopenia, neutropenia, impaired wound healing, or death related to an adverse event.
Conclusion.
Treatment with bevacizumab was associated with a slightly higher risk for any severe (grade 3 or 4) adverse event in patients with cancer. The result may impact individual benefit–risk assessments and policy guidelines.
Introduction
The molecularly targeted agent bevacizumab is a humanized recombinant monoclonal antibody against vascular endothelial growth factor (VEGF). Bevacizumab blocks the binding of VEGF to its receptors on vascular endothelial and other cells and was the first antiangiogenic agent approved by the U.S. Food and Drug Administration (in 2004) and the European Medicines Agency (in 2005). Bevacizumab treatment has led to longer survival times for patients with metastatic colon cancer when used in combination with cytotoxic chemotherapy [1–3], and for patients with non-small cell lung cancer [4], and it has led to longer progression-free survival times for metastatic breast cancer [5] and renal cell carcinoma [6] patients. The role of bevacizumab is also being explored for other tumor types: pancreatic cancer, ovarian cancer, hepatocellular carcinoma, soft-tissue sarcoma, prostate cancer, melanoma, and acute myelogenous leukemia [7–13].
In contrast to traditional cytotoxic chemotherapeutic agents, bevacizumab is generally well tolerated. However, in some trials, bevacizumab was associated with a higher risk for adverse events (AEs) as defined by the Common Toxicity Criteria of the National Cancer Institute (NCI-CTC): grade 1 (mild AE) to grade 4 (life-threatening AE) and grade 5 (death related to AE) [14]. Across all oncologic clinical trials, the grade 3–5 AEs were: gastrointestinal (GI) perforation, hemorrhage, and arterial/venous thromboembolic events. The most frequently observed AEs (overall incidence ≥10%) were hypertension, proteinuria, asthenia, and diarrhea [15]. However, the significance of these summary findings is controversial for two reasons: first, the assessment of multiple safety endpoints complicates the interpretation of the statistical significance of positive findings; and second, single trials were not powered to assess rare AEs, limiting the interpretation of negative findings. A meta-analysis assessing proteinuria and hypertension found a significant dose-dependent higher risk for these AEs with bevacizumab [16]. A pooled analysis of individual-level data from five randomized controlled trials showed a higher risk for arterial thromboembolism with bevacizumab [17]. Moreover, a recent meta-analysis concluded that the use of bevacizumab was significantly associated with a higher risk for developing venous thromboembolism in cancer patients [18]. However, other clinically relevant AEs such as GI perforation, bleeding, and leukopenia have not yet been systematically evaluated.
Therefore, we performed a comprehensive systematic meta-analysis on the safety of bevacizumab for the primary composite endpoint of any severe (grade 3 or 4) AE and explored individual AEs as secondary endpoints.
Methods
The Quality of Reporting of Meta-Analyses of Randomized Controlled Trials statement was followed for reporting the methods and results [19].
Endpoint Definition
The primary endpoint of our meta-analysis was “any grade 3 or 4 AE.” The secondary endpoints were analyzed separately and were individual AEs of grade ≥1 such as proteinuria, hypertension, cardiac events, any thrombotic events, any bleeding events, GI perforation or hemorrhage, hemoptysis, epistaxis, leukopenia, neutropenia, thrombocytopenia, diarrhea, impaired wound healing, and asthenia, or death related to an AE (grade 5). Individual AEs were chosen because either: (a) they had been reported previously as the most serious AEs (high incidence of grades 3–5) leading to treatment discontinuation, additional treatment, or death or (b) they were observed in ≥10% of all patients across oncologic clinical trials with bevacizumab [15, 20].
Literature Search
A systematic literature search of electronic databases (Embase, 1988 through August 2007; Medline, 1950 through October 2007; ISI Web of Science, 1990 through June 2007) was performed. The last search update in Medline was performed on May 12, 2008. The Medical Subject Headings, Emtree terms, and text key words for the search strategy were: “bevacizumab,” “anti-VEGF antibody,” “cancer,” “neoplasm,” and “clinical trial.” There were no language restrictions. The full search strategy is available upon request. We hand-searched the reference lists of review articles and of included studies to identify additional potentially eligible studies.
Inclusion Criteria
All randomized controlled trials comparing a treatment arm containing bevacizumab with chemotherapy alone (or with placebo) in patients with cancer were potentially eligible. Trials published in abstract form only were excluded because of the lack of precise AE data available. Trials were potentially eligible regardless of line of treatment and clinical trial phase. All randomized clinical trials (RCTs) reporting the primary endpoint or at least one of the secondary endpoints were included. Assessment of eligibility criteria for inclusion or exclusion was performed independently by two investigators (S.G.G., B.S.). Differences were resolved by consensus or were arbitrated by a medical oncology expert (R.M.).
Data Extraction and Quality Assessment
Extraction of study characteristics and AE data from the text, tables, and figures of included studies was performed independently by two investigators (S.G.G., U.S.) and differences were resolved by consensus. Study characteristics (first author, journal, year of publication), trial design characteristics (study design, outcome measures, type of cancer, therapy regime for each arm), study population (mean age, number of patients evaluated for efficacy and safety analysis in each arm), and AE results (type and number of AEs in the bevacizumab and control groups) were recorded. The Jadad score was assessed to evaluate study quality features for randomization, double blinding, and dropout rate/loss to follow-up [21].
Statistical Methods
A series of meta-analyses was performed for any severe (grade 3 or 4) AE (primary endpoint) and for individual AEs (secondary endpoints). For each endpoint, the AE relative risk (RR) and risk difference (RD) with 95% confidence interval (95% CI) for bevacizumab compared with chemotherapy alone (or with placebo) were calculated. The Q-test statistic was determined to examine heterogeneity between trials (at a p < .1 level) [22]. In addition, the I2 value, representing the percentage of total variability attributed to between-study heterogeneity, was calculated [23]. As a result of substantial heterogeneity of effects on most endpoints, a random-effects model was used to pool data across RCTs for each endpoint reported by at least two studies. The Mantel-Haenszel approach, as recommended by the Cochrane Collaboration for rare events and data from small studies, was used [24]. On the basis of pooled RDs, the number of patients treated with bevacizumab needed to harm (NNH) one patient was calculated for each endpoint. Funnel plots were visually assessed for the potential of publication bias. A funnel plot is a scatter plot of the intervention effect estimates (RRs) from individual studies against a measure of each study's precision (standard errors). In the absence of publication bias, the plot should approximately resemble a symmetrical (inverted) funnel [24]. A subgroup analysis was performed for the phase of clinical trial (phase II versus phase III) and for the dosage of bevacizumab (high dose versus low dose). The robustness of the result for the primary endpoint was tested in sensitivity analyses by reperforming the analyses using a fixed-effects model or by leaving one study out. In addition, we performed a sensitivity analysis using a composite endpoint of any severe AE of grade 3 or 4 or death related to an AE (grade 5). All statistical analyses were performed with Review Manager 5 (Cochrane Collaboration) [25].
Results
Systematic Literature Search
The literature search yielded 710 abstracts describing the use of bevacizumab as an anticancer agent. One hundred twenty-six articles were potentially eligible and the full-text publications were retrieved for further evaluation. One hundred thirteen publications did not meet all eligibility criteria. The two primary reasons for exclusion were: (a) not reporting a clinical trial (e.g., reviews) and (b) the reported trial was not randomized and controlled. Thirteen individual RCTs were finally included in the meta-analysis. In total, 6,436 patients were investigated in these trials and they had a variety of cancers: colorectal cancer (six trials), non-small cell lung cancer (three trials), breast cancer (two trials), and renal cell carcinoma (two trials). All included trials evaluated only patients with advanced or recurrent cancer. The selection process is summarized in Figure 1.
Figure 1.
Flow chart of selection process.
Study Characteristics
The 13 included RCTs were performed in 1998–2005 and the results were published in 2003–2008. The Jadad score ranged from two (poor) to five (excellent), with a median of three (good). Only five trials were explicitly double blinded. Sample sizes were in the range of 99–1,400 patients, with six trials including >500 patients each. The median follow-up time was in the range of 15.8–28 months for the five studies reporting this parameter. Five trials were phase II studies and eight were phase III studies. Six trials used overall survival as the primary endpoint; for the remaining trials, progression-free survival was the primary endpoint. Trial treatment regimens varied by tumor type, and the bevacizumab dose was in the range of 3–15 mg/kg. The mean age of study participants was in the range of 51.5–71 years (some studies only reported the median age). Table 1 reports the study and patient characteristics for the included trials.
Table 1.
Characteristics of randomized controlled trials of bevacizumab included in meta-analysis
Table 1.
(Continued)
aIFL–placebo arm already included in Hurwitz et al. (2004).
bThe bevacizumab plus erlotinib arm was not used for the meta-analysis.
Abbreviations: BV, bevacizumab; FOLFOX4, oxaliplatin + FU + LV; FU, fluorouracil; IFL, irinotecan + LV + FU; ITT, intention-to-treat analysis; LV, leucovorin; mBC, metastatic breast cancer; mCRC, metastatic colorectal cancer; mRCC, metastatic renal cell carcinoma; NR, not reported; NSCLC, non-small cell lung cancer; RCT, randomized controlled trial; XELOX, capecitabine + oxaliplatin.
AEs Reported in Trials
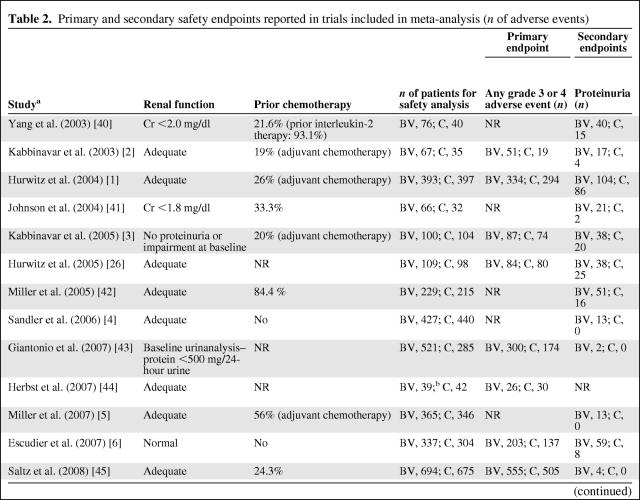
Reported individual AEs and grade 3 (severe) and 4 (life-threatening) AEs differed among studies (Table 2). In addition, we found considerable differences in the completeness of toxicity reporting among studies: only eight studies reported the primary endpoint of “any grade 3 or 4 AE.” AE data were available for hypertension, any thrombotic event, and bleeding events in all 13 studies, whereas only 12 studies reported data for proteinuria and 11 studies reported grade 5 events (death related to an AE). Nine studies described GI hemorrhage or perforation, seven studies reported data for epistaxis and diarrhea, and six studies described data for leukopenia and thrombocytopenia. Fewer than six studies provided data on neutropenia, hemoptysis, and cardiac events, with impaired wound healing reported in three studies and asthenia reported in two studies.
Table 2.
Primary and secondary safety endpoints reported in trials included in meta-analysis (n of adverse events)
Table 2.
(Continued)
Table 2.
(Continued)
aYear of publication.
bThe bevacizumab plus erlotinib arm was not used for the meta-analysis.
Abbreviations: BV, bevacizumab; C, control; GI, gastrointestinal; NR, not reported.
Heterogeneity
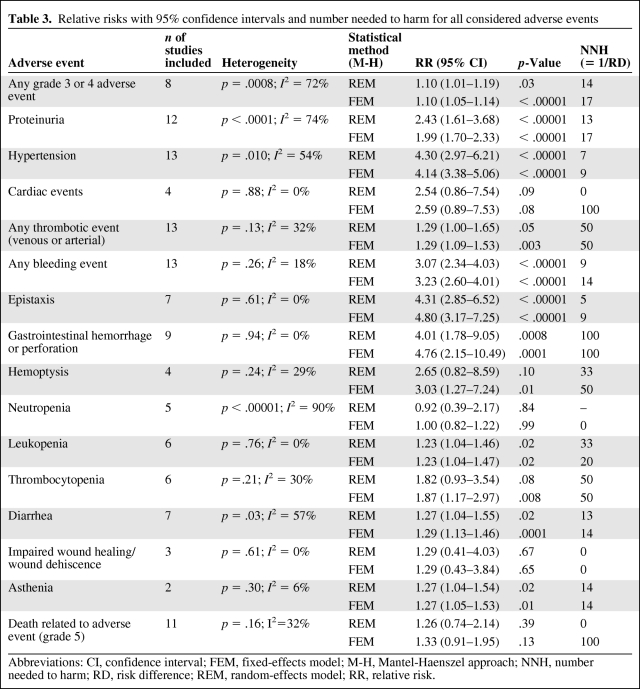
There was significant heterogeneity (Q-test p = .0008) for the primary endpoint “any grade 3 or 4 AE,” with an I2 value of 72%, indicating large differences in the RR among trials. In addition, there was substantial and statistically significant heterogeneity (I2 >50%; Q-test p < .1) across studies for the individual AEs of proteinuria, hypertension, neutropenia, and diarrhea. Based on this evidence of heterogeneity, random-effects models were employed for the meta-analysis.
Pooled Effects
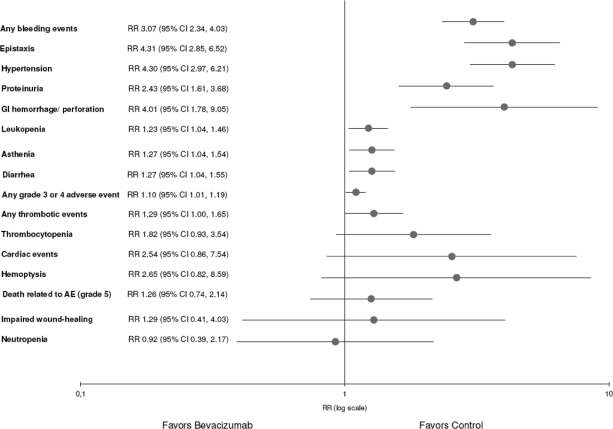
Compared with controls, bevacizumab was associated with a slightly higher risk for any grade 3 or 4 AE (Fig. 2). The pooled RR was 1.10 (95% confidence interval [CI], 1.01–1.19) and the pooled RD was 7% (95% CI, 1%–13%). Therefore, for every 14 patients treated with bevacizumab, one is harmed by a grade 3 or 4 AE (NNH = 14). An exploratory analysis showed that bevacizumab was associated with a fourfold higher risk for hypertension (RR, 4.30; 95% CI, 2.97–6.21), epistaxis (RR, 4.31; 95% CI, 2.85–6.52), and GI hemorrhage or perforation (RR, 4.01; 95% CI, 1.78–9.05). In addition, bevacizumab was associated with a threefold higher risk for any bleeding event (RR, 3.07; 95% CI, 2.34–4.03). Furthermore, bevacizumab was associated with a higher risk for proteinuria (RR, 2.43; 95% CI, 1.61–3.68), leukopenia (RR, 1.23; 95% CI, 1.04–1.46), diarrhea (RR, 1.27; 95% CI, 1.04–1.55), and asthenia (RR, 1.27; 95% CI, 1.04–1.54). No statistically significant differences were found for any thrombotic event (arterial or venous), hemoptysis, cardiac events, thrombocytopenia, neutropenia, impaired wound healing, or death related to an AE. The pooled RR for each AE and NNH are summarized in Table 3. In summary, an exploratory analysis showed a statistically significant higher risk for eight of the 15 evaluated individual secondary endpoints. Figure 3 provides an overview of the pooled RRs (sorted by p-value) and 95% CIs from the random-effects model meta-analyses.
Figure 2.
Forest plot for “any grade 3 or 4 adverse event,” with relative risk (random-effects model).
Abbreviations: CI, confidence interval; M-H, Mantel Haenszel.
Table 3.
Relative risks with 95% confidence intervals and number needed to harm for all considered adverse events
Abbreviations: CI, confidence interval; FEM, fixed-effects model; M-H, Mantel-Haenszel approach; NNH, number needed to harm; RD, risk difference; REM, random-effects model; RR, relative risk.
Figure 3.
Overview plot of pooled RRs (95% CIs) of the primary and all secondary endpoints (each line represents a meta-analysis of an adverse event).
Abbreviations: AE, adverse event; CI, confidence interval; GI, gastrointestinal; RR, relative risk.
Publication Bias
The funnel plot of the RR for “any grade 3 or 4 AE” (Fig. 4) was symmetric. Therefore, there is no evidence for publication bias. Similarly, most funnel plots for secondary endpoints did not indicate publication bias (data not shown).
Figure 4.
Funnel plot for the primary endpoint “any grade 3 or 4 adverse event.”
Abbreviations: RR, relative risk; SE, standard error.
Subgroup and Sensitivity Analyses
A subgroup analysis showed more severe AEs in both phase II trials (RR, 1.17; 95% CI, 0.97–1.42) and phase III trials (RR, 1.08; 95% CI, 0.98–1.8). However, the subgroup results were not statistically significant. The subgroup analysis for bevacizumab dose showed very similar results, and therefore heterogeneity cannot be explained by the bevacizumab dose. The pooled RR from studies with low-dose bevacizumab (<10 mg/kg) was 1.10 (95% CI, 1.02–1.20), whereas the pooled RR from studies with high-dose bevacizumab (10–15 mg/kg) was 1.13 (95% CI, 0.89–1.42).
We performed a sensitivity analysis for “any grade 3 or 4 AE,” excluding the publication of Hurwitz and colleagues [26] because their study included a control arm that was also used by another study included in our meta-analysis [1]. The sensitivity analysis excluding that study yielded a pooled RR of 1.12 (95% CI, 1.03–1.23), which was similar to the primary result. In an additional sensitivity analysis, the primary endpoint analysis was repeated using a fixed-effects model. The pooled result from the fixed-effects model (RR, 1.10; 95% CI, 1.05–1.14) was similar to the result from the random-effects model (Table 3). In our sensitivity analysis for the composite endpoint of “any severe AE of grade 3 or 4 or death related to an AE (grade 5),” only six studies could be included because of insufficient reporting of grade 5 events. This sensitivity analysis showed a pooled RR (1.14; 95% CI, 1.07–1.23) similar to that of our main analysis.
Discussion
Although randomized controlled trials investigating the addition of bevacizumab to chemotherapy demonstrated longer overall survival times in colorectal and non-small cell lung cancer [1–4] patients and longer progression-free survival times in breast cancer [5] and renal cell carcinoma [6] patients, the results of our meta-analysis suggest that, compared with controls, bevacizumab was also associated with a slightly higher risk for any grade 3 or 4 AE. In addition, our exploratory analysis suggested a likely elevated risk for several individual AEs: bleeding, epistaxis, GI perforation or hemorrhage, hypertension, proteinuria, leukopenia, asthenia, and diarrhea. In the following, some of these findings are described in detail and are compared with previously published results.
Although we identified 126 publications on our topic of interest, only 13 studies met the inclusion criteria. We consider it a strength of our study that we excluded nonrandomized studies, because in such studies confounding cannot be excluded. Our meta-analysis of studies across different indications indicated the potential for a higher risk for bleeding events of all grades for patients treated with bevacizumab than for controls. This finding is consistent with those of prior reviews on this topic, which were largely qualitative and focused on colorectal cancer patients [27–30]. Although none of the nine individual studies included in the meta-analysis reported a statistically significant difference for GI perforation or hemorrhage, our meta-analyses found a fourfold higher risk for these AEs in patients treated with bevacizumab. This result extends previous findings for GI perforation in colorectal cancer patients by evaluating bevacizumab for all tumor types [31]. Our analysis also increased the precision of the estimated risk for hematological events. Whereas six RCTs reported a statistically nonsignificant RR for leukopenia, our meta-analysis suggested a slightly higher risk. To our knowledge, this finding has not been reported previously. We confirmed previous findings of a higher risk for hypertension and proteinuria [16] by including six additional studies in our meta-analysis. A recent analysis of pooled data from five RCTs found that combination treatment with bevacizumab in patients with metastatic carcinoma was associated with a higher risk for arterial thromboembolism, but not venous thromboembolism [17], and a recent meta-analysis assessing venous thromboembolism found a significantly higher risk for this AE with bevacizumab treatment in cancer patients [18]. Our meta-analysis including aggregated data from all 13 RCTs for the composite endpoint “any thrombotic event” (including arterial and venous thrombotic events) resulted in a slightly higher risk in the bevacizumab group, with borderline statistical significance (p = .05).
Our meta-analysis differs in several aspects from prior studies. We analyzed a broader spectrum of AEs and, by using data from all published studies, we were able to include more studies than those analyses using pooled patient-level data [17, 32–34]. Furthermore, we assessed pooled RDs in order to calculate: (a) absolute rather than only relative figures and (b) NNH, a useful measure in clinical practice. Finally, we systematically assessed heterogeneity and publication bias.
Our study has several important limitations. First, our search was restricted to aggregated data from published studies. Therefore, AE categories were broad. For example, arterial and venous thromboembolic events had to be combined. Second, there was considerable variability in the type and extent of AE reporting among trials. Therefore, we used a composite endpoint that captured the most clinically important AEs: all NCI-CTC grade 3 or 4 AEs [15, 34]. Third, our analyses were based on trials primarily designed to demonstrate efficacy. Therefore, the sample size and time horizon of individual RCTs may not have been sufficient to detect rare AEs. By combining the best available evidence, we aimed to address at least the sample size limitation of individual trials to provide more precise risk estimates. Fourth, each patient may have contributed to multiple exploratory analyses of individual secondary endpoints. Therefore, these exploratory results are merely hypothesis generating rather than statistically testing hypotheses. However, it must be noted that eight of the 15 endpoints were statistically significant, exceeding the number that would be expected to occur by chance alone. Fifth, our meta-analysis pooled trials with heterogeneous cancer types; for example, intestinal perforation is more likely to occur in patients who have had abdominal surgery for colorectal cancer, whereas hemoptysis is more likely to occur in patients with lung cancer. We also pooled different patient populations, bevacizumab doses, treatment regimes, and clinical trial stages. To consider this heterogeneity in our analysis, we used random-effects models for all endpoints. In addition, we performed subgroup and sensitivity analyses for key differences. Finally, as in all meta-analyses, our results may be biased as a result of potential publication bias. However, a funnel plot evaluation for the primary endpoint did not indicate publication bias.
Our meta-analysis of bevacizumab AEs highlights a number of areas for further research. First, uniform and comprehensive reporting of safety data, even in phase II trials, would aid future meta-analyses of AEs. Second, detailed AE data are important to further palliative treatment goals in advanced cancer. A recently published health technology assessment [35] evaluated the relative clinical effectiveness of bevacizumab in terms of overall survival and also in terms of health-related quality of life, compared with current standard therapies. Because of the lack of quality-of-life data for bevacizumab therapy, the authors concluded that further RCTs should include assessments of the impact of treatment with bevacizumab on health-related quality of life. Although our analysis does not provide quality-of-life values, it provides the currently best available estimates for AE risks, which can be integrated with the positive quality-of-life effects of bevacizumab treatment. In addition, our results provide the most comprehensive and precise AE relative frequencies, which can be used to evaluate the net quality-of-life effect of bevacizumab as well as the trade-off among overall and progression-free survival, AEs, and cost-effectiveness for existing and future indications for bevacizumab [36–39].
Conclusion
This meta-analysis suggests that cancer therapy with bevacizumab is associated with a slightly elevated risk for developing any serious AE. The individual AE results provide comprehensive information for clinicians about the safety profile of bevacizumab and underline the importance of individual benefit–risk assessments and personalized decision making. The higher risk for AEs in patients treated with bevacizumab should be weighed against its benefits and be considered in clinical guidelines, bedside decision tools, and health technology assessments of bevacizumab-based cancer treatment. Future studies should be conducted to investigate the prevention and management of severe AEs resulting from bevacizumab treatment in cancer patients.
Acknowledgments
This study was partly supported by the Ludwig Boltzmann Institute of Health Technology Assessment, Vienna, Austria, and UMIT-University for Health Sciences, Medical Informatics and Technology, Hall i.T., Austria.
Author Contributions
Conception/Design: Sabine Geiger-Gritsch, Rebecca Miksad, Uwe Siebert, Claudia Wild
Collection and/or assembly of data: Sabine Geiger-Gritsch, Beate Guba
Data analysis and interpretation: Sabine Geiger-Gritsch, Rebecca Miksad, Uwe Siebert, Bjoern Stollenwerk
Manuscript writing: Sabine Geiger-Gritsch, Rebecca Miksad, Uwe Siebert
Final approval of manuscript: Sabine Geiger-Gritsch, Beate Guba, Rebecca Miksad, Uwe Siebert, Bjoern Stollenwerk, Claudia Wild
References
- 1.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 3.Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: Results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 4.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 5.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 7.Cohn DE, Valmadre S, Resnick KE, et al. Bevacizumab and weekly taxane chemotherapy demonstrates activity in refractory ovarian cancer. Gynecol Oncol. 2006;102:134–139. doi: 10.1016/j.ygyno.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 8.D'Adamo DR, Anderson SE, Albritton K, et al. Phase II study of doxorubicin and bevacizumab for patients with metastatic soft-tissue sarcomas. Journal of Clinical Oncology Oct. 2005;23(28):7135–7142. doi: 10.1200/JCO.2005.16.139. [DOI] [PubMed] [Google Scholar]
- 9.Karp JE, Gojo I, Pili R, et al. Targeting vascular endothelial growth factor for relapsed and refractory adult acute myelogenous leukemias: Therapy with sequential 1-beta-D-arabinofuranosylcytosine, mitoxantrone, and bevacizumab. Clin Cancer Res. 2004;10:3577–3585. doi: 10.1158/1078-0432.CCR-03-0627. [DOI] [PubMed] [Google Scholar]
- 10.Kindler HL, Friberg G, Singh DA, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033–8040. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 11.Rini BI, Weinberg V, Fong L, et al. Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting cells (Provenge) plus bevacizumab in patients with serologic progression of prostate cancer after definitive local therapy. Cancer. 2006;107:67–74. doi: 10.1002/cncr.21956. [DOI] [PubMed] [Google Scholar]
- 12.Varker KA, Biber JE, Kefauver C, et al. A randomized phase 2 trial of bevacizumab with or without daily low-dose interferon alfa-2b in metastatic malignant melanoma. Ann Surg Oncol. 2007;14:2367–2376. doi: 10.1245/s10434-007-9389-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhu AX, Blaszkowsky LS, Ryan DP, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:1898–1903. doi: 10.1200/JCO.2005.04.9130. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Common Terminology Criteria for Adverse Events. [accessed October 22, 2010]. Available at http://ctep.info.nih.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 15.Avastin® (Bevacizumab) - FDA Drug Label. 2008. [accessed October 22, 2010]. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/125085s145lbl.pdf.
- 16.Zhu X, Wu S, Dahut WL, et al. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: Systematic review and meta-analysis. Am J Kidney Dis Feb. 2007;49:186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 17.Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 18.Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA. 2008;300:2277–2285. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: The QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 20.European Medicines Agency. Avastin® (Bevacizumab) - Summary of Product Characteristics. [accessed October 22, 2010]. Available at http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000582/WC500029271.pdf.
- 21.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 22.Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care. 1990;6:5–30. doi: 10.1017/s0266462300008916. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.2 [updated September 2009] - The Cochrane Collaboration. [accessed April 18, 2008]. Available at http://www.cochrane-handbook.org.
- 25.Review Manager (RevMan) [Computer program]. Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. [accessed October 22, 2010]. Available at http://ims.cochrane.org/revman/download.
- 26.Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: An active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502–3508. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Hurwitz H, Saini S. Bevacizumab in the treatment of metastatic colorectal cancer: Safety profile and management of adverse events. Semin Oncol. 2006;33(suppl 10):S26–S34. doi: 10.1053/j.seminoncol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Lyseng-Williamson KA, Robinson DM. Bevacizumab: A review of its use in advanced colorectal cancer, breast cancer, and NSCLC. Am J Cancer. 2006;5:43–60. doi: 10.2165/00063030-200620030-00007. [DOI] [PubMed] [Google Scholar]
- 29.Saif MW, Mehra R. Incidence and management of bevacizumab-related toxicities in colorectal cancer. Expert Opin Drug Saf. 2006;5:553–566. doi: 10.1517/14740338.5.4.553. [DOI] [PubMed] [Google Scholar]
- 30.Motl S. Bevacizumab in combination chemotherapy for colorectal and other cancers. Am J Health Syst Pharm. 2005;62:1021–1032. doi: 10.1093/ajhp/62.10.1021. [DOI] [PubMed] [Google Scholar]
- 31.Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol Jun. 2007;14(6):1860–1869. doi: 10.1245/s10434-006-9337-9. [DOI] [PubMed] [Google Scholar]
- 32.Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: The addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:3706–3712. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- 33.Scappaticci FA, Fehrenbacher L, Cartwright T, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol. 2005;91:173–180. doi: 10.1002/jso.20301. [DOI] [PubMed] [Google Scholar]
- 34.European Medicines Agency. Avastin® (Bevacizumab) - European Public Assessment Report (EPAR) [accessed October 22, 2010]. Available at http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000582/WC500029260.pdf.
- 35.Tappenden P, Jones R, Paisley S, et al. Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer. Health Technol Assess. 2007;11:1–128. iii–iv. doi: 10.3310/hta11120. [DOI] [PubMed] [Google Scholar]
- 36.Drummond MF, Mason AR. European perspective on the costs and cost-effectiveness of cancer therapies. J Clin Oncol. 2007;25:191–195. doi: 10.1200/JCO.2006.07.8956. [DOI] [PubMed] [Google Scholar]
- 37.Earle CC, Chapman RH, Baker CS, et al. Systematic overview of cost-utility assessments in oncology. J Clin Oncol. 2000;18:3302–3317. doi: 10.1200/JCO.2000.18.18.3302. [DOI] [PubMed] [Google Scholar]
- 38.Earle CC, Coyle D, Evans WK. Cost-effectiveness analysis in oncology. Ann Oncol. 1998;9:475–482. doi: 10.1023/a:1008292128615. [DOI] [PubMed] [Google Scholar]
- 39.Siebert U. When should decision-analytic modeling be used in the economic evaluation of health care [editorial]? Eur J Health Econ. 2003;4:143–150. [Google Scholar]
- 40.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 42.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 43.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 44.Herbst RS, O'Neill VJ, Fehrenbacher L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol. 2007;25:4743–4750. doi: 10.1200/JCO.2007.12.3026. [DOI] [PubMed] [Google Scholar]
- 45.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]