The safety and tolerability of a continuous regimen of sorafenib combined with transarterial chemoembolization are assessed. Continuous administration of sorafenib at a dose of 400 mg bid combined with TACE was tolerable.
Keywords: Chemoembolization, Liver cancer, Phase I, Safety, Sorafenib, VEGF
Abstract
Background and Aim.
It is unknown whether sorafenib can be combined with transarterial chemoembolization (TACE) in patients with hepatocellular carcinoma. This study assesses the safety and tolerability of a continuous regimen of sorafenib combined with TACE.
Methods.
This was an open-label phase I study testing a continuous administration of sorafenib (dose escalation from 200 mg twice daily [bid] to 400 mg bid) starting 7 days prior to TACE with doxorubicin (50 mg).
Results.
Twenty-one patients were screened and 14 received sorafenib combined with TACE. Because there were no dose-limiting toxicities in the first three patients who received sorafenib at a dose of 200 mg bid, subsequent patients received 400 mg bid. Twenty-seven procedures were performed (median, two per patient) and two local therapy–related severe adverse events occurred. The median duration of sorafenib therapy was 246 days (range, 14–547 days). Sorafenib-related adverse events of grade ≥3 were hand–foot skin reaction (n = 3), weight loss (n = 2), diarrhea (n = 1), abdominal pain (n = 1), and thrombocytopenia (n = 3). After treatment with sorafenib and TACE, there was a significant decrease in the concentration of plasma vascular endothelial growth factor (VEGF) from 93 ng/l to 67 ng/l.
Conclusions.
Continuous administration of sorafenib at a dose of 400 mg bid combined with TACE was tolerable. The adverse event profile of this regimen was comparable with that of sorafenib monotherapy with the exception of thrombocytopenia, which may be more frequent. There were no increases in the circulating VEGF levels after TACE with this combined regimen. (Swiss Association for the Study of the Liver study number 25; ClinicalTrials.gov trial identifier, NCT00478374).
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and third most common cause of cancer-related death worldwide [1]. Untreated patients who are categorized as having Barcelona Clinical Liver Cancer (BCLC) stage B (i.e., intermediate stage) HCC have an estimated overall survival duration of 16 months [2]. Data from meta-analyses of randomized controlled trials have shown that, in selected patients with BCLC stage B HCC, transarterial chemoembolization (TACE) prolongs overall survival [3, 4]. However, in some patients, disease recurrence can occur; thus, strategies to further improve the survival of these patients are needed. Treatment with TACE elicits the secretion of growth factors, such as vascular endothelial growth factor (VEGF), from hypoxic cells at the periphery of the treated lesion [5]. This increase in the circulating VEGF level [5, 6] may further promote tumor growth [7]. It is therefore conceptually attractive to consider combining TACE with a systemic targeted therapy such as sorafenib, which is known to block the effects of these growth factors.
Sorafenib was the first systemic therapy to demonstrate a significant survival benefit in HCC patients [8, 9]. Sorafenib exerts its therapeutic benefit by inhibiting the Raf kinases CRAF, BRAF, and V600 BRAF, platelet-derived growth factor receptor β, Flt-3, and c-KIT and the kinase activity of VEGF receptor (VEGFR)-2 and VEGFR-3 [10]. The approval of sorafenib for treating HCC patients was based on data from a phase III randomized control trial, the Sorafenib HCC Assessment Randomized Protocol (SHARP) trial [8]. When a systemic targeted therapy is combined with doxorubicin, there is greater antitumoral activity than with monotherapy with doxorubicin, as shown in an experimental model [11] and in a clinical study (overall survival time, 13.7 months for sorafenib plus doxorubicin and 6.5 months for doxorubicin and placebo [12]), or with sorafenib alone (overall survival time, 10.7 months) [8]. This suggests that there is a rationale for combining sorafenib and doxorubicin TACE for the treatment of HCC.
There are theoretically three different strategies for combining sorafenib with TACE: the sequential approach, the interrupted approach, and the continuous approach [13]. The sequential approach involves treating patients with TACE, then initiating sorafenib treatment (as an adjuvant therapy) once the TACE sessions have finished. The interrupted approach involves placing patients on treatment with sorafenib between TACE sessions, and pausing sorafenib during TACE to avoid possible adverse events. In the continuous approach, patients are prescribed sorafenib without interruption before, during, and after TACE. Because growth factors are secreted in the hours following TACE, continuous treatment with sorafenib combined with TACE may be the most effective approach [7].
As with all anticancer therapies, both TACE and sorafenib monotherapy are known to be associated with complications and adverse events; what is unknown is how the combination of continuous sorafenib and TACE with doxorubicin is tolerated by patients with HCC. We designed a phase I study to determine the safety and tolerability of combining the continuous administration of sorafenib with TACE. The results of this study may form the basis of future phase II studies investigating the effectiveness of this combination for treating HCC.
Methods
Patients
Eligible patients were aged >18 years, had confirmed HCC (according to European Association for the Study of the Liver criteria [14]), were eligible for TACE, and were allowed prior treatment with TACE if delivered >1 month prior to study enrollment. Additional eligibility criteria were an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1 [15], a Child-Pugh liver function class of <10 points (Child Pugh class A or B) [16, 17], a life expectancy ≥12 weeks, adequate hematological function (platelet count, ≥60 × 109/l; hemoglobin, ≥9 g/dl; prothrombin time international normalized ratio ≤1.5; alanine aminotransferase [ALT] and aspartate aminotransferase [AST] ≤5× the upper limit of normal), and adequate renal function (serum creatinine ≤1.5× the upper limit of normal). Patients were excluded if they had previously received any other systemic treatment for HCC or had congestive heart failure (New York Heart Association class ≥2) [18], active coronary artery disease, cardiac arrhythmias requiring antiarrhythmic therapy, or thrombotic or embolic events within the past 6 months. Other exclusion criteria were: hypertension (defined as systolic blood pressure >150 mmHg or diastolic pressure >90 mmHg, despite optimal medical management); proteinuria (defined as a 24-hour urine protein excretion >1,000 mg); therapeutic anticoagulation with coumarin, heparins, or heparinoids; serious nonhealing wounds; and organ allograft. The protocol was amended on July 27, 2008 to ensure a more homogeneous patient population by restricting inclusion to patients with BCLC stage B HCC.
All patients provided written informed consent before enrollment into the study. The study was approved by the institutional review board and complied with the provisions of the Good Clinical Practice guidelines and the Declaration of Helsinki and local laws.
Study Design
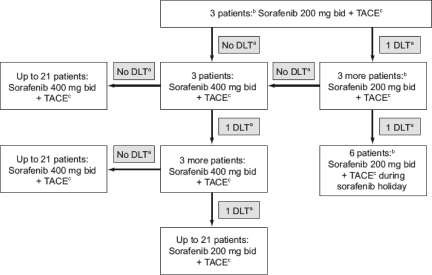
This was a phase I dose-escalation study designed to investigate the safety and tolerability of continuous administration of sorafenib combined with TACE for treating patients with HCC. The design of the study is depicted in Figure 1. All eligible patients were assigned to receive continuous oral treatment with sorafenib starting 7 days prior to TACE without a pause for TACE treatment. After obtaining arterial access, a diagnostic visceral arteriogram was performed using a 5-F Cobra-1 catheter or a 5-F VS-2 catheter (both Cook Medical Inc., Bloomington, IN) and a power injector to demonstrate arterial supply to the tumor and the presence of variant arterial anatomy, and to confirm portal vein patency. A catheter was then advanced selectively into the right or left hepatic artery, distal to the cystic artery. Using a microcatheter (2.7-F Progreat; Terumo, Somerset, NJ; or 2.8-F Renegade; Boston Scientific, Natick, MA) and a microwire (SilverSpeed .014”; ev3 Endovascular Inc., Plymouth, MN) to select a branch of the right or left hepatic artery, patients with unifocal tumors were treated with selective chemoembolization. Patients with multiple or diffuse lesions received lobar embolizations. Having safely positioned the catheter within the artery feeding the tumor, the chemoembolization mixture (emulsion of 50 mg doxorubicin mixed in a water-soluble contrast medium [Iomeron 300; Bracco, Milan, Italy] and an equivalent volume of ethiodized oil [lipiodol]) was infused into the artery using 3-cc syringes until stagnant flow was observed. As a peri-interventional prophylactic treatment to avoid the occurrence of infections, patients were treated with a single-dose of ceftriaxone (2 g i.v.) immediately prior to the procedure.
Figure 1.
Design of the phase I study.
aGrade 4 neutropenia and thrombocytopenia lasting >7 days; grade >3 febrile neutropenia; grade >3 nonhematological toxicities (excluding alanine aminotransferase and aspartate aminotransferase levels, alopecia, and nonpremedicated nausea or vomiting).
bOne month after their last session of TACE, these patients experienced an increase in the dose of sorafenib they received from 200 mg to 400 mg bid.
cTACE is repeated as often as necessary (determined by computed tomography review).
Abbreviations: bid, twice daily; DLT, dose-limiting toxicity; TACE, transarterial chemoembolization.
According to the study design, the first cohort of three patients was to receive 200 mg of sorafenib bid; if these patients experienced no dose-limiting toxicities during the 4-week period following TACE, these three patients were to be dose escalated to receive 400 mg bid. If dose-limiting toxicities were experienced by the first patient cohort, a further three patients were to be included in the study to receive sorafenib at 200 mg bid. Dose-limiting toxicities were defined as grade 4 neutropenia and thrombocytopenia lasting >7 days, febrile neutropenia grade >3, and nonhematological toxicities grade >3 (excluding ALT and AST levels, alopecia, and nonpremedicated nausea or vomiting).
If the first patient cohort went on to receive 400 mg bid of sorafenib without any dose-limiting toxicities, then up to 21 patients were to be included in the study to receive 400 mg sorafenib bid. Patients were monitored for the occurrence of adverse events on a monthly basis and received computed tomography (CT) scans 4 weeks after treatment with TACE. TACE was repeated if indicated in the 2 weeks following radiological assessment. When further treatment with TACE was not indicated based on the absence of hypervascularity, CT scans were scheduled at 3-month intervals after the first CT assessment. In the event of drug-related adverse events, treatment interruptions and dose reductions from 400 mg to 200 mg of sorafenib bid or 200 mg bid every other day were permitted. If further dose reductions were required, patients were to be withdrawn from the study. Study treatment continued until there was a deterioration in the patient's ECOG performance status score to 4, consent was withdrawn, or there was an occurrence of unacceptable adverse events or death. The study was designed by the principal investigator (J.F.D.) (Swiss Association for the Study of the Liver study number 25; ClinicalTrials.gov trial identifier, NCT00478374).
Outcomes and Assessments
The primary objective of the study was to assess the safety and tolerability of the continuous administration of sorafenib combined with TACE for treating patients with HCC by determining the maximum-tolerated dose. Safety was assessed according to the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 3.0 [19], in all patients who received at least one session of TACE. Patients visited the clinic every 4 weeks and at the end of treatment for the assessment of compliance and safety and for the determination of the occurrence and severity of adverse events. Compliance was monitored by counting the number of sorafenib pills patients had left at the end of each monthly period and by interviewing patients. Safety assessments included documentation of adverse events, clinical laboratory tests (hematological and biochemical analyses), a physical examination, and measurement of vital signs.
A secondary outcome of this study was the blood concentration of circulating VEGF prior to and after treatment with sorafenib and TACE.
Blood Sampling and Enzyme Immunoassay of VEGF Concentration
Peripheral venous blood was collected before and 20 hours after treatment with TACE and was combined with 1.6 mg EDTA per ml blood. Plasma was separated by centrifugation at 200 rpm for 15 minutes to eliminate platelets. Plasma was then stored at −70°C. VEGF levels were measured by commercially available enzyme-linked immunosorbent assay kits (Quantikine VEGF, DVE00; R&D Systems Europe, Ltd., Abingdon, U.K.). According to the manufacturer, the minimal detectable dose of VEGF was 9.0 ng/l. Samples were assayed in duplicate, and tests were performed according to the manufacturer's instructions on a microplate reader at 450 nm.
Results
Patients
From May 10, 2007 to January 27, 2009 (date of entry of last patient), 21 patients from one center in Switzerland were screened for inclusion in the study. Of these 21 patients, 15 met the eligibility criteria and started therapy with sorafenib. Six patients were excluded from the study during the screening period because of deterioration in liver function (n = 1), development of a second tumor (n = 1), impractical distance from the study center (n = 2), unconfirmed diagnosis of HCC (n = 1), and development of portal vein thrombosis (n = 1). A further patient was excluded from the study because TACE was not possible as a result of the presence of intrahepatic arteriovenous shunts. Fourteen patients received sorafenib during at least one session of TACE.
During the course of the study, TACE was delivered 27 times in total; the median number of TACE treatments was two per patient (range, 1–4). Doxorubicin (50 mg) was delivered 20 times and was given in combination with mitomycin twice; on five occasions embolization with lipiodol (5 ml) alone was performed because of the presence of thrombocytopenia (platelet count <60 × 109/l). The duration of treatment with sorafenib was in the range of 14–547 days, with a median of 246 days. Baseline and treatment characteristics of the 14 cirrhotic patients treated with sorafenib and TACE are given in Tables 1 and 2.
Table 1.
Baseline characteristics of the study population
aMaximum: 7 points.
bThe maximum number of tumor nodules in a single patient was 5.
Abbreviations: BCLC, Barcelona Clinical Liver Cancer; ECOG, Eastern Cooperative Oncology Group; INR, international normalized ratio.
Table 2.
Treatment characteristics for each patient
Safety
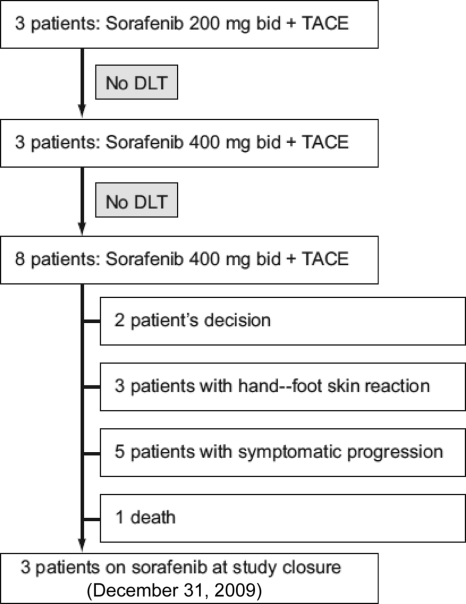
There were no dose-limiting toxicities in the first three patients who received 200 mg sorafenib bid combined with TACE; therefore, the dose of sorafenib was increased to 400 mg bid in these patients. Furthermore, none of the three subsequent patients who went on to receive 400 mg sorafenib bid combined with TACE developed any dose-limiting toxicities. This meant that the remaining eight patients were enrolled to receive 400 mg sorafenib bid plus TACE. An overview of the outcomes of the 14 patients who took part in this study is presented in Figure 2. Two patients (14%) withdrew their consent, three patients (21%) discontinued sorafenib therapy because of hand–foot skin reaction, five patients (36%) had symptomatic progression and stopped treatment with sorafenib, and one patient (7%) died from rapid deterioration. Three patients (3%) were still on treatment with sorafenib when the study closed on December 31, 2009.
Figure 2.
Patient enrollment and outcomes.
Abbreviations: bid, twice daily; DLT, dose-limiting toxicity; TACE, transarterial chemoembolization.
There were two severe adverse events that occurred after the first session of TACE (one acute cholecystitis, one hospitalization because of pain in the right thigh), and there was no prolongation of hospitalization resulting from post-TACE syndrome. The performance of TACE was not hindered by concurrent administration of sorafenib, and on no occasion was the transarterial approach or the intrahepatic catheterization of the tumor-feeding arteries precluded by vasoconstriction or vessel rarefaction.
The dose of sorafenib was reduced in three patients because of diarrhea (n = 1) and hand–foot skin reaction (n = 2). Treatment with sorafenib was interrupted in four patients because of weight loss (n = 1), diarrhea (n = 1), abdominal pain (n = 1), and surgery (n = 1). All drug-related adverse events are listed in Table 3 and laboratory abnormalities are reported in Table 4.
Table 3.
All drug-related adverse events reported during the study
Table 4.
Laboratory abnormalities reported during the study
VEGF Concentration
Prior to TACE, the circulating level of plasma VEGF was 93 ng/l (range, 12–219 ng/l), and after TACE it decreased significantly to 67 ng/l (range, 0–173 ng/l; p < .02, paired t-test).
Discussion
This is the first phase I study of its kind to assess the tolerability of the continuous administration of sorafenib in patients with inoperable HCC treated by TACE with doxorubicin. Because the number of recruited patients in our study was small, it is limited by its size. However, this study was intentionally designed to be small because it was a phase I study aiming to demonstrate the feasibility of combining sorafenib with TACE. Whether the profile of the adverse events and their incidence will be the same in larger populations remains to be investigated in phase II clinical trials.
The incidence of drug-related adverse events reported in our study was comparable with the frequency of adverse events reported with sorafenib [8] or TACE [20] monotherapy. Comparing the occurrence of adverse events reported in our study with those reported in a comparable patient population in the SHARP trial [8], hand–foot skin reaction occurred in three of 14 patients (21%) in our study, which is in line with the 21% observed in the SHARP trial. Diarrhea was noted in seven of 14 patients (50%) in our study, which was 11% more than the 39% reported in the SHARP trial; however, grade 3 diarrhea occurred in one patient (7%) in our study, which is similar to the 8% reported in the SHARP trial. In our study, 7% of patients experienced acute cholecystitis following TACE; this reflects the 5% of patients experiencing ischemic complications after TACE reported in the literature [20]. Abdominal pain was reported at a higher incidence in our study (28%, four of 14) than in the SHARP trial (8%); however, grade 3 abdominal pain only occurred in one patient in our study. In our study, no patient reported any episode of bleeding or the development of liver abscesses. Our study reported a treatment discontinuation rate of 21% (three of 14). This is lower than the 38% rate of treatment discontinuation reported in the SHARP trial.
Grade 3 thrombocytopenia was the only adverse event that appeared to be more frequent with sorafenib–TACE combination therapy than with sorafenib monotherapy, occurring at incidences of 21% and 4% with combination therapy and monotherapy, respectively. One study that investigated combination treatment with sorafenib and TACE for treating HCC (n = 14) reported a mean duration of the combined treatment of 12 months without any grade 3 or 4 adverse events [21]. However, that was a retrospective analysis in which sorafenib treatment was administered several months after the first session of TACE.
The rationale for administering sorafenib without pause during treatment with TACE was to prevent tumor growth in response to increased levels of VEGF as a result of the hypoxia elicited by TACE. Li et al. [22] reported that the plasma level of VEGF significantly increased from 64 ng/l to 103 ng/l after TACE. In contrast, we observed a significant decrease in the concentration of plasma VEGF from 93 ng/l to 67 ng/l after treatment with sorafenib and TACE. This suggests that sorafenib is able to prevent an increase in the circulating levels of this growth factor in response to TACE, and may therefore prevent tumor growth after TACE, which may translate into greater survival benefits than with TACE monotherapy. This study has limitations. The number of patients was small and precludes any efficacy analysis. But, this was a phase I study and its aim was to address feasibility and safety, not efficacy. The second limitation is the heterogeneity of the locoregional procedures. Five of the 27 locoregional therapies were without chemotherapy and have less significance in terms of safety than those delivering chemotherapy. However, none of them took place in the dose-escalation cohort. TACE was not done with drug-eluting beads, as it is performed most of the time now. A third limitation of the study is the lack of prospective collection of data on well-being just after locoregional treatments.
In conclusion, the results of this phase I study show that the combination of sorafenib at a dose of 400 mg bid and TACE is well tolerated in patients with HCC and may result in greater efficacy than with monotherapy with TACE or sorafenib in this patient population. Results from this study pave the way for phase II clinical trials into combined TACE and continuous sorafenib to be performed.
Acknowledgments
We are indebted to Bayer Schering Pharmaceuticals for financing this investigator-driven study and to N. Maradan and P. Weiss from Bayer Switzerland for their constant support.
Author Contributions
Conception/Design: Jean-François Dufour, Markus Borner, Daniel Candinas, Bettina Saar
Provision of study material or patients: Jean-François Dufour, Hanno Hoppe, Zsolt Szucs-Farkas, Ralph Kickuth, Markus H. Heim, Beat Helbling
Collection and/or assembly of data: Jean-François Dufour
Data analysis and interpretation: Jean-François Dufour, Olivier Maurhofer, Bettina Saar
Manuscript writing: Jean-François Dufour
Final approval of manuscript: Jean-François Dufour
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 3.Camma C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: Meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Feng GS, Zheng CS, et al. Influence of transarterial chemoembolization on angiogenesis and expression of vascular endothelial growth factor and basic fibroblast growth factor in rat with Walker-256 transplanted hepatoma: An experimental study. World J Gastroenterol. 2003;9:2445–2449. doi: 10.3748/wjg.v9.i11.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): The role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–921. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 7.Dufour JF, Johnson P. Liver cancer: From molecular pathogenesis to new therapies: Summary of the EASL single topic conference. J Hepatol. 2010;52:296–304. doi: 10.1016/j.jhep.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 9.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 10.Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 11.Piguet AC, Semela D, Keogh A, et al. Inhibition of mTOR in combination with doxorubicin in an experimental model of hepatocellular carcinoma. J Hepatol. 2008;49:78–87. doi: 10.1016/j.jhep.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Abou-Alfa GK, Huitzil-Melendez FD, O'Reilly EM, et al. Current management of advanced hepatocellular carcinoma. Gastrointest Cancer Res. 2008;2:64–70. [PMC free article] [PubMed] [Google Scholar]
- 13.Strebel BM, Dufour JF. Combined approach to hepatocellular carcinoma: A new treatment concept for nonresectable disease. Expert Rev Anticancer Ther. 2008;8:1743–1749. doi: 10.1586/14737140.8.11.1743. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 15.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 16.Child CG, editor. The liver and portal hypertension. Philadelphia: W.B. Saunders; 1964. Surgery and portal hypertension; pp. 50–64. [Google Scholar]
- 17.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 18.Heart Failure Society of America. The Stages of Heart Failure – NYHA Classification. [accessed April 23, 2010]. Available at http://www.abouthf.org/questions_stages.htm.
- 19.National Cancer Institute. National Cancer Institute's Common Terminology Criteria for Adverse Events v3.0. [accessed April 23, 2010]. Available at http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf.2006.
- 20.Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: Which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6–25. doi: 10.1007/s00270-006-0062-3. [DOI] [PubMed] [Google Scholar]
- 21.Sinakos E, Dedes I, Papalavrentios L, et al. Safety of transarterial chemoembolization plus sorafenib combination treatment in unresectable hepatocellular carcinoma. Scandinavian J Gastroenterology. 2010;45:511–512. doi: 10.3109/00365521003628335. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Feng GS, Zheng CS, et al. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10:2878–2882. doi: 10.3748/wjg.v10.i19.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]