Discordance in estrogen and human epidermal growth factor receptor 2 status between the primary breast cancer and metastasis is often interpreted in the context of changing tumor biology; however, a substantial number of these apparent changes in receptor status can be explained on technical grounds. False-negative results in metastatic biopsies can harm patients.
Abstract
Discordance in estrogen receptor and human epidermal growth factor receptor 2 receptor status between the primary tumor and recurrence is frequently reported in the literature. This is frequently interpreted as evidence for a change in the biology of breast cancer during the course of the disease. This commentary discusses some of the caveats of this interpretation. Discordant receptor results can be caused by any of 3 factors: (a) a genuine switch in the biology of the disease, (b) sampling error in focally receptor-positive cancers, and (c) limited accuracy and reproducibility of receptor assays. The relative contribution of each of these factors to discordant results is unknown. A switch in molecular class between primary and recurrent cancer (or residual cancer after therapy) appears to be a rare event based on the available limited molecular profiling data. Small pockets of strongly focally receptor-positive tumor nests in a larger receptor-negative cancer are also relatively infrequently seen. Discordance resulting from inherent limitations in assay reproducibility is evident from the frequently discordant receptor results even when the same samples are assessed in different laboratories (e.g., central versus local laboratory). A repeat tumor biopsy is clearly justified when it is suspected, on clinical grounds, that the original receptor results may have been false negative or when the diagnosis of metastatic disease is in question. However, routine repeat biopsy for receptor re-evaluation does not necessarily improve diagnostic accuracy and have a potential to harm through a false-negative result. For patients with clinical courses consistent with hormone responsiveness, or with prior positive hormone receptor results, a course of endocrine therapy is reasonable regardless of the most recent hormone receptor assay result.
Background
Breast cancer biopsies of local or distant recurrences often yield estrogen (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER)-2 results discordant from the original primary tumor specimen [1–11]. Discordance rates are in the range of 10% to 35%–40% and are also reported in studies that examine circulating tumor cells or serial biopsies during therapy [1–14]. These results are often interpreted as “true” alterations in receptor status and linked to changes in tumor biology. Frequently evoked biological explanations for changes in receptor status include: (a) intratumor heterogeneity (i.e., sampling error), (b) clonal selection, and, (c) variable ER-lineage differentiation of a putative disseminated breast cancer stem cell during the course of the disease [15].
High-throughput gene expression and comparative genomic hybridization studies revealed large-scale molecular differences between ER+ and ER− cancers, and to a lesser extent between HER-2+ and HER-2−, cancers [16, 17]. The limited amount of data that currently exists suggests that these large-scale genomic features of breast cancers remain stable during the course of the disease [18–20]. However, this does not preclude the possibility of acquiring smaller scale genomic changes such as mutations and alterations in the expression of individual genes during the evolution of the disease [21]. Although changes in receptor expression are biologically possible, the purpose of this paper is to draw attention to an important technical explanation for discordance in receptor status between primary and recurrent tumors.
Estimated Er Discordance Rates Resulting from Technical Variability in Repeated Measurements
Any two measurements of the same variable are expected to yield discordant results unless the method is 100% accurate and perfectly reproducible. Unfortunately, immunohistochemistry-based determination has unknown accuracy, because of the lack of an independent gold standard method to establish ER status. However, we know that current methods have less than perfect technical reproducibility. Variable staining results are a result of differences in tissue fixation, antigen retrieval, and staining methods [22–25]. Subjective scoring of results also contributes to less than perfect interobserver reproducibility. False-negative rates for ER status (relative to a reference laboratory) could be as high as 60%, even when the same specimens are analyzed by different laboratories [22]. The length of fixation can have a profound influence on ER positivity rates: some strongly ER+ tumors can become completely negative if the fixation time is reduced [25]. Despite important quality control initiatives, ER and HER-2 determinations remain variable in routine clinical practice even today [26, 27].
A simple statistical rule of combining probabilities gives an estimate of expected concordance rates based on correctly classified cases when the same assay is repeated on the same cohort of specimens and the misclassification error is assumed to be random: concordance = accuracy × accuracy. Therefore, a 90% accurate test that correctly identifies the true receptor status 90% of the time would yield 81% (0.9 × 0.9) concordance when repeated a second time on the same cases. Nineteen percent of receptor assignments would be discordant by chance alone. A greater technical discordance rate is expected if the original and repeat measurements use different methods and have different accuracies. For example, if the first test is 80% accurate and the second is 95% accurate, the expected discordance rate is 24%. The limited reproducibility of ER, PR, and HER-2 results across laboratories when the same samples are retested indicates that the technical variability of these assays is substantial. This certainly contributes to the discrepant receptor results reported in the literature. It is also important to recognize that, if the measurement error is not random but a result of a bias inherent to the sample (i.e., inappropriate preanalytical processing), the concordance rate would be higher but the diagnostic accuracy would not improve by repeating the assay because the same cases would be misclassified repeatedly.
Importantly, even if the most recent measurement is more accurate than the first assay, a discordant result does not necessarily indicate any real change in the biology of the disease. Unfortunately, a more recent measurement does not necessarily imply a more accurate test, particularly in hospital case series and retrospective chart reviews. In these instances, high rates of discordant results are expected because of the time differences between the two measurements. This often implies that different laboratories may have performed the assays using different preanalytical and staining procedures, and different pathologists have interpreted the results. These technical confounders are even more relevant in studies that examine receptor status concordance between circulating tumor cells and primary tumors because of the entirely different tissue sampling, fixation, and measurement methods that are applied to these specimens. Performing simultaneous repeated receptor measurement on the original primary tumor specimen and the repeat biopsy can minimize some of the analytical variability (e.g., antigen retrieval and staining method and scoring) but still cannot adjust for preanalytical variables (e.g., tissue handling before fixation and quality and length of fixation).
Clinical Implications
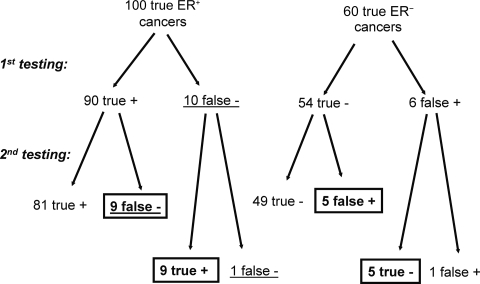
An important clinical question is whether or not a single repeat biopsy would improve diagnostic accuracy in terms of establishing the “true” receptor status of a recurrent cancer. If the original and repeat assay have similar sensitivities, specificities, and overall accuracies, the diagnostic precision will not improve (Fig. 1). The same number of cases will be misclassified during the second biopsy as during the first. A repeat measurement with an assay that has 90% sensitivity and specificity will correctly identify about 90% of cases that were incorrectly assigned to a negative status during the first time but will also misclassify 10% of the originally correctly assigned positive cases. The diagnostic conundrum is that considering only the two separate test results, one cannot determine whether the first or the second measurement is correct.
Figure 1.
Expected concordance rate when the same assay is repeated on the same patient cohort. In this example, the test has 90% sensitivity and specificity (overall accuracy of 90%), the measurement error is random, and the test is applied to 160 tumors. Cases in the boxes represent discordant results between two measurements: 9 + 9 + 5 + 5 = 28, corresponding to a 17.5% discordance rate. After the second test, the same number of cases remain false negative or false positive as after the first test, n = 10 (cases underlined), resulting in no overall improvement in diagnostic accuracy. If repeat testing is limited only to the false-negative cases of the first test, the discordance rate would be high (90%) but the overall diagnostic accuracy would improve substantially.
Abbreviation: ER, estrogen receptor.
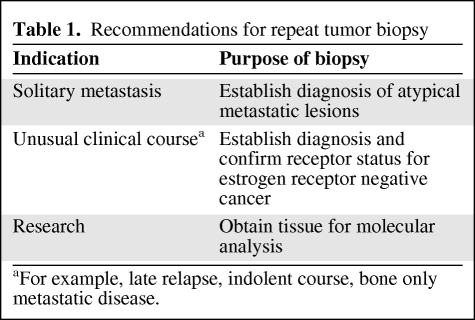
The utility of the repeat measurement changes if (a) the second assay is more accurate or (b) the cases that were most likely to be misclassified during the first test can be selectively identified for repeat testing. If a quality control problem is identified during the initial testing process, repeating the assay is necessary. Repeating hormone receptor measurements if the clinical course of the disease is not consistent with the typical biological behavior of ER− cancers (i.e., suspected false negative) is also appropriate. Similarly, if the diagnosis of metastatic disease is in question, a diagnostic biopsy is essential. Table 1 lists unequivocal indications for repeat tumor biopsies.
Table 1.
Recommendations for repeat tumor biopsy
aFor example, late relapse, indolent course, bone only metastatic disease.
An important caveat against routine repeat measurements of receptor status on all recurrent breast cancers needs to be considered. All studies show that patients with an ER− recurrence when the primary tumor was ER+ have shorter survival than patients with a concordant ER+ recurrence [3, 6–8]. This is often interpreted as proof that the biology of the disease has changed for the worse. However, shorter survival for patients with ER− cancer on a second biopsy is expected without any change in tumor biology. If the ER status of the primary tumor was false positive and the second biopsy is correct, these truly ER− patients are expected to have a worse prognosis than appropriately treated ER+ patients. If the repeat measurement is a false-negative ER result that leads to withholding endocrine therapy from a truly ER+ cancer patient, this will again result in an outcome inferior to that of properly treated patients with ER+ cancers. In a large institutional series in which repeat ER measurements were performed on metastatic cancer, 80% of the patients with an ER+ recurrence received endocrine therapy, compared with 12% of those with an ER− recurrence (p < .0001), even though in this latter group 46% had an ER+ primary tumor [8]. The potential for denying endocrine therapy because of a false-negative ER result on a second biopsy is troubling and should dampen enthusiasm for routine receptor evaluation on every patient, most notably when one positive result already exists.
It could be argued that this mistake is offset by the benefit of identifying potentially endocrine-sensitive cancers that were originally falsely labeled as ER−. Indeed, some studies report a better outcome for such patients than for patients with consistently receptor-negative cancers [7]; however, other studies have not observed a better outcome in this cohort [8]. Patients with ER− primary tumors who have ER+ cancers on repeat biopsy include some false-positive cases that would not benefit from endocrine therapy and some true positive cases who may. The relative contributions of these two types of result may influence the power of a retrospective analysis to show differences in outcome. In one study, a time-dependent effect was observed that may explain the conflicting reports. Patients with ER− primary tumors who had an ER+ recurrence within 1 year of diagnosis had the same poor survival as patients with ER− primary tumors, whereas those who had an ER+ recurrence at a later time had longer survival than patients with consistently ER− cancers [3]. These observations do not prove, but are consistent with, the possibility that the majority of receptor results were false positive in the early recurrence cohort, whereas the second cohort was enriched for true ER+ cases that were false negative at the time of the first biopsy.
Conclusion
In summary, a discordance in receptor status between the primary tumor and recurrence is a result of a combination of at least three factors: (a) limited accuracy and reproducibility of receptor assays, (b) sampling error in focally receptor-positive cancers, and (c) a genuine switch in the biology of the disease. The relative contribution of each of these factors to the overall discordance rate is unclear. Discordance resulting from the inherent technical limitations of the assays is common and is evident from the frequently discordant results obtained on the same samples assessed in different laboratories. Sampling error resulting from focal positivity is less common because cancers with small pockets of focally ER+ (or PR+ or HER-2+) tumor nests are relatively infrequent. A change in ER status resulting from a switch in the molecular class of breast cancer appears to be a rare event based on the available gene expression data. Repeat biopsy for receptor re-evaluation does not necessarily improve the diagnostic accuracy and has the potential to harm through a false-negative result. However, careful selection of patients to enrich for those who are most likely to have false-negative results in the primary tumor can result in a potential clinical benefit. As a practical hint for pathologists who perform ER and/or HER-2 assessment for a recurrent tumor, we suggest repeating the test simultaneously on both the primary and recurrent tumor specimens, and also considering using a confirmatory test (e.g., a fluorescence in situ hybridization assay for HER-2 or an mRNA-based measurement for ER) in cases of discordant results before rendering the final diagnosis. Though this policy will not completely eliminate false-positive and false-negative results (because of preanalytical variables), it can reduce the technical discordance rate. Finally, clinical judgment remains important. For patients with clinical courses consistent with hormone receptor–positive breast cancer, or with prior positive hormone receptor results, a course of endocrine therapy is reasonable regardless of the most recent hormone receptor assay result.
Author Contributions
Conception/Design: Lajos Pusztai
Collection and/or assembly of data: Lajos Pusztai, Giuseppe Viale, Catherine M. Kelly, Clifford A. Hudis
Data analysis and interpretation: Lajos Pusztai, Giuseppe Viale, Catherine M. Kelly, Clifford A. Hudis
Manuscript writing: Lajos Pusztai, Giuseppe Viale, Catherine M. Kelly, Clifford A. Hudis
Final approval of manuscript: Lajos Pusztai, Giuseppe Viale, Catherine M. Kelly, Clifford A. Hudis
References
- 1.Osborne CK. Heterogeneity in hormone receptor status in primary and metastatic breast cancer. Semin Oncol. 1985;12:317–326. [PubMed] [Google Scholar]
- 2.Li BD, Byskosh A, Molteni A, et al. Estrogen and progesterone receptor concordance between primary and recurrent breast cancer. J Surg Oncol. 1994;57:71–77. doi: 10.1002/jso.2930570202. [DOI] [PubMed] [Google Scholar]
- 3.Spataro V, Price K, Goldhirsch A, et al. Sequential estrogen receptor determinations from primary breast cancer and at relapse: Prognostic and therapeutic relevance. The International Breast Cancer Study Group (formerly Ludwig Group) Ann Oncol. 1992;3:733–740. doi: 10.1093/oxfordjournals.annonc.a058330. [DOI] [PubMed] [Google Scholar]
- 4.Simon R, Nocito A, Hübscher T, et al. Patterns of her-2/neu amplification and overexpression in primary and metastatic breast cancer. J Natl Cancer Inst. 2001;93:1141–1146. doi: 10.1093/jnci/93.15.1141. [DOI] [PubMed] [Google Scholar]
- 5.Holdaway IM, Bowditch JV. Variation in receptor status between primary and metastatic breast cancer. Cancer. 1983;52:479–485. doi: 10.1002/1097-0142(19830801)52:3<479::aid-cncr2820520317>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Guarneri V, Giovannelli S, Ficarra G, et al. Comparison of HER-2 and hormone receptor expression in primary breast cancers and asynchronous paired metastases: impact on patient management. The Oncologist. 2008;13:838–844. doi: 10.1634/theoncologist.2008-0048. [DOI] [PubMed] [Google Scholar]
- 7.Lower EE, Glass EL, Bradley DA, et al. Impact of metastatic estrogen receptor and progesterone receptor status on survival. Breast Cancer Res Treat. 2005;90:65–70. doi: 10.1007/s10549-004-2756-z. [DOI] [PubMed] [Google Scholar]
- 8.Liedtke C, Broglio K, Moulder S, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009;20:1953–1958. doi: 10.1093/annonc/mdp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simmons C, Miller N, Geddie W, et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol. 2009;20:1499–1504. doi: 10.1093/annonc/mdp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amir S, Ooi WS, Simmons C, et al. Discordance between receptor status in primary and metastatic breast cancer: An exploratory study of bone and bone marrow biopsies. Clin Oncol. 2008;20:763–768. doi: 10.1016/j.clon.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Aitken SJ, Thomas JS, Langdon SP, et al. Quantitative analysis of changes in ER, PR and HER2 expression in primary breast cancer and paired nodal metastases. Ann Oncol. 2009 doi: 10.1093/annonc/mdp427. [DOI] [PubMed] [Google Scholar]
- 12.Vincent-Salomon A, Pierga JY, Couturier J, et al. HER2 status of bone marrow micrometastasis and their corresponding primary tumours in a pilot study of 27 cases: A possible tool for anti-HER2 therapy management? Br J Cancer. 2007;96:654–659. doi: 10.1038/sj.bjc.6603584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neubauer H, Gall C, Vogel U, et al. Changes in tumour biological markers during primary systemic chemotherapy (PST) Anticancer Res. 2008;28:1797–1804. [PubMed] [Google Scholar]
- 14.Ignatiadis M, Xenidis N, Perraki M, et al. Different prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancer. J Clin Oncol. 2007;25:5194–5202. doi: 10.1200/JCO.2007.11.7762. [DOI] [PubMed] [Google Scholar]
- 15.Prat A, Perou CM. Mammary development meets cancer genomics. Nat Med. 2009;15:842–844. doi: 10.1038/nm0809-842. [DOI] [PubMed] [Google Scholar]
- 16.Gruvberger S, Ringnér M, Chen Y, et al. Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res. 2001;61:5979–5984. [PubMed] [Google Scholar]
- 17.Pusztai L, Ayers M, Stec J, et al. Gene expression profiles obtained from fine-needle aspirations of breast cancer reliably identify routine prognostic markers and reveal large-scale molecular differences between estrogen-negative and estrogen-positive tumors. Clin Cancer Res. 2003;9:2406–2415. [PubMed] [Google Scholar]
- 18.Weigelt B, Glas AM, Wessels LF, et al. Gene expression profiles of primary breast tumors maintained in distant metastases. Proc Natl Acad Sci U S A. 2003;100:15901–15905. doi: 10.1073/pnas.2634067100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacroix M, Toillon RA, Leclercq G, et al. Stable ‘portrait’ of breast cancer during, progression: Data from biology, pathology and genetics. Endocr Relat Cancer. 2004;11:497–522. doi: 10.1677/erc.1.00758. [DOI] [PubMed] [Google Scholar]
- 20.Bernards R, Weinberg RA. A progression puzzle. Nature. 2002;418:823. doi: 10.1038/418823a. [DOI] [PubMed] [Google Scholar]
- 21.Shah SP, Morin RD, Khattra J, et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461:809–813. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- 22.Rhodes A, Jasani B, Barnes DM, et al. Reliability of immunohistochemical demonstration of oestrogen receptors in routine practice: Interlaboratory variance in the sensitivity of detection and evaluation of scoring systems. J Clin Pathol. 2000;53:125–130. doi: 10.1136/jcp.53.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rd̈iger T, Höfler H, Kreipe HH, et al. Quality assurance in immunohistochemistry: results of an interlaboratory trial involving 172 pathologists. Am J Surg Pathol. 2002;26:873–882. doi: 10.1097/00000478-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein NS, Ferkowicz M, Odish E, et al. Minimum formalin fixation time for consistent estrogen receptor immunohistochemical staining of invasive breast carcinoma. Am J Clin Pathol. 2003;120:86–92. doi: 10.1309/QPHD-RB00-QXGM-UQ9N. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes A, Jasani B, Balaton AJ, et al. Study of interlaboratory reliability and reproducibility of estrogen and progesterone receptor assays in Europe. Documentation of poor reliability and identification of insufficient microwave antigen retrieval time as a major contributory element of unreliable assays. Am J Clin Pathol. 2001;115:44–58. doi: 10.1309/H905-HYC1-6UQQ-981P. [DOI] [PubMed] [Google Scholar]
- 26.Miller K, Ibrahim M, Barnett S, et al. Technical aspects of predictive and prognostic markers in breast cancer: What UK NEQAS data shows. Curr Diagn Pathol. 2007;13:135–149. [Google Scholar]
- 27.Alred DC. Commentary: Hormone receptor testing in breast cancer: A distress signal from Canada. The Oncologist. 2008;13:1134–1136. doi: 10.1634/theoncologist.2008-0184. [DOI] [PubMed] [Google Scholar]