The article examines fatigue associated with current targeted therapies for renal cell carcinoma.
Keywords: Fatigue, Renal cell carcinoma, Targeted therapy
Abstract
Fatigue is one of the most common symptoms associated with cancer. Persistent fatigue can impair multiple aspects of daily functioning and quality of life, and patients report that treatment-related fatigue has a greater impact than other symptoms, including pain, nausea, and depression. Thus, management of fatigue is recognized as an important component of care for patients with cancer. Treatment of advanced and metastatic renal cell carcinoma (RCC) was, until recently, limited to cytokine-based therapies, which are associated with modest response rates and significant toxicity, including high rates of treatment-related fatigue. The paradigm for RCC treatment has shifted dramatically in the last 5 years with the advent of efficacious targeted therapies. These agents provide the promise of better tolerability because of their more selective mechanisms of action. However, there is considerable variation in the selectivity of targeted agents for RCC, and a review of randomized clinical trials in patients with advanced and/or metastatic disease reveals that there is considerable variation in the tolerability of these agents. Fatigue remains a prominent toxicity with current targeted therapies. Future agents that show better selectivity and potency than current targeted therapies should help to provide better efficacy and tolerability.
Introduction
Fatigue has been estimated to affect 70%–100% of patients treated for cancer [1]. The causes of fatigue in patients with cancer are multifactorial and interrelated, although the precise underlying pathophysiology of the development of fatigue has yet to be elucidated [2]. Fatigue can arise as a result of the cancer itself or as a side effect of cancer treatment. The disruption of several physiological and biochemical systems is proposed to influence the development of cancer-related fatigue. Serotonin dysregulation, alterations in muscle and ATP metabolism, hypothalamic–pituitary–adrenal axis dysfunction, disruption of circadian rhythms, and increased cytokine production have been implicated [2]. Comorbid conditions can also contribute to the development of fatigue and include anemia, cachexia, depression, and sleep disorders [2]. Myelosuppression is a common side effect of many cancer treatments, and patients with myelosuppression often experience fatigue as a result of anemia [3]. Immunologic and targeted agents for cancer may also induce hypothyroidism, which can lead to fatigue [4, 5].
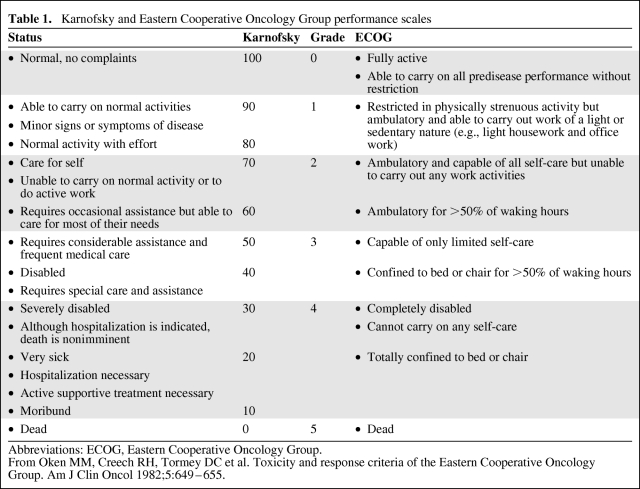
Fatigue is a multifaceted, subjective symptom [6]. Patients may associate fatigue with an overall lack of energy, cognitive impairment, somnolence, mood disturbance, or muscle weakness [7]. In clinical trials, fatigue is commonly graded using the National Cancer Institute Common Toxicity Criteria (NCI-CTC) and ranges from grade 1, whereby fatigue is greater than at baseline but with no impact on daily living, to grade 4, whereby patients are bedbound or experience severe fatigue-related disability [8]. The grading of fatigue is dependent on the functional assessment of patients, which is typically quantified using either the Eastern Cooperative Oncology Group (ECOG) scale or the Karnofsky performance status scale [9]. A comparison of these two scales is shown in Table 1 [10] and reveals that clear differences exist. In particular, the discrete increment for the Karnofsky scale is in contrast to the relatively broad grading system employed by the ECOG scale. The disparity between these two measures may contribute to the wide variation in reported incidence rates of fatigue observed among clinical trials (see below).
Table 1.
Karnofsky and Eastern Cooperative Oncology Group performance scales
Abbreviations: ECOG, Eastern Cooperative Oncology Group.
From Oken MM, Creech RH, Tormey DC et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–655.
Moderate fatigue (NCI-CTC grade 2), which is defined as a reduction in performance status (by one ECOG level or by 20% in Karnofsky score) or difficulties in carrying out daily activities [8], can place a considerable burden on patients when symptoms persist over time. Indeed, patients report that fatigue is the longest lasting and most intrusive side effect of chemotherapy, persisting longer than pain, nausea, and depression, and having a greater effect on daily life [11]. Persistent fatigue can impair multiple aspects of daily functioning and quality of life, including simple day-to-day physical activities, that impact on patients' ability to care for themselves [11, 12]. Reduced emotional and mental functioning, with feelings of hopelessness, isolation, lack of motivation, sadness, and frustration, and negative effects on social activities are also commonly reported [11, 12]. The onset of fatigue can have serious implications for treatment, requiring reductions in treatment dose until symptoms have resolved [13], which may impair the overall efficacy of therapeutic regimens.
Because grade 2 fatigue can impact many aspects of a patient's ability to function on a daily basis [11, 12], the tolerability of treatment-related grade 2 fatigue may be dependent on whether patients are likely to receive cancer treatment indefinitely or for a defined time period. Patients receiving time-limited cycles of therapy, in either the adjuvant or palliative setting, may be more willing to endure treatment-related grade 2 fatigue because they know these symptoms will cease once treatment is completed. For patients receiving chronic therapy, for advanced or metastatic disease, the aim of treatment is to control disease progression, thus maximizing quality of life. These patients are likely to remain on treatment until near the time of death, and, therefore, the experience of grade 2 fatigue may be less tolerable, particularly if combined with other treatment-related toxicities. Thus, efficacious treatment modalities that can be used long term with a low incidence of treatment-related fatigue could represent attractive treatment options for patients with advanced and or metastatic disease.
Despite its undoubted impact, fatigue in cancer patients is often underreported, underdiagnosed, and undermanaged [1]. This may be a result of the fact that the symptoms that characterize fatigue—feelings of tiredness, exhaustion, depression, feeling unwell, loss of motivation, and reduced capacity for mental work [6, 14, 15]—may not be recorded as fatigue per se. Further, the lack of a medical treatment for fatigue is likely to contribute to its undermanagement [11].
Renal Cell Carcinoma: An Overview
GLOBOCAN data from the International Agency for Research on Cancer showed that, in 2002, cancer of the kidney accounted for 1.9% of all new cases of cancer diagnosed and was responsible for 102,000 deaths worldwide [16]. It has been estimated that 90% of cancers of the kidney are renal cell carcinoma (RCC), and 70%–85% of these are of the clear-cell histology [17–19]. Approximately 30% of patients with RCC present with metastatic disease at initial diagnosis, and 40% of patients diagnosed with localized tumors subsequently develop metastases [20, 21]. Historically, advanced metastatic RCC has been one of the most treatment-resistant malignancies and was associated with a 5-year survival rate <10% [17]. However, over the past decade, this has increased to approximately 20% for metastatic RCC [22], and, if diagnosed early enough, the prognosis is generally good, with a 5-year survival rate of 96% if diagnosed at stage I [19].
Before 2006, treatment options for patients with metastatic RCC were limited to cytokine-based therapies, such as interleukin (IL)-2 and interferon (IFN)-α, which are associated with modest response rates (typically <20%) and significant toxicity [23]. Thus, there was a need for more efficacious and better tolerated therapeutic options. Since 2006, a number of new therapies have been developed that target and inhibit activity of the mammalian target of rapamycin (mTOR) pathway—temsirolimus (Torisel®; Wyeth Pharmaceuticals, Inc., Madison, NJ [24]) and everolimus (Afinitor®; Novartis Pharmaceuticals Corporation, East Hanover, NJ [25])—the vascular endothelial growth factor (VEGF) pathway—bevacizumab (Avastin®; Genentech, Inc., South San Francisco, CA) [26]—or multiple tyrosine kinase receptors, including VEGF receptors (VEGFRs) and platelet-derived growth factor receptors (PDGFRs). These multiple tyrosine kinase receptor inhibitors (TKIs) include sunitinib (Sutent®; Pfizer, Inc., New York) [27], sorafenib (Nexavar®; Bayer Pharmaceuticals Corporation, West Haven, CT) [28], and pazopanib (Votrient®; GlaxoSmithKline, Philadelphia) [29]. Sorafenib also inhibits the BRAF and CRAF serine/threonine kinases [30].
Assessment and Management Strategies for Cancer-Related Fatigue
The National Comprehensive Cancer Network (NCCN) guidelines define fatigue as a distressing, persistent, and subjective sense of tiredness related to cancer or cancer treatment that is not proportional to recent activity or usual functioning [1]. However, there is no standard definition of cancer-related fatigue, making consistent measurement and estimates of prevalence difficult [31]. Furthermore, the experience of fatigue in individual patients is subjective and can occur during different stages of the disease or treatment. No specific guidelines have been developed for the assessment and management of fatigue in RCC patients, although general guidelines for cancer-related fatigue are available.
The lack of a consensus definition of fatigue is coupled with the lack of well-validated assessment tools. Numerous assessment tools have been developed and include unidimensional and multidimensional scales and subscales that take into account measures of quality of life, psychosocial adjustment, mood, or self-reported health status [32]. A systematic review by Minton and Stone (2009) highlighted that a substantial number of these tools have not been well validated in cancer patients or assessed in a great number of studies [31]. Unidimensional scales confer the advantage of being easy to apply in clinical practice because of their short length [31]. Multidimensional scales are able to assess more aspects of the multifactorial nature of fatigue, but their length can hinder their clinical application [31]. Further validation of these tools in RCC patients is needed before any scale can be recommended for routine use.
Until recently, management of treatment-related fatigue was underrecognized by health care professionals. In a survey of 379 cancer patients, 40% of patients undergoing chemotherapy were not offered any treatment or advice for reducing fatigue, whereas bed rest was recommended for 37% of patients [12]. Growing focus on the quality of life of patients with cancer has led to a re-evaluation of this distressing side effect. The NCCN has developed guidelines for the standard of care of cancer-related fatigue, which recommend that fatigue be systematically screened and assessed in all patients using appropriate tools. When identified, fatigue should be managed according to clinical practice guidelines, which should be initiated from the start of therapy and continued after completion of therapy, as clinically indicated [1]. Etiologic factors that could contribute to fatigue experienced by cancer patients, such as anemia, pain, sleep disturbances, and depression, should also be assessed, because they are potentially treatable [32].
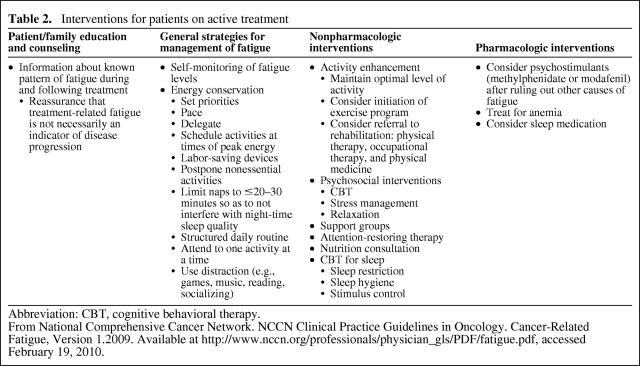
The NCCN advocates four types of intervention for fatigue (Table 2). The first strategy is education and counseling of the patient and family, which involves providing patients with guidance on the physical symptoms they are likely to experience prior to starting cancer therapies, so they know what to expect, and reassuring patients that fatigue is not a sign of disease progression [1, 33]. General strategies, such as self-monitoring, energy conservation, and distraction represent the second type of intervention for fatigue, and can also benefit patients experiencing treatment-related fatigue (Table 2) [1]. Energy conservation and management strategies teach patients to plan adequate periods of rest and inactivity into their daily routine, allowing them to maintain energy levels in order to carry out valued tasks [32, 33].
Table 2.
Interventions for patients on active treatment
Abbreviation: CBT, cognitive behavioral therapy.
From National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Cancer-Related Fatigue, Version 1.2009. Available at http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf, accessed February 19, 2010.
The third type of intervention advocated by the NCCN is nonpharmacologic. Such interventions include activity enhancement, psychosocial interventions, attention-restoring therapy, nutritional consultation, and sleep therapy [1]. Activity enhancement ranges from short periods of low-intensity exercise to structured rehabilitation programs that combine intensive exercise with physical training and therapy [32, 33]. Psychosocial interventions include cognitive–behavioral therapy, motivational coaching, and education on patterns of fatigue, coping strategies, and self-management approaches that may enhance patient morale by giving a sense of control over symptoms [32]. Pharmacologic interventions comprise the fourth NCCN-advocated strategy and include psychostimulants (for the treatment of depression, such as methylphenidate and modafinil), treatment for anemia (such as erythropoiesis-stimulating agents), and sleep medication (Table 2). The effectiveness of these management strategies in RCC patients and cancer patients in general needs to be fully evaluated in randomized controlled trials.
Fatigue Associated with the Current Treatments for RCC
Kinase inhibitor therapies are typically developed to inhibit a discrete number of key tumor-sustaining targets, such as PDGFRs, VEGFRs, and the receptor for stem cell factor (c-KIT), but most have a propensity to bind to a range of off-target kinases. The safety and tolerability of an agent is influenced by both on- and off-target kinase binding. Among the VEGF-targeted TKIs, most are associated with some degree of hypertensive effect, suggesting that this is an on-target side effect mediated by the VEGF pathway [34]. However, off-target binding affinities vary considerably among agents [35] and this variation may explain differences in their tolerability [13, 36]. Given the fact that these TKIs are often given chronically, unlike cytokine-based therapies, the management of side effects that may last over a long period of time becomes an important consideration for both patients and health care professionals. Although this limitation of some TKIs does not apply to bevacizumab or the mTOR inhibitors (temsirolimus and everolimus), because of their different mode of action, side effects still arise from nonspecific inhibition of the VEGF pathway (bevacizumab) and cell cycle arrest (mTOR inhibitors) [13, 36].
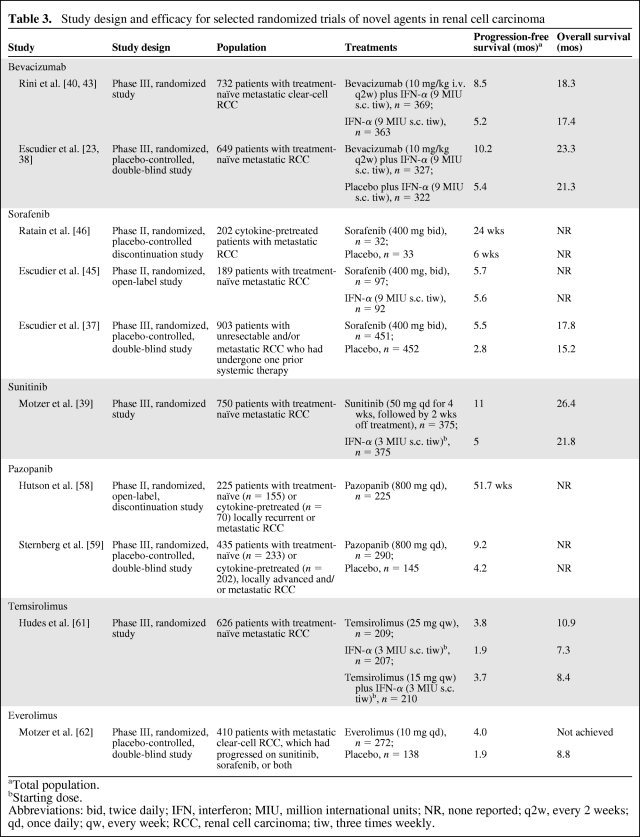
Recent clinical trials in RCC listed in Table 3 provide insight into the efficacy of these new targeted agents, and highlight the incidence of associated fatigue [37–40].
Table 3.
Study design and efficacy for selected randomized trials of novel agents in renal cell carcinoma
aTotal population.
bStarting dose.
Abbreviations: bid, twice daily; IFN, interferon; MIU, million international units; NR, none reported; q2w, every 2 weeks; qd, once daily; qw, every week; RCC, renal cell carcinoma; tiw, three times weekly.
IFN-α
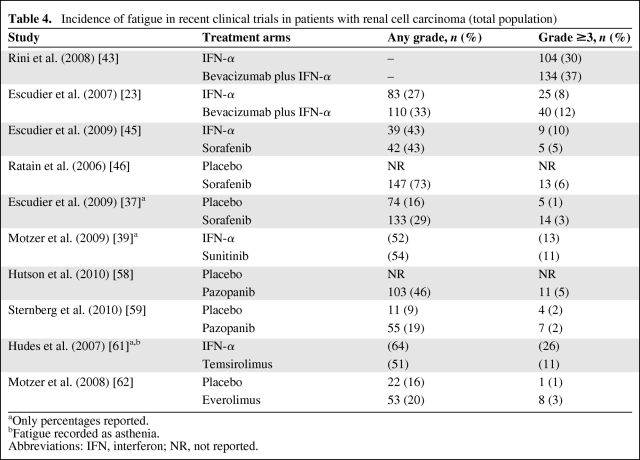
Until recently, IFN-α was one of the few treatment options available for RCC patients. However, it is associated with a poor tolerability profile, and treatment-related fatigue is a well-known and frequently severe side effect [4, 41]. Recent clinical trials have shown that up to two thirds of patients report fatigue during IFN-α therapy (Table 4), and earlier evidence suggested that dose reduction is necessary in 10%–40% of cases [4], making fatigue an almost inevitable and potentially therapeutically detrimental side effect of IFN-α treatment. The etiology of IFN-α treatment–related fatigue is multifactorial, with endocrine failure, neuropsychiatric disturbance, autoimmunity, and cytokine dysregulation reported to contribute to the onset of fatigue in these patients [4].
Table 4.
Incidence of fatigue in recent clinical trials in patients with renal cell carcinoma (total population)
aOnly percentages reported.
bFatigue recorded as asthenia.
Abbreviations: IFN, interferon; NR, not reported.
Bevacizumab
Bevacizumab is a monoclonal antibody that sequesters VEGF, thereby preventing signaling through VEFGRs [42]. Two phase III studies—the Cancer and Leukemia Group B (CALGB) 90206 trial and the Avastin for Renal Cell Cancer (AVOREN) trial—have evaluated the clinical utility of bevacizumab plus IFN-α versus IFN-α alone in patients with metastatic RCC [23, 43] (Table 3). The progression-free survival (PFS) interval was significantly longer with bevacizumab plus IFN-α than with IFN-α alone in both studies (CALGB 90206: 8.5 months versus 5.2 months; p < .0001. AVOREN: 10.2 months versus 5.4 months; p = .0001) [23, 43].
In the CALGB 90206 trial, bevacizumab plus IFN-α was associated with significantly more grade 3 fatigue than with IFN-α alone (35% [n = 127] versus 28% [n = 98]), whereas the rate of grade 4 fatigue was similar between groups (2% [n = 7] versus 2% [n = 6]) [43]. The total rate of all-grade fatigue was not reported. In contrast, the AVOREN study found that the rates of all-grade fatigue were 33% versus 27%, whereas the rates of grade 3 or 4 fatigue were 12% and 8% for bevacizumab plus IFN-α and IFN-α alone, respectively [23] (Table 4). The markedly lower rate of grade 3 or 4 fatigue reported for patients receiving bevacizumab plus IFN-α in that study relative to the Rini et al. [43] report (12% versus 37%) is surprising given the similarities between the study regimens and populations. This difference may be indicative of variations in the recording of fatigue between studies and suggests that comparison of fatigue rates between trials may not be reliable.
The study design also allowed for reduction of the IFN-α dose from 9 million international units (MIU) s.c. three times weekly to 6 or 3 MIU in the event of IFN-attributable toxicity [23]. A retrospective subgroup analysis of 136 patients receiving bevacizumab plus IFN-α who underwent dose reduction was conducted to generate hypotheses for future prospective studies. That analysis showed a concomitant reduction in the rate of all-grade fatigue from 21% to 8% of patients after 6 weeks, with a further reduction to 1% upon complete IFN-α withdrawal [44].
Sorafenib
Sorafenib, an oral multikinase inhibitor, is approved in several countries worldwide for the treatment of advanced RCC in patients who have failed prior IFN-α– or IL-2–based therapy or are considered unsuitable for such therapy [28]. It targets receptor tyrosine kinases, including VEGFR-2, VEGFR-3, PDGFR-β, Flt-3, and c-KIT [30].
Sorafenib has been evaluated in several studies in patients with metastatic or advanced RCC (Table 3). In a phase II study comparing sorafenib alone (n = 97) with IFN-α alone (n = 92) in first-line patients with metastatic RCC similar efficacies were observed (5.7 months versus 5.6 months) (Table 3) [45]. Fatigue was reported by 42 (43.3%) and 39 (43.3%) patients, respectively, including five (5.2%) and nine (10.0%) cases of grade 3 or 4 fatigue [45] (Table 4). Sorafenib-treated patients reported fewer kidney cancer–related symptoms than did IFN-α–treated patients (p = .015) [45]. Similarly, differences in quality of life, time to health status deterioration, and treatment satisfaction all favored sorafenib over IFN-α.
In a phase II randomized discontinuation study, 202 patients with metastatic RCC who had undergone prior surgery and/or cytokine therapy received open-label sorafenib for 12 weeks [46]. At the end of this period, patients with >25% tumor shrinkage continued on open-label sorafenib, patients with >25% tumor growth discontinued therapy, and the remainder were randomized to double-blind treatment with sorafenib or placebo for an additional 12 weeks. In total, 65 patients were randomized to sorafenib (n = 32) or placebo (n = 33), with results demonstrating a significantly longer PFS interval in the sorafenib arm than in the placebo arm (6.0 months versus 1.5 months; p = .0087) [46]. In the total population, fatigue was the most common treatment-related adverse event, reported at any grade for 73% of patients, including 6% with grade 3 or 4 fatigue (Table 4).
The phase III Treatment Approaches in Renal cancer Global Evaluation Trial (TARGET) of patients with unresectable and/or metastatic RCC who had undergone one prior systemic therapy and were randomized to either sorafenib (n = 451) or placebo (n = 452) demonstrated that the PFS time was significantly longer in the sorafenib arm than in the placebo arm (5.5 months versus 2.8 months; p < .000001) [37]. Overall, among patients receiving sorafenib and placebo, fatigue was experienced by 133 (29%) and 74 (16%) patients, respectively, including 14 (3%) and five (1%) patients with grade 3 or 4 fatigue [37] (Table 4). Of the 216 patients who crossed over from placebo to sorafenib, 53 patients (25%) reported any-grade fatigue whereas 10 patients (5%) had grade 3 or 4 fatigue [37]. Dose reductions or interruptions were relatively infrequent, with only 28% of patients receiving sorafenib having two or more such events, compared with 22% of patients receiving placebo.
Sunitinib
Sunitinib is an orally administered multitargeted TKI of, among others, VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-α, PDGFR-β, Flt-3, and c-KIT. The efficacy of sunitinib in metastatic RCC underwent clinical evaluation in a large-scale, randomized, phase III study that compared sunitinib (n = 375) with IFN-α (n = 375) in treatment-naïve patients (Table 3). In the intention-to-treat population, the PFS time was longer in the sunitinib-treated group than in those treated with IFN-α (PFS, 11 months versus 5 months; p < .001) [39]. Fatigue was the second most common adverse event (after diarrhea), reported by 54% of patients on sunitinib and by 52% of patients on IFN-α [39] (Table 4). Grade 3 fatigue was observed in 11% and 13% of patients, respectively. As with the comparison between sorafenib and IFN-α [45], these studies appear to show that fatigue is still common among patients receiving sunitinib alone.
Patient quality of life was also assessed using multiple measures [39, 47]. Patients receiving sunitinib reported better quality of life scores at each cycle than those receiving IFN-α, with significant differences in the overall scores. Patients treated with sunitinib experienced significantly less severe symptoms of lack of energy, weight loss, bone pain, fatigue, breathlessness, coughing, and fever than those receiving IFN-α (all p < .01). Updated results based on the final dataset confirmed these initial findings [48].
This pivotal study used an interrupted dosing schedule of 4 weeks on and 2 weeks off therapy, developed to reduce the potential impact of the toxicities of the treatment observed in animal models [49–51]. Some patients appreciate the 2-week interruption in sunitinib treatment, and many of the adverse events associated with the treatment are resolved during the “drug holiday,” including treatment-emergent fatigue [52, 53], which allows patients to plan/attend activities and events that they would otherwise be unable to participate in. However, a minority of patients experience tumor flare while off treatment [53, 54], which may lead to increased levels of fatigue as a consequence of tumor growth [52].
Pazopanib
The newest TKI to be approved in the U.S. for the treatment of advanced RCC is pazopanib [19, 29, 55], which is also currently undergoing phase III evaluation for the treatment of advanced/metastatic RCC. Although pazopanib is a potent inhibitor of VEGFR-2, it also shows activity against VEGFR-1, VEGFR-3, PDGFR-α, PDGFR-β, and c-KIT [56, 57]. Pazopanib showed markedly less activity against Flt-3 kinase in vitro than sunitinib and sorafenib [56]. Flt-3 is implicated in the differentiation and proliferation of hematopoietic stem cells, and the lack of its inhibition has been proposed as a rationale for the low rates of myelosuppression observed in clinical trials with pazopanib [56].
Pazopanib was evaluated in one phase II and one phase III study, both in advanced and/or metastatic RCC patients [58, 59] (Table 3). The phase II study was originally designed as a randomized discontinuation trial, but was changed to an open-label trial after a planned interim analysis gave an early indication of activity [58]. Overall, 225 patients (155 treatment naïve, 70 cytokine or bevacizumab pretreated) received open-label pazopanib. Pazopanib demonstrated promising efficacy results and was generally well tolerated. The incidence of grade 3 or 4 fatigue was low (5%) and fatigue/asthenia rarely led to discontinuation of pazopanib (<1% each) [58].
In the phase III study of treatment-naïve and cytokine-pretreated RCC patients, pazopanib was clinically efficacious, as demonstrated by a longer PFS interval in the overall study population (9.2 months versus 4.2 months; p < .0001), with outcomes being influenced by prior therapy (treatment naïve, 11.1 months versus 2.8 months; cytokine pretreated, 7.4 months versus 4.2 months) [59]. Any-grade fatigue was reported by 19% of patients, with the rate of grade 3 fatigue being very low (2%), and two patients in the placebo group (versus no patients in the pazopanib group) reported grade 4 fatigue (Table 4). Fourteen percent of patients in the pazopanib arm discontinued study treatment because of adverse events, compared with 3% of patients in the placebo arm. This study also assessed quality of life, the results of which showed no differences in the three preselected health-related quality of life endpoints between patients treated with pazopanib and those given placebo (p > .05) at any of the five assessment time points.
Temsirolimus
Temsirolimus is a specific inhibitor of the mTOR pathway, which is involved in cancer progression and metastasis [60]. The efficacy and safety of temsirolimus alone, IFN-α alone, and combination therapy were assessed in a phase III study (Global Advanced Renal Cell Carcinoma [Global ARCC]) of 626 poor-risk patients with advanced RCC [61]. The median PFS time was slightly longer with temsirolimus than with IFN-α alone (3.8 months versus 1.9 months; p < .001). All-grade asthenia was the most frequent adverse event, recorded for 51% of patients (grade 3 or 4, 11%) receiving temsirolimus alone (Table 4); 64% and 26% of IFN-α only, and 62% and 28% of IFN-α plus temsirolimus–treated patients reported all-grade and grade 3 or 4 asthenia, respectively. In the temsirolimus only and IFN-α only arms, 23% and 39% of patients, respectively, underwent at least one dose reduction. Based on these data, temsirolimus is advocated as first-line treatment in metastatic RCC patients with a poor prognosis [19].
Everolimus
To date, one randomized study of everolimus in advanced RCC patients has been published [62]. Patients who were refractory to sunitinib and/or sorafenib were randomized to either everolimus (n = 272) or placebo (n = 138). Patients treated with everolimus had a significantly longer PFS interval than patients given placebo (4.0 months versus 1.9 months; p < .0001) [62]. Following this finding, the independent data monitoring committee ended the study and patients who were initially randomized to placebo were crossed over to everolimus [63]; however, median overall survival times have yet to be reached. Analysis of the safety results showed that fatigue was the third most common adverse event, reported by 53 (20%) and 22 (16%) patients, respectively (Table 4), which suggests that everolimus treatment does not have as marked an effect on patient-reported fatigue as the treatments described above. Nonetheless, fatigue was among the four most common reasons for study discontinuation [62], suggesting that, in those patients who do experience this adverse event, the consequences can include interruption of cancer treatment.
Health-related quality of life assessments showed no significant clinically meaningful differences between everolimus and placebo in terms of physical functioning or global health status. Quality of life was sustained during treatment with everolimus relative to placebo, irrespective of the adverse effects that were experienced during the study [62].
Comparing Rates of Fatigue with the Current Treatments for RCC
Although it is tempting to speculate that some differences do exist between targeted therapies—for example, pazopanib and sorafenib generally demonstrated lower rates of severe fatigue than other treatments (Table 4)—such crosstrial comparisons can only provide information that is useful for hypothesis generation, which can only be validated by properly conducted, prospective, randomized, controlled trials [64]. Additionally, aside from the inherent limitations associated with crosstrial comparisons, it is notable that the incidence of fatigue associated with IFN-α varied considerably (any grade, 27%–64%; grade ≥3, 8%–30%) in the randomized trials of targeted agents versus IFN-α (Table 4). This variation means that a comparison between targeted therapies would not in any case provide any meaningful information, given that there is no similar “baseline” incidence of fatigue for IFN-α among the different studies. Therefore, head-to-head trials are essential to enable a robust comparison of targeted agents for the treatment of RCC. Indeed, several comparator phase III clinical trials are currently ongoing in patients with RCC, the results of which are much awaited.
The COMParing the efficacy, sAfety and toleRability of paZopanib (COMPARZ) versus sunitinib in first-line advanced and/or metastatic RCC trial (NCT00720941) is a randomized, open-label, phase III study in locally advanced and/or metastatic RCC patients [65]. Approximately 876 patients (aged ≥18 years with a Karnofsky performance scale score ≥70%) who received no prior systemic therapy for advanced or metastatic RCC will be randomized in a 1:1 ratio to receive either oral pazopanib (800 mg once daily) or oral sunitinib (50 mg), administered in 6-week cycles (4 weeks on treatment, followed by 2 weeks off treatment) [65].
The REnal Cell cancer treatment with Oral RAD001 given Daily-3 (RECORD-3) trial is an open-label, multicenter, phase II study that will assess the sequential activity of everolimus and sunitinib in metastatic RCC patients [66]. The study will compare the efficacy and safety of everolimus first line followed by second-line sunitinib versus sunitinib first line followed by second-line everolimus in 390 patients aged ≥18 years with a Karnofsky performance status score ≥70% [66].
Similarly, a third trial will investigate the efficacy and safety of temsirolimus versus sorafenib as second-line therapy in patients with advanced RCC who have failed first-line sunitinib [67]. This randomized, open-label, active control, single group trial will include approximately 480 patients aged ≥18 years with metastatic RCC, irrespective of histology or nephrectomy status, who progressed following first-line sunitinib therapy.
Finally, the phase III AXItinib versus Sorafenib (AXIS) trial is under way to compare axitinib—an oral selective inhibitor of VEGFR-1, VEGFR-2, VEGFR-3, and PDGFR—with sorafenib in approximately 540 patients with metastatic RCC who experienced failure on a first-line treatment (including sunitinib, bevacizumab plus IFN-α, temsirolimus, or cytokines) [68].
Need for Better Tolerated Therapies for RCC
As evidenced by the tolerability data from the studies described previously, fatigue continues to be a significant problem associated with the majority of available RCC treatments, with approximately half of patients reporting all-grade fatigue, and up to one third of patients reporting fatigue of grade 3 or 4 in severity. Furthermore, the effectiveness of the currently recommended fatigue management strategies in RCC patients needs to be evaluated in well-designed clinical trials.
There is a clear unmet need to improve the management of fatigue. This improvement can be partially achieved through the use of more efficacious screening, diagnosis, and management strategies [1], but to make a significant impact during treatment, the development of novel agents with better safety profiles than the currently approved agents for RCC is also required.
The findings of the clinical trials described above clearly demonstrate that the majority of the currently available agents for RCC treatment are significantly limited by their association with very high rates of patient-reported fatigue, which is particularly evident for the multi-TKIs. These agents are also frequently associated with gastrointestinal events, particularly nausea (any grade, active versus comparator, 7%–52% versus 4%–41%), vomiting (12%–31% versus 4%–28%), and diarrhea (20%–63% versus 3%–20%) [23, 37, 39, 43, 45, 46, 58, 59, 61, 62], which were more pronounced than with IFN-α in head-to-head studies [20, 45].
Conclusions
Fatigue represents one of the most common symptoms associated with cancer and its treatment. Despite its profound detrimental effect on the daily activities and subsequent quality of life of cancer patients, the etiology and mechanisms underlying fatigue remain poorly understood. Consequently, treatment options for fatigue are very limited, with most physicians recommending to their patients methods aimed at managing fatigue.
In contrast, the options available for treating RCC patients have evolved considerably, from cytokine-based therapies to targeted TKI agents. Nevertheless, these newer treatments are still associated with significant toxicities, including fatigue. Moreover, the long-term administration of these targeted therapies means that the management of these side effects over time is an important consideration for physicians and patients alike. Future TKIs that show higher selectivity and potency than the current targeted therapies should help to provide better efficacy and tolerability.
Future research should focus on elucidating the mechanisms by which fatigue occurs with different treatment modalities for RCC. More trials validating tools for the assessment of fatigue in RCC patients are also needed. Well-designed, adequately powered studies need to assess the impact of fatigue on quality of life from the patient perspective and should fully evaluate the effectiveness of fatigue management strategies.
Acknowledgments
This article was supported by an unrestricted educational grant from GlaxoSmithKline.
Author Contributions
Conception/Design: James M.G. Larkin, Linda M. Pyle, Martin E. Gore
Collection and/or assembly of data: James M.G. Larkin, Linda M. Pyle, Martin E. Gore
Data analysis and interpretation: James M.G. Larkin, Linda M. Pyle, Martin E. Gore
Manuscript writing: James M.G. Larkin, Linda M. Pyle, Martin E. Gore
Final approval of manuscript: James M.G. Larkin, Linda M. Pyle, Martin E. Gore
The authors take full responsibility for the content of the paper but thank Philip Li, Ph.D. (Medicus International, supported by GlaxoSmithKline) for his assistance in preparation of references, data/fact checking, grammatical assistance, assembling of tables for the manuscript, and collation and incorporation of comments and revisions at each draft stage.
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Cancer-Related Fatigue, Version 1.2009. [accessed February 19, 2010]. Available at http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf.
- 2.Ryan JL, Carroll JK, Ryan EP, et al. Mechanisms of cancer-related fatigue. The Oncologist. 2007;12(suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 3.Montoya L. Managing hematologic toxicities in the oncology patient. J Infus Nurs. 2007;30:168–172. doi: 10.1097/01.NAN.0000270676.59180.c3. [DOI] [PubMed] [Google Scholar]
- 4.Malik UR, Makower DF, Wadler S. Interferon-mediated fatigue. Cancer. 2001;92(6 suppl):1664–1668. doi: 10.1002/1097-0142(20010915)92:6+<1664::aid-cncr1494>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Torino F, Corsello SM, Longo R, et al. Hypothyroidism related to tyrosine kinase inhibitors: An emerging toxic effect of targeted therapy. Nat Rev Clin Oncol. 2009;6:219–228. doi: 10.1038/nrclinonc.2009.4. [DOI] [PubMed] [Google Scholar]
- 6.Iop A, Manfredi AM, Bonura S. Fatigue in cancer patients receiving chemotherapy: An analysis of published studies. Ann Oncol. 2004;15:712–720. doi: 10.1093/annonc/mdh102. [DOI] [PubMed] [Google Scholar]
- 7.Portenoy RK. Cancer-related fatigue: An immense problem. The Oncologist. 2000;5:350–352. doi: 10.1634/theoncologist.5-5-350. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute. Common Toxicity Criteria (CTC), Version 2.0. [accessed February 19, 2010]. Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4–30-992.pdf.
- 9.Conill C, Verger E, Salamero M. Performance status assessment in cancer patients. Cancer. 1990;65:1864–1866. doi: 10.1002/1097-0142(19900415)65:8<1864::aid-cncr2820650832>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 11.Curt GA. The impact of fatigue on patients with cancer: Overview of FATIGUE 1 and 2. The Oncologist. 2000;5(suppl 2):9–12. doi: 10.1634/theoncologist.5-suppl_2-9. [DOI] [PubMed] [Google Scholar]
- 12.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. The Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 13.Hutson TE, Figlin RA, Kuhn JG, et al. Targeted therapies for metastatic renal cell carcinoma: An overview of toxicity and dosing strategies. The Oncologist. 2008;13:1084–1096. doi: 10.1634/theoncologist.2008-0120. [DOI] [PubMed] [Google Scholar]
- 14.Cella D, Peterman A, Passik S, et al. Progress toward guidelines for the management of fatigue. Oncology (Williston Park) 1998;12:369–377. [PubMed] [Google Scholar]
- 15.Ahlberg K, Ekman T, Gaston-Johansson F, et al. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362:640–650. doi: 10.1016/S0140-6736(03)14186-4. [DOI] [PubMed] [Google Scholar]
- 16.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 18.Nelson EC, Evans CP, Lara PN., Jr Renal cell carcinoma: Current status and emerging therapies. Cancer Treat Rev. 2007;33:299–313. doi: 10.1016/j.ctrv.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Kidney Cancer, Version 2.2010. [accessed February 19, 2010]. Available at http://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf.
- 20.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 21.Kroog GS, Motzer RJ. Systemic therapy for metastatic renal cell carcinoma. Urol Clin North Am. 2008;35:687–701. doi: 10.1016/j.ucl.2008.07.007. ix. [DOI] [PubMed] [Google Scholar]
- 22.Belldegrun AS, Klatte T, Shuch B, et al. Cancer-specific survival outcomes among patients treated during the cytokine era of kidney cancer (1989–2005): A benchmark for emerging targeted cancer therapies. Cancer. 2008;113:2457–2463. doi: 10.1002/cncr.23851. [DOI] [PubMed] [Google Scholar]
- 23.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 24.Wyeth Pharmaceuticals. Torisel Summary of Product Characteristics. [accessed February 19, 2010]. Available at http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000799/WC500039912.pdf.
- 25.Novartis Everolimus Summary of Product Characteristics. [accessed February 19, 2010]. Available at http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001038/WC500022814.pdf.
- 26.Roche Pharma AG. Avastin Summary of Product Characteristics. [accessed February 19, 2010]. Available at http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000582/WC500029271.pdf.
- 27.Pfizer Sutent Summary of Product Characteristics. [accessed February 19, 2010]. Available at http://www.medicines.org.uk/emc/medicine/18531,
- 28.Bayer Healthcare. Nexavar Summary of Product Characteristics. [accessed February 19, 2010]. Available at http://www.medicines.org.uk/emc/document.aspx?documentid=18520&docType=SPC.
- 29.GlaxoSmithKline Votrient. Full Prescribing Information. [accessed February 19, 2010]. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022465lbl.pdf.
- 30.Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 31.Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF) Ann Oncol. 2009;20:17–25. doi: 10.1093/annonc/mdn537. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell SA. Cancer-related fatigue: State of the science. PM R. 2010;2:364–383. doi: 10.1016/j.pmrj.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Barsevick AM, Newhall T, Brown S. Management of cancer-related fatigue. Clin J Oncol Nurs. 2008;12(5 suppl):21–25. doi: 10.1188/08.CJON.S2.21-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Launay-Vacher V, Deray G. Hypertension and proteinuria: A class-effect of antiangiogenic therapies. Anticancer Drugs. 2009;20:81–82. doi: 10.1097/CAD.0b013e3283161012. [DOI] [PubMed] [Google Scholar]
- 35.Karaman MW, Herrgard S, Treiber DK, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 36.Porta C, Szczylik C. Tolerability of first-line therapy for metastatic renal cell carcinoma. Cancer Treat Rev. 2009;35:297–307. doi: 10.1016/j.ctrv.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the Phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 38.Escudier BJ, Bellmunt J, Negrier S, et al. Final results of the phase III, randomized, double-blind AVOREN trial of first-line bevacizumab (BEV) + interferon-α2a (IFN) in metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2009;27(15 suppl):5020. [Google Scholar]
- 39.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rini BI, Halabi S, Rosenberg J, et al. Bevacizumab plus interferon-alpha versus interferon-alpha monotherapy in patients with metastatic renal cell carcinoma: Results of overall survival for CALGB 90206. J Clin Oncol. 2009;27(18 suppl):LBA5019. [Google Scholar]
- 41.Dean GE, Spears L, Ferrell BR, et al. Fatigue in patients with cancer receiving interferon alpha. Cancer Pract. 1995;3:164–172. [PubMed] [Google Scholar]
- 42.Rini BI, Rathmell WK. Biological aspects and binding strategies of vascular endothelial growth factor in renal cell carcinoma. Clin Cancer Res. 2007;13:741s–746s. doi: 10.1158/1078-0432.CCR-06-2110. [DOI] [PubMed] [Google Scholar]
- 43.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melichar B, Koralewski P, Ravaud A, et al. First-line bevacizumab combined with reduced dose interferon-α2a is active in patients with metastatic renal cell carcinoma. Ann Oncol. 2008;19:1470–1476. doi: 10.1093/annonc/mdn161. [DOI] [PubMed] [Google Scholar]
- 45.Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1280–1289. doi: 10.1200/JCO.2008.19.3342. [DOI] [PubMed] [Google Scholar]
- 46.Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 47.Cella D, Li JZ, Cappelleri JC, et al. Quality of life in patients with metastatic renal cell carcinoma treated with sunitinib or interferon alfa: Results from a phase III randomized trial. J Clin Oncol. 2008;26:3763–3769. doi: 10.1200/JCO.2007.13.5145. [DOI] [PubMed] [Google Scholar]
- 48.Cella D, Michaelson MD, Cappelleri JC, et al. Quality of life (QOL) with sunitinib versus interferon-alfa (IFN-α) as first-line therapy in patients with metastatic renal cell carcinoma (mRCC): Final results. J Clin Oncol. 2009;27:6529. [Google Scholar]
- 49.Fiedler W, Serve H, Döhner H, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105:986–993. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- 50.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 51.Le Tourneau C, Raymond E, Faivre S. Sunitinib: A novel tyrosine kinase inhibitor. A brief review of its therapeutic potential in the treatment of renal carcinoma and gastrointestinal stromal tumors (GIST) Ther Clin Risk Manag. 2007;3:341–348. doi: 10.2147/tcrm.2007.3.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Speca J, Yenser S, Creel P, et al. Improving outcomes with novel therapies for patients with newly diagnosed renal cell carcinoma. Clin Genitourin Cancer. 2006;5(suppl 1):S24–S30. doi: 10.3816/cgc.2006.s.004. [DOI] [PubMed] [Google Scholar]
- 53.Wolter P, Beuselinck B, Pans S, et al. Flare-up: An often unreported phenomenon nevertheless familiar to oncologists prescribing tyrosine kinase inhibitors. Acta Oncol. 2009;48:621–624. doi: 10.1080/02841860802609574. [DOI] [PubMed] [Google Scholar]
- 54.Liu G, Jeraj R, Perlman S, et al. Pharmacodynamic study of FLT-PET imaging in patients treated with sunitinib. J Clin Oncol. 2008;26(15 suppl):3515. [Google Scholar]
- 55.U.S. Food and Drug Administration. Pazopanib. [accessed February 19, 2010]. Available at http://www.fda.gov/AboutFDA/CentersOffices/CDER/ucm187509.htm.
- 56.Kumar R, Crouthamel MC, Rominger DH, et al. Myelosuppression and kinase selectivity of multikinase angiogenesis inhibitors. Br J Cancer. 2009;101:1717–1723. doi: 10.1038/sj.bjc.6605366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sonpavde G, Hutson TE. Pazopanib: A novel multitargeted tyrosine kinase inhibitor. Curr Oncol Rep. 2007;9:115–119. doi: 10.1007/s11912-007-0007-2. [DOI] [PubMed] [Google Scholar]
- 58.Hutson TE, Davis ID, Machiels JP, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2010;28:475–480. doi: 10.1200/JCO.2008.21.6994. [DOI] [PubMed] [Google Scholar]
- 59.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 60.Kapoor A. Inhibition of mTOR in kidney cancer. Curr Oncol. 2009;16(suppl 1):S33–S39. doi: 10.3747/co.v16i0.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 62.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 63.Kay A, Motzer R, Figlin R, et al. Updated data from a phase III randomized trial of everolimus (RAD001) versus PBO in metastatic renal cell carcinoma (mRCC) [abstract 278]. Presented at the American Society of Clinical Oncology Genitourinary Cancers Symposium; Orlando, Florida. February 26–28, 2009. [Google Scholar]
- 64.Markman M. The dangers of “cross-trial” and “cross-retrospective experience” comparisons: Examples employing data in the peer-reviewed ovarian cancer literature. Cancer. 2007;109:1929–1932. doi: 10.1002/cncr.22645. [DOI] [PubMed] [Google Scholar]
- 65.U.S. National Institutes of Health. Pazopanib Versus Sunitinib in the Treatment of Locally Advanced and/or Metastatic Renal Cell Carcinoma (COMPARZ) [accessed February 19, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00720941.
- 66.U.S. National Institutes of Health. Efficacy and Safety Comparison of RAD001 Versus Sunitinib in the First-Line and Second-Line Treatment of Patients With Metastatic Renal Cell Carcinoma (RECORD-3) [accessed February 19, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00903175?term=record-00903173&rank=00903172.
- 67.U.S. National Institutes of Health. Temsirolimus Versus Sorafenib as Second-Line Therapy in Patients With Advanced RCC Who Have Failed First-Line Sunitinib. [accessed February 19, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00474786?term=temsirolimus+sorafenib&rank=00474785.
- 68.U.S. National Institutes of Health. Axitinib (AG 013736) as Second Line Therapy for Metastatic Renal Cell Cancer. [accessed February 19, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00678392?term=axitinib+sorafenib&rank=00678392.