Results of the trial that led to the U.S. Food and Drug Administration's recent approval of erlotinib as first-line maintenance treatment in patients with locally advanced or metastatic non-small cell lung cancer whose disease has not progressed (including stable disease) on first-line treatment with platinum-based chemotherapy are presented.
Keywords: Erlotinib, Non-small cell lung cancer, Maintenance treatment
Learning Objectives
After completing this course, the reader will be able to:
Evaluate the relationship between EGFR mutation status and clinical outcomes reported in this study.
Identify patients with NSCLC who may be appropriate candidates for first-line maintenance therapy with erlotinib.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Abstract
On April 16, 2010, the U. S. Food and Drug Administration (FDA) approved erlotinib tablets (Tarceva®; OSI Pharmaceuticals, Inc., Melville, NY) for maintenance treatment of patients with stage IIIB/IV non-small cell lung cancer (NSCLC) whose disease had not progressed after four cycles of platinum-based first-line chemotherapy.
In total, 889 patients received either erlotinib (150 mg) or placebo once daily. Progression-free survival (PFS), in all patients and in patients with epidermal growth factor receptor (EGFR)+ tumors by immunohistochemistry (IHC), was the primary efficacy endpoint. Overall survival (OS) was a secondary sponsor endpoint but was the primary regulatory endpoint.
Median PFS times were 2.8 months and 2.6 months in the erlotinib and placebo arms, respectively (hazard ratio [HR], 0.71; 95% confidence interval [CI], 0.62–0.82; p < .001). Median OS times were 12.0 months and 11.0 months, favoring erlotinib (HR, 0.81; 95% CI, 0.70–0.95). The PFS and OS HRs in patients with EGFR+ tumors by IHC were 0.69 (95% CI, 0.58–0.82) and 0.77 (95% CI, 0.64–0.93), respectively. The PFS and OS HRs in patients with EGFR− tumors by IHC were 0.77 (95% CI, 0.51–1.14) and 0.91 (95% CI, 0.59–1.38), respectively.
Following disease progression, 57% of placebo-treated patients received additional chemotherapy, compared with 47% of erlotinib-treated patients. Fourteen percent of placebo-treated patients received erlotinib or gefitinib, 31% received docetaxel, and 14% received pemetrexed. In total, 59% of placebo-treated patients who received treatment received FDA approved second-line NSCLC drugs.
The most common adverse reactions in patients receiving erlotinib were rash and diarrhea.
Introduction
Algorithms for the treatment of patients with locally advanced or metastatic (stage IIIB and stage IV) non-small cell lung cancer (NSCLC) are changing [1, 2]. For some U.S. Food and Drug Administration (FDA)-approved drugs, such as pemetrexed and bevacizumab, tumor histology is relevant, with treatment restricted to nonsquamous NSCLC patients [3–9]. For drugs such as erlotinib and gefitinib, clinical and molecular markers predictive of treatment benefit, including gender, nationality, histology, smoking history, and epidermal growth factor receptor (EGFR) mutation status, are being evaluated [10]. Also, the practice of a drug holiday after four to eight cycles of chemotherapy in patients with an objective tumor response or stable disease (SD) in favor of immediate institution of maintenance therapy is being evaluated [11–13].
The present manuscript concerns the FDA's recent approval of erlotinib as first-line maintenance treatment in patients with locally advanced or metastatic NSCLC whose disease has not progressed (including SD) on first-line treatment with platinum-based chemotherapy. This is the FDA's second NSCLC maintenance therapy approval. Pemetrexed (Alimta®; Eli Lilly and Company, Indianapolis, IN) was approved in July, 2009 for the maintenance treatment of patients with nonsquamous NSCLC [14, 15].
Conduct of the erlotinib maintenance trial was originally an FDA-requested postmarketing commitment following erlotinib's accelerated approval on November 18, 2004 for the treatment of locally advanced or metastatic NSCLC after failure of at least one prior chemotherapy regimen. This study was also to evaluate the relationship between EGFR protein expression and clinical outcomes. The sponsor's primary efficacy endpoint was progression-free survival (PFS) in the total patient population and in the EGFR+ by immunohistochemistry (IHC) population. The sponsor's secondary and the FDA's primary efficacy endpoint was overall survival (OS).
Patients and Methods
A single multicenter, double-blind, randomized study was submitted to investigate the efficacy and safety of erlotinib in the maintenance setting following four cycles of first-line platinum-based chemotherapy in all study patients and in patients whose tumors were EGFR+ by IHC. All patients included in the study were required to provide a tumor tissue sample within 3 weeks prior to commencing chemotherapy. IHC for EGFR protein expression was performed using the EGFR pharmDX™ Kit (Dako, Glostrup, Denmark), according to the package insert. A positive EGFR expression status was defined as at least 10% of tumor cells stained for EGFR.
All patients successfully completed four cycles of an acceptable standard platinum-based chemotherapy combination in the absence of unacceptable toxicity and/or disease progression. Acceptable initial chemotherapy regimens included cisplatin or carboplatin with gemcitabine, paclitaxel and docetaxel, and also a cisplatin plus vinorelbine regimen. Doses and schedules of drug administration for these doublets were acceptable.
Patients without progressive disease (i.e., complete response [CR], partial response [PR], or SD) after four cycles of chemotherapy were eligible for randomization if they had a life expectancy ≥12 weeks, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0 or 1, a granulocyte count ≥1,500/mm3, a platelet count >100,000/mm3, a hemoglobin level ≥9.0 g/dl, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels <2.5× the upper limit of normal (ULN) in the absence of liver metastases or up to 5× ULN in cases of liver metastases, an alkaline phosphatase (ALP) level <2.5× ULN (if ALP was ≥2.5× ULN, AST and ALT were required to be <1.5× ULN; if ALP was ≥2.5× ULN in the presence of liver metastases, AST and ALT were required to be <5× ULN), and a normal serum calcium. Females of childbearing potential had to have a negative pregnancy test within 48 hours before starting erlotinib/placebo treatment. Male and female patients with reproductive potential had to use two effective methods of contraception. Written (signed) informed consent must be obtained. Patients with prior exposure to agents directed at the human epidermal growth factor receptor axis (e.g., gefitinib, cetuximab, trastuzumab) were excluded.
Randomization was conducted using an adaptive method (minimization) that ensured a balance between the treatment arms for the following factors: (a) EGFR protein expression by IHC (EGFR+ versus EGFR− versus EGFR undetermined), (b) stage of disease at the start of chemotherapy (IIIB versus IV), 3) ECOG PS score (0 versus 1), (d) chemotherapy regimen (gemcitabine plus cisplatin versus carboplatin plus docetaxel versus other), (e) smoking status (current smoker versus former smoker versus never smoked [current smokers included patients who had stopped smoking less than 1 year prior to randomization]), and (f) region (North America, South America, western Europe, eastern Europe, southeast Asia, and Africa).
The study was powered to perform two primary analyses, to compare PFS, from day 1 of chemotherapy, between the two treatment arms in all patients and in patients who had EGFR+ tumors by IHC. The required number of events and the related number of patients were calculated based on the PFS analysis for all patients. The median PFS time in the placebo arm was estimated to be 4 months. This estimate was based on the median PFS interval from the time of completion of chemotherapy in placebo arm patients with a CR, a PR, or SD as their best response in Study BO16411 (the Tarceva® Lung Cancer Investigation trial), which investigated the effect of chemotherapy with or without erlotinib as first-line therapy in advanced NSCLC patients. In order to detect a 25% improvement in the median PFS interval with erlotinib compared with placebo (hazard ratio [HR], 0.8) with an 80% power at a two-sided 3% significance level and one efficacy interim analysis after 50% events, 731 events (progression or death) were required. Assuming 18 months' accrual, 6 months' follow-up of the last patient, and a 5% dropout rate over 2 years, 427 randomized patients per arm were required. If 50% of patients had EGFR+ tumors by IHC (based on a phase III erlotinib trial in patients with advanced NSCLC after failure of at least one prior chemotherapy regimen, wherein 47% of the patients with known EGFR status by IHC were EGFR−), this would lead to approximately 215 randomized patients per arm for testing the treatment difference in terms of PFS for the EGFR+ by IHC population. The HR was expected to be smaller in this subset of patients. A test of the difference in PFS duration for the EGFR+ by IHC population at a two-sided significance level of 2% would have an 85% power to detect an HR of 0.7 (360 events expected) and a 66% power detect an HR of 0.75 (365 events expected).
The study was also powered for OS, assuming a 10-month median survival duration in the placebo arm. In order to detect a 25% improvement in the median survival time with erlotinib, compared with placebo (HR, 0.8), with an 80% power at a two-sided 5% significance level, 641 events (deaths) were required. Assuming 18 months' accrual, 427 randomized patients per arm, and a 5% dropout rate over 3 years, 641 events were expected to occur by approximately 15 months after the last patient was randomized.
Patients had clinical tumor assessments every 6 weeks during treatment for the first 48 weeks and then every 12 weeks. Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors. Tumor assessments were performed by computed tomography (CT), spiral CT, or magnetic resonance imaging (MRI). X-rays or ultrasound were not permitted to assess target lesions. For all patients eligible to be randomized to erlotinib/placebo, all MRI and CT scans collected both during chemotherapy and during the study were to be provided for central review. Patients experiencing a global deterioration in health status that required discontinuation of treatment without objective evidence of disease progression were classified as having “symptomatic deterioration.”
Biomarker analyses on patients' tumor and blood specimens were performed using technically validated assays to identify predictive indicators of clinical benefit from erlotinib therapy. In the present study, the EGFR protein expression level (by IHC), EGFR gene copy number by fluorescence in situ hybridization (FISH), polymorphism in EGFR intron 1, EGFR mutation status, and K-ras mutation status were investigated in preplanned and predefined analyses. Because of limited amounts of tumor tissue, the molecular analyses on patients' tumors were prioritized in the following order: protein expression level of EGFR (IHC), EGFR gene copy number (FISH), K-ras mutation status by DNA sequencing, and EGFR mutation status by DNA sequencing. The biomarker analysis from blood specimens examined EGFR intron 1 polymorphism (CA repeats).
Disease-related lung cancer symptoms were assessed using the Functional Assessment of Cancer Therapy–Lung (FACT-L), version 4, questionnaire. The FACT-L consists of 27 general health questions and nine lung cancer questions (FACT-L subscale). General health questions are grouped into four general health subscales, which include physical well-being, emotional well-being, social well-being, and functional well-being. The FACT-L subscale score consists of nine items, seven of which make up the Lung Cancer Subscale, which assesses symptoms commonly reported by lung cancer patients (e.g., shortness of breath, loss of weight, tightness in chest).
The FACT-L was given to patients at baseline and every 6 weeks until week 48 (or disease progression) and every 12 weeks after week 48. Patients who terminated treatment prior to the end of the study were given the FACT-L at their treatment termination visit. Patients were given the FACT-L prior to all other assessments and before they were given disease/tumor status information, to avoid potential biasing of patient responses.
All clinical adverse experiences (AEs) encountered during the clinical study were reported on the AE page of the case report from (CRF). The intensity of AEs was graded on a five-point scale (mild, moderate, severe, life-threatening, death resulting from AE) according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) for AEs (version 3) and was reported in detail on the CRF.
Results
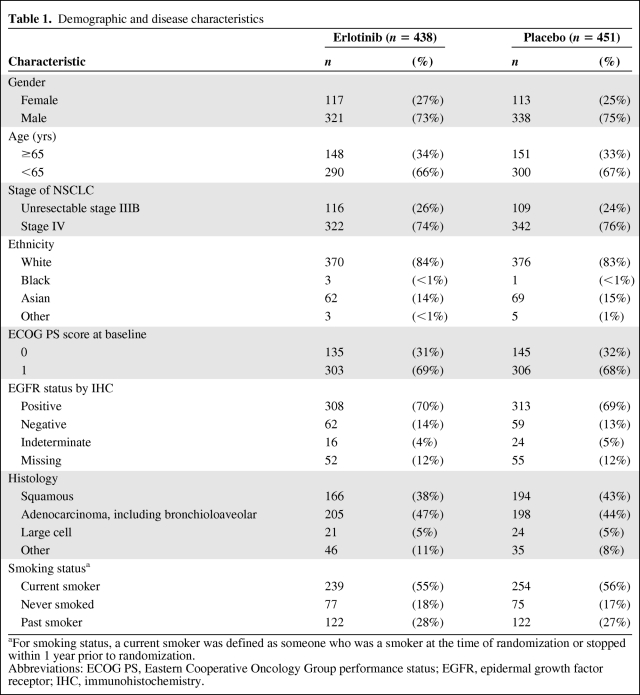
Demographic characteristics were balanced between the two treatment groups (Table 1). Approximately 15% of patients were Asian.
Table 1.
Demographic and disease characteristics
aFor smoking status, a current smoker was defined as someone who was a smoker at the time of randomization or stopped within 1 year prior to randomization.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; IHC, immunohistochemistry.
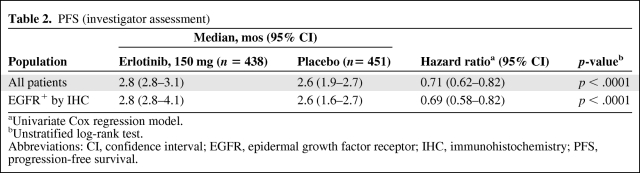
PFS times, based on investigator assessment, for the intent-to-treat (ITT) population (all randomized patients), are summarized in Table 2. There were 438 erlotinib-treated patients and 451 placebo-treated patients. Median PFS values in patients with EGFR+ tumors by IHC (erlotinib, 308; placebo, 313) were identical to those for the ITT population. The HRs were 0.71 (95% CI, 0.62–0.82) in the ITT population and 0.69 (95% CI, 0.58–0.82) in the EGFR+ by IHC population, with p < .0001 for both populations. The central review corroborated the assessment of PFS conducted by the investigators for all patients (HR, 0.71; 95% CI, 0.61–0.84; p < .0001) and for the EGFR+ by IHC population (HR, 0.66; 95% CI, 0.54–0.80; p < .0001).
Table 2.
PFS (investigator assessment)
aUnivariate Cox regression model.
bUnstratified log-rank test.
Abbreviations: CI, confidence interval; EGFR, epidermal growth factor receptor; IHC, immunohistochemistry; PFS, progression-free survival.
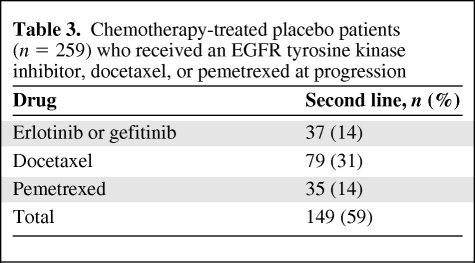
Table 3 indicates the percentage of placebo-treated patients who received an FDA approved second-line NSCLC drug at the time of disease progression. Of the 259 patients who received any drug, only 14% received erlotinib or gefitinib, 31% received docetaxel, and 14% received pemetrexed. Among Asian placebo-treated patients, 22% received either erlotinib or gefitinib at progression.
Table 3.
Chemotherapy-treated placebo patients (n = 259) who received an EGFR tyrosine kinase inhibitor, docetaxel, or pemetrexed at progression
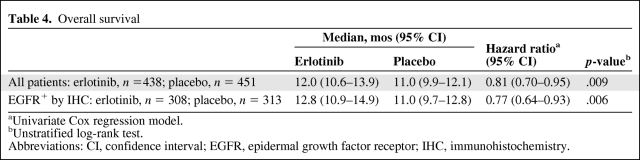
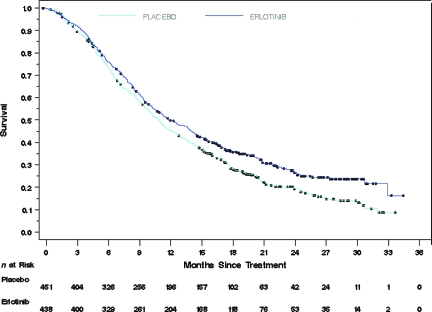
Table 4 summarizes the OS results for all study patients and for patients whose tumors were EGFR+ by IHC. As noted, the OS time was significantly longer in both erlotinib-treated patient populations than in placebo-treated patients. Figure 1 depicts the Kaplan–Meier curves for survival in the ITT population. Similar survival curves were observed in the EGFR+ by IHC population.
Table 4.
Overall survival
aUnivariate Cox regression model.
bUnstratified log-rank test.
Abbreviations: CI, confidence interval; EGFR, epidermal growth factor receptor; IHC, immunohistochemistry.
Figure 1.
Overall survival in the intent-to-treat population.
Time to symptom progression was a primary quality of life endpoint. There was no significant difference between erlotinib- and placebo-treated patients in this outcome (HR, 0.91; 95% CI, 0.74–1.12).
Non-prespecified analyses of outcome in relevant subgroups, that is, EGFR− by IHC patients, squamous cell patients, and patients with an EGFR-activating mutation produced tentative outcomes because of small sample sizes. Most striking were results in 49 patients (erlotinib, 22; placebo, 27) with an EGFR-activating mutation. The median PFS intervals were 10.3 months versus 3.0 months, favoring erlotinib (HR, 0.10; 95% CI, 0.04–0.25). However, the median survival difference was 0.2 months, favoring placebo (23.8 months versus 23.6 months; HR, 1.01; 95%, CI, 0.47–2.16).
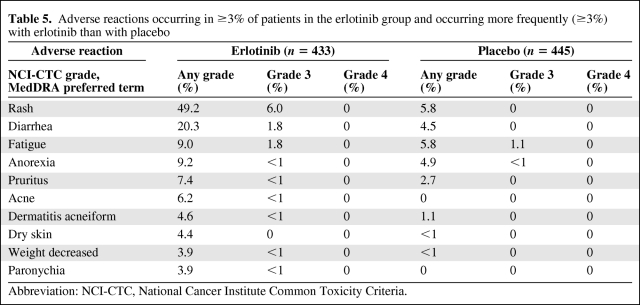
Adverse reactions, regardless of causality, that occurred in at least 3% of patients treated with single-agent erlotinib and at least 3% more often than in the placebo group in the randomized maintenance trial are summarized by NCI-CTC (version 3.0) grade in Table 5. The most common adverse reactions in patients receiving erlotinib were rash and diarrhea. Grade 3 rash and diarrhea occurred in 6.0% and 1.8% of patients, respectively. There was no grade 4 toxicity. Rash and diarrhea resulted in study discontinuation in 1.2% and 0.5% of erlotinib-treated patients, respectively. Dose reduction or interruption for rash and diarrhea was needed in 5.1% and 2.8% of patients, respectively. The onset of erlotinib-associated rash was within 2 weeks of treatment initiation in 66% of patients and within 1 month of treatment in 81% of patients.
Table 5.
Adverse reactions occurring in ≥3% of patients in the erlotinib group and occurring more frequently (≥3%) with erlotinib than with placebo
Abbreviation: NCI-CTC, National Cancer Institute Common Toxicity Criteria.
Liver function abnormalities (including elevated ALT, AST, and bilirubin) were observed in both erlotinib- and placebo-treated patients. Grade 2 (>2.5–5.0× ULN) ALT elevations occurred in 2% and 1% of patients, respectively, and grade 3 (>5.0–20.0× ULN) ALT elevations were observed in 1% and 0% of patients, respectively. Four percent of erlotinib-treated patients had grade 2 (>1.5–3.0× ULN) bilirubin elevations and <1% had grade 3 (>3.0–10.0× ULN) bilirubin elevations, compared with <1% for both grade 2 and grade 3 in the placebo group.
Discussion
When the reported trial was initiated, the standard of care for regionally advanced or metastatic NSCLC treatment was to administer four to six cycles of platinum-based doublet chemotherapy and then to discontinue treatment while continuing to regularly observe the patient until disease progression. At that time, depending on the patient's clinical status, additional treatment could be offered [16].
The present study, based on the above standard of care, is an attempt to determine the worth of adding erlotinib treatment immediately after completion of four cycles of platinum-based doublet chemotherapy in patients who do not have progressive disease. The proposed primary efficacy endpoint was PFS. The FDA's regulatory endpoint was OS.
In considering approval of this application, one of the issues that the FDA had to consider was the regulatory precedent. Bevacizumab was approved in October 2006 for the first-line treatment of patients with metastatic nonsquamous NSCLC in combination with carboplatin and paclitaxel. In that study, patients were randomized to either six cycles of paclitaxel and carboplatin (CP) or the same chemotherapy plus bevacizumab (BV/CP). After completion or discontinuation of chemotherapy, patients in the bevacizumab arm continued bevacizumab until disease progression or unacceptable toxicity. The study was not designed to determine whether continuing bevacizumab after chemotherapy is beneficial. In addition, some type of second-line treatment was given to 66% of CP-treated patients and 62% of BV/CP-treated patients. Chemotherapy was administered to approximately 50% of patients in each group. The median OS times were 12.3 in the bevacizumab arm and 10.3 months in the chemotherapy alone arm (HR, 0.80; 95% CI, 0.68–0.94; p = .013) [17].
Pemetrexed was approved in July 2009 for the maintenance treatment of patients with locally advanced or metastatic nonsquamous NSCLC whose disease has not progressed after four cycles of platinum-based first-line chemotherapy. In the study used to support that approval, patients with a response or SD were randomized to receive either pemetrexed or placebo after completion of chemotherapy. The median OS times in the overall population were 13.4 months in the pemetrexed arm and 10.6 months in the placebo arm (HR, 0.79; 95% CI, 0.65–0.95; p = .012) [14, 15].
Another issue was trial design. The current FDA preferred study design for a maintenance trial is that NSCLC patients who have an objective response or SD after four cycles of a platinum-containing doublet chemotherapy regimen are then randomized to either immediate maintenance therapy or delayed therapy to be started at the time of disease progression. An important point in the study design is that the identical drug or a closely related drug should be given to placebo-treated patients at disease progression so as to demonstrate that early maintenance therapy is better than delayed therapy. A published trial comparing immediate with delayed docetaxel after frontline NSCLC treatment is an example of this design [18]. In the current study, a greater proportion of patients in the placebo group (57%) received second-line treatment for NSCLC than in the erlotinib group (47%). Of the 259 patients in the placebo group who received second-line treatment, 37 (14%) received either erlotinib or gefitinib at first progression, 31% received docetaxel, and 14% received pemetrexed. In total, 59% of patients in the placebo group who received treatment at the time of tumor progression received FDA-approved second-line NSCLC drugs. In the pemetrexed maintenance trial, 67% of placebo-treated patients received second-line chemotherapy. Pemetrexed was received by 18.5%, docetaxel was received by 29% and erlotinib or gefitinib was received by 31%. As described in recent first-line and maintenance studies, approximately 30%–60% of patients with advanced NSCLC go on to receive second-line therapy after disease progression [19–21]. Therefore, in both the erlotinib and pemetrexed trials, an adequate percentage of patients who did not receive maintenance therapy did subsequently receive postprogression therapy with active NSCLC drugs.
Another issue concerns the fact that the placebo arm patients would be receiving “second-line and beyond” therapy, whereas erlotinib patients would be receiving “third-line and beyond” therapy. The possible influence of additional chemotherapy in the erlotinib arm compared with the placebo arm is uncertain, although it might be expected that third-line therapy would not have a large survival effect.
Exploratory nonprespecified subgroup analyses raised several questions that could not be definitively answered because of small patient numbers in the various groups. These included the role of maintenance erlotinib in patients with EGFR− tumors by IHC, whether patients with squamous cell cancer benefit from erlotinib maintenance chemotherapy, and the lack of survival benefit associated with the presence of an EGFR-activating mutation despite a dramatic benefit in terms of PFS. The latter finding might be a result of the small sample size (49 patients) and a limited number of events.
In summary, the present study demonstrated a survival benefit associated with erlotinib maintenance therapy, compared with placebo therapy, in the total study population and in patients with EGFR+ tumors by IHC. Although the trial design was not optimal in that it did not fully test the question of whether maintenance therapy is better than giving the identical or a similar drug at the time of progression, an additional postmarketing trial agreed to by the sponsor will answer that question. In addition, going forward, the FDA will recommend that maintenance trials in NSCLC randomize patients to either maintenance treatment or treatment at progression with the same drug regimen. OS will remain the regulatory endpoint for approval.
Acknowledgment
The views expressed are the result of independent work and do not necessarily represent the views and findings of the U.S. Food and Drug Administration.
Author Contributions
Conception/Design: Martin H. Cohen, John R. Johnson, Robert Justice, Richard Pazdur, Somesh Chattopadhyay, Shenghui Tang, Rajeshwari Sridhara
Collection and/or assembly of data: Martin H. Cohen, John R. Johnson, Robert Justice, Richard Pazdur, Somesh Chattopadhyay, Shenghui Tang, Rajeshwari Sridhara
Data analysis and interpretation: Martin H. Cohen, John R. Johnson, Robert Justice, Richard Pazdur, Somesh Chattopadhyay, Shenghui Tang, Rajeshwari Sridhara
Manuscript writing: Martin H. Cohen, John R. Johnson, Robert Justice, Richard Pazdur, Somesh Chattopadhyay, Shenghui Tang, Rajeshwari Sridhara
Final approval of manuscript: Martin H. Cohen, Robert Justice, Richard Pazdur
References
- 1.Gandara DR, Mack PC, Li T, et al. Evolving treatment algorithms for advanced non-small-cell lung cancer: 2009 looking toward 2012. Clin Lung Cancer. 2009;10:392–394. doi: 10.3816/CLC.2009.n.074. [DOI] [PubMed] [Google Scholar]
- 2.Stinchcombe TE, Socinski MA. Treatment paradigms for advanced stage non-small cell lung cancer in the era of multiple lines of therapy. J Thorac Oncol. 2009;4:243–250. doi: 10.1097/JTO.0b013e31819516a6. [DOI] [PubMed] [Google Scholar]
- 3.Peterson P, Park K, Fossella F, et al. Is pemetrexed more effective in adenocarcinoma and large cell lung cancer than in squamous cell carcinoma? A retrospective analysis of a phase III trial of pemetrexed vs docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC) J Thorac Oncol. 2007;2(suppl 4):s316–s317. [Google Scholar]
- 4.Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: A review of two phase III studiesr. The Oncologist. 2009;14:253–263. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 5.Ceppi P, Volante M, Saviozzi S, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer. 2006;107:1589–1596. doi: 10.1002/cncr.22208. [DOI] [PubMed] [Google Scholar]
- 6.Sigmond J, Backus HH, Wouters D, et al. Induction of resistance to the multitargeted antifolate pemetrexed (ALIMTA) in WiDr human colon cancer cells is associated with thymidylate synthase overexpression. Biochem Pharmacol. 2003;66:431–438. doi: 10.1016/s0006-2952(03)00287-9. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto H, Ozeki Y, Sato M, et al. Significance of thymidylate synthase gene expression level in patients with adenocarcinoma of the lung. Cancer. 2006;106:1595–1601. doi: 10.1002/cncr.21777. [DOI] [PubMed] [Google Scholar]
- 8.Gridelli C, Ardizzoni A, Douillard JY, et al. Recent issues in first-line treatment of advanced non-small-cell lung cancer: Results of an International Expert Panel Meeting of the Italian Association of Thoracic Oncology. Lung Cancer. 2010;68:319–331. doi: 10.1016/j.lungcan.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 11.Coate LE, Shepherd FA. Maintenance therapy in advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:723–734. doi: 10.1097/JTO.0b013e3181d86e8b. [DOI] [PubMed] [Google Scholar]
- 12.Mok TS, Ramalingam SS. Maintenance therapy in nonsmall-cell lung cancer: A new treatment paradigm. Cancer. 2009;115:5143–5154. doi: 10.1002/cncr.24563. [DOI] [PubMed] [Google Scholar]
- 13.Owonikoko TK, Ramalingam SS, Belani CP. Maintenance therapy for advanced non-small cell lung cancer: Current status, controversies, and emerging consensus. Clin Cancer Res. 2010;16:2496–2504. doi: 10.1158/1078-0432.CCR-09-2328. [DOI] [PubMed] [Google Scholar]
- 14.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: A randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 15.Gridelli C, Di Maio M. The role of pemetrexed as maintenance treatment in advanced NSCLC: A phase III randomized trial. Expert Opin Pharmacother. 2010;11:321–324. doi: 10.1517/14656560903485672. [DOI] [PubMed] [Google Scholar]
- 16.Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, 1997 by the American Society of Clinical Oncology. J Clin Oncol. 1997;15:2996–3018. doi: 10.1200/JCO.1997.15.8.2996. [DOI] [PubMed] [Google Scholar]
- 17.Cohen MH, Gootenberg J, Keegan P, et al. FDA drug approval summary: Bevacizumab (Avastin) plus carboplatin and paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. The Oncologist. 2007;12:713–718. doi: 10.1634/theoncologist.12-6-713. [DOI] [PubMed] [Google Scholar]
- 18.Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:591–598. doi: 10.1200/JCO.2008.17.1405. [DOI] [PubMed] [Google Scholar]
- 19.Scagliotti GV, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–4291. doi: 10.1200/JCO.2002.02.068. [DOI] [PubMed] [Google Scholar]
- 20.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 21.Brodowicz T, Krzakowski M, Zwitter M, et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: A phase III trial. Lung Cancer. 2006;52:155–163. doi: 10.1016/j.lungcan.2006.01.006. [DOI] [PubMed] [Google Scholar]