This paper summarizes the scientific review of the application leading to approval of ofatumumab for the treatment of chronic lymphocytic leukemia in the European Union.
Keywords: Ofatumumab, Chronic lymphocytic leukemia, CLL, EMA, European Medicines Agency
Learning Objectives
After completing this course, the reader will be able to:
Evaluate the evolving risk-benefit profile of ofatumumab when considering treatment in patients with CLL.
Identify patients with CLL who may be appropriate candidates for treatment with ofatumumab.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Abstract
On April 19, 2010, the European Commission issued a conditional marketing authorization valid throughout the European Union (EU) for ofatumumab (Arzerra®; Glaxo Group Ltd, Greenford, Middlesex, U.K.). The decision was based on the favorable opinion of the Committee for Medicinal Products for Human Use recommending a conditional marketing authorization for ofatumumab for the treatment of chronic lymphocytic leukemia (CLL) in patients refractory to fludarabine and alemtuzumab. A conditional marketing authorization means that additional data to confirm the benefit–risk balance of ofatumumab are awaited. The active substance of Arzerra® is ofatumumab, a monoclonal antibody medicinal product (ATC code L01XC10). The recommended dose is 300 mg of atumumab for the first infusion and 2,000 mg of atumumab for all subsequent infusions. The infusion schedule is eight consecutive weekly infusions, followed 4–5 weeks later by four consecutive monthly (i.e., every 4 weeks) infusions. Ofatumumab targets CD20, a cell surface marker of B lymphocytes, which is followed by cell lysis via complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity. The benefit of ofatumumab is the control of CLL in patients who are refractory to both fludarabine and alemtuzumab, which was indicated by a high response rate. The most common side effects are infections and infusion reactions. The objective of this paper is to summarize the scientific review of the application leading to approval in the EU. The detailed scientific assessment report and product information, including the summary of product characteristics, are available on the EMA website (http://www.ema.europa.eu).
Introduction
B-cell chronic lymphocytic leukemia (CLL) is a subtype of mature peripheral B-cell neoplasm characterized by the accumulation of circulating malignant lymphocytes that typically express the cell surface markers CD5, CD20, and CD23. The purine analog fludarabine, alone or in combination with other agents, can be considered as the backbone of CLL therapy in both the frontline and subsequent lines of therapy [1, 2]. Chlorambucil may still be a suitable first-line treatment for elderly patients with CLL [1]. Alemtuzumab, a humanized monoclonal antibody (mAb) directed against the CD52 antigen that is expressed on the cell surface of both B cells and T cells, has been approved for the treatment of patients with B-cell CLL for whom fludarabine combination chemotherapy is not appropriate. Recently, rituximab, an anti-CD20 mAb, was approved for the first-line treatment of patients with CLL in combination with chemotherapy. Although most patients with CLL achieve responses with initial therapy, nearly all patients relapse and require further treatments. Advanced age, more than two prior therapies [3], and the presence of chromosomal abnormalities such as 17p and 11q deletions [4] are associated with a poorer response to therapy. Therapy for second and subsequent lines depends on the response and duration of response to prior therapy. If the duration of response is at least 12–18 months, the same regimen can be tried again. If the duration of response is short or lacking, a different therapeutic approach is needed.
In February 2008, Glaxo Group Ltd. (Greenford, Middlesex, U.K.) applied for a marketing authorization via the European Medicines Agency (EMA) centralized procedure for ofatumumab with the trade name Arzerra®. Ofatumumab is designated as an orphan medicinal product in the EU. A review was conducted by the Committee for Medicinal Products for Human Use (CHMP), the scientific committee of the EMA responsible for providing a scientific opinion on the granting of a marketing authorization. The review was started on February 25, 2009 and a positive opinion was issued on January 20, 2010. The approved indication in the EU is: “treatment of chronic lymphocytic leukemia (CLL) in patients refractory to fludarabine and alemtuzumab.”
This publication summarizes the assessment of the scientific data submitted for the initial marketing authorization application in the EU. The detailed scientific assessment report and product information such as the summary of product characteristics, including any updated information on the marketing authorization, are available on the EMA website (http://www.ema.europa.eu).
Nonclinical Aspects
The active substance, ofatumumab, is a fully human mAb directed against CD20 and binds specifically to epitopes that encompass the amino residues 163 and 166 in the small extracellular loop of the CD20 molecule on human B cells.
Primary pharmacology studies were conducted in immunodeficient mice. Based on crossreactivity studies and cDNA analysis, it was determined that the cynomolgus monkey was suitable as a test species in the nonclinical safety studies, whereas the mouse, rat, and dog were not.
Pharmacology
Based on immunohistochemistry, ofatumumab and rituximab display similar cellular binding (B-cell follicles) in the tonsils of humans and cynomolgus monkeys. Specific, positive, membrane-bound staining was recorded in lymphocytes of the lymph node, spleen, thymus, and tonsil and also in the mucosa-associated lymphoid tissue of the small and large intestines in donors in whom lymphoid tissue had been sampled.
In a mutation and epitope mapping study, ofatumumab bound to an epitope distinct from that of rituximab. Replacement of the amino acid residues at positions 163 and 166 strongly reduced the binding of ofatumumab, whereas binding of rituximab was unaffected.
Cells from different tumor B-cell lines were preincubated with CD20-specific antibodies before the addition of normal human serum, which induces complement-dependent cytotoxicity (CDC). Ofatumumab lysed >80% of cells in all tested cell lines.
In order to investigate the impact of CD20 expression levels on ofatumumab-induced CDC, tests were performed in CD20-transfected human CEM T cells, which lack endogenous CD20 expression. Ofatumumab achieved complete lysis of any cell line expressing >60,000 molecules of CD20 and lysed approximately 18% of cells expressing as few as 4,500 CD20 molecules per cell. Rituximab did not reach maximal lysis against clones expressing the highest levels of CD20 and showed CDC activity toward cells expressing at least 30,000 CD20 molecules per cell.
Various types of target cells were incubated with ofatumumab or rituximab in the presence of different effector cells. In the presence of mononuclear cells, ofatumumab and rituximab mediated lysis of ARH-77 cells, CLL cells, B-cell acute lymphocytic leukemia cells and hairy cell leukemia cells to a similar extent.
Rapid complement activation has been suggested to underlie the first-dose side effects of rituximab resulting from the release of inflammatory mediators such as the complement split products (anaphylotoxins) C4a, C3a, and C5a [5]. Analysis using C4a, C3a, and C5a antibody-coated cytometric beads and flow cytometry showed that ofatumumab had a tendency to induce higher levels of C4a, C3a, and C5a during complement activation than rituximab.
Induction of CD20 translocation into lipid rafts by anti-CD20 antibodies is considered to be an important mechanism for inducing cell signaling and complement activation [6, 7]. Following binding to CD20, both rituximab and ofatumumab clustered into lipid rafts within the cell membrane, leading to complement activation.
Ofatumumab increased and prolonged survival (60% survival at the end of the study) and inhibited tumor growth in different B-cell tumor xenograft models in severe combined immunodeficient mice. In the same xenograft models, rituximab resulted in similar tumor growth inhibition and prolonged survival during the study, but there was no apparent prolongation of survival at the end of the study. Further, in one experiment, ofatumumab showed a clear dose–effect relationship, whereas rituximab led to only a slightly longer survival time at the highest dose.
Toxicology
Six toxicology studies were conducted in male and female cynomolgus monkeys and involved doses of ofatumumab up to 100 mg/kg given i.v. or s.c. for periods of 4 days to 7 months, with a maximum once-weekly frequency for the studies of longer duration.
The majority of treated animals experienced slowly developing, reversible, hemolytic anemia during both the dosing and recovery periods. The hemolytic anemia is likely to have arisen as a result of binding of ofatumumab–antiofatumumab antibody complexes to RBCs and sequestration in the spleen by macrophages. Ofatumumab is a human recombinant antibody, and formation of antiofatumumab antibodies in cynomolgus monkeys is expected. In general, antibody formation against human antibodies in monkeys is not predictive of antibody formation in humans.
Intravenous administration of ofatumumab to pregnant cynomolgus monkeys at a dose of 100 mg/kg once weekly on days 20–50 of gestation did not elicit maternal or fetal toxicity or teratogenicity. At day 100 of gestation, depletion of B cells related to the pharmacological activity of ofatumumab was observed in fetal cord blood and fetal splenic tissues. Pre- and postnatal development studies have not been performed. Postnatal recovery has therefore not been demonstrated. Because ofatumumab is a mAb, genotoxicity and carcinogenicity studies have not been conducted.
The activation and release of inflammatory and coagulation parameters were investigated in an immunotoxicity study following administration of ofatumumab to cynomolgus monkeys. The results suggest that administration of ofatumumab may be associated with cytokine release syndrome. However, the severity of this syndrome in monkeys was limited and clinical manifestations were not observed.
Clinical Aspects
The clinical efficacy data submitted in support of this application comprised primarily data from a preplanned interim analysis of a single-arm, open-label, multicenter ongoing pivotal study, Hx-CD20–406. Additional supportive evidence was provided by a phase I/II study, Hx-CD20–402.
Pharmacokinetics
Pharmacokinetics were assessed in the course of the efficacy/safety studies supporting the current application as well as in the development of ofatumumab for other indications. Ofatumumab was administered by i.v. infusion. Ofatumumab had a small volume of distribution (geometric mean steady-state volume values were in the range of 1.7–5.1l across studies, dose levels, and infusion numbers). This is consistent with distribution largely in the systemic circulation. Ofatumumab is eliminated in two ways: a target-independent route, as with other IgG molecules, and a target-mediated route, which is related to binding to B cells.
There was rapid and sustained depletion of CD20+ B cells after the first ofatumumab infusion, leaving a lower number of CD20+ cells available for the antibody to bind at subsequent infusions. As a result, ofatumumab clearance values were lower and half-life values were significantly larger after later infusions than after the initial infusion. Similarly, mean maximal concentration (Cmax) and area under the curve extrapolated to infinity (AUC0-∞) values for ofatumumab after the first dose seemed to increase by dose more than expected in most studies. Pharmacokinetic data were available from 146 patients with refractory CLL. The geometric mean Cmax value was 63 μg/ml after the first infusion (300 mg); after the eighth weekly infusion (seventh infusion of 2,000 mg), the geometric mean Cmax value was 1,482 μg/ml and the geometric mean AUC0-∞ value was 674,463 μg·h/ml; after the twelfth infusion (fourth monthly infusion; 2,000 mg), the geometric mean Cmax value was 881 μg/ml and geometric mean AUC0-∞ was 265,707 μg·h/ml.
Weight, body surface area, age (range, 21–86 years), and gender had no clinically relevant effects, and no dose adjustment is recommended based on these factors. No dose adjustment is recommended for mild-to-moderate renal impairment (creatinine clearance >30 ml/minute). There are no pharmacokinetic data in patients with severe renal impairment (creatinine clearance <30 ml/minute). No pharmacokinetic data are available in patients with hepatic impairment. Changes in hepatic function are unlikely to have any effect on the elimination of ofatumumab.
Pharmacodynamics
Primary and secondary pharmacology parameters were measured in the course of the efficacy/safety studies. CD19+ B-cell counts were determined before, during, and after ofatumumab therapy to assess B-cell depletion. The B-cell surface antigen CD19 was used as a marker for CD20, because the two antigens have similar expression profiles on B cells and the presence of anti-CD20 antibody binding to CD20+ cells can interfere with flow cytometric measurement of CD20+ cells. In patients with refractory CLL in study Hx-CD20–406, the median decrease in the B-cell count was 23% after the first infusion (300 mg) and 92% after the eighth infusion (2,000 mg). Peripheral B-cell counts remained low throughout therapy in most patients and gradually increased after the end of ofatumumab therapy, with the median B-cell count remaining 68% below baseline 3 months after the last infusion.
In general, serum IgG and IgA concentrations were stable and serum IgM concentrations declined, consistent with long-term B-cell depletion; in study Hx-CD20–406 (CLL), there appeared to be a modest decrease in the mean IgG concentration from baseline to week 16. No clinically significant changes in serum complement levels, T-cell counts, or natural killer cell counts were observed. There did not appear to be a relationship between ofatumumab dose and the incidence of infection across the clinical development studies, as an indirect indicator of potential immune impairment.
Clinical Efficacy
Dose–Response Study (Hx-CD20–402)
Thirty-three patients were enrolled in the Hx-CD20–402 dose-escalation study [8]. Patients received four weekly infusions of ofatumumab:
Group A (n = 3) received a first infusion of 100 mg and three subsequent infusions of 500 mg.
Group B (n = 3) received a first infusion of 300 mg and three subsequent infusions of 1,000 mg.
Group C (n = 27) received a first infusion of 500 mg and three subsequent infusions of 2,000 mg.
The absolute doses applied in this study were selected based on previous studies with rituximab in follicular lymphoma (FL) and CLL, and on previous experience with ofatumumab in FL patients. One patient in group C was withdrawn from treatment after the first infusion because of a serious adverse event (SAE) of cytolytic hepatitis (elevation of transaminases, maximal Common Toxicity Criteria grade 3) that was considered related to study medication. Patients had a median age of 61 years (range, 27–82 years), and 42% of patients were female. All patients were white. Most patients were in the low- or intermediate-risk stage of their disease, with Rai stage I or stage II (39% and 45%, respectively) or Binet stage A or stage B (21% and 67%, respectively). No patients in group A or group B had constitutional symptoms. In group C, 6% of patients experienced extreme fatigue and 15% experienced night sweats. The median number of prior CLL therapies was four in group A, three in group B, and two in group C.
Rapid, efficient, and sustained depletion of malignant and normal B cells was observed in all patients in group C during the study period. The median time to progression in group C was 4.4 months in the full analysis population and 5.3 months in the subgroup of responders. The duration of response was 4.4 months, and the time to next CLL therapy was 12.1 months. (Main efficacy results are shown in Table 1.) The response definition was based on the National Cancer Institute (NCI) Working Group criteria [9]. Based on the results of this study, a dose of 2,000 mg was chosen for the pivotal study. Higher doses of ofatumumab were not tested in this or any subsequent clinical study nor were any other phase I/II studies submitted in support of the choice of dose or frequency and duration of treatment.
Table 1.
Summary of primary and secondary efficacy endpoints, study Hx-CD20–402
Abbreviations: CI, confidence interval; CLL, chronic lymphocytic leukemia; NA, not available; RR, response rate.
Main Study (Hx-CD20–406)
This was an ongoing, single-arm, open-label, multicenter (41 sites in 10 countries) study of ofatumumab in patients with B-cell CLL who were either refractory to both fludarabine and alemtuzumab or refractory to fludarabine and considered inappropriate for alemtuzumab treatment because of bulky lymphadenopathy [10].
Adult patients were eligible if they had active CLL and were refractory to prior therapy, defined as a minimum of two cycles of fludarabine and at least 12 administrations of alemtuzumab (double refractory [DR]). Patients were also eligible if they were refractory to prior therapy and were considered inappropriate candidates for alemtuzumab treatment because of the presence of bulky lymphadenopathy, defined as lymph node size >5 cm (bulky fludarabine refractory [BFR]).
Refractoriness was defined as failure to achieve at least a partial response or disease progression while on treatment, or disease progression in responders within 6 months of treatment.
The intended treatment consisted of eight weekly infusions of ofatumumab (first dose, 300 mg; second to eighth dose, 2,000 mg) followed by four monthly infusions of ofatumumab (ninth to twelfth dose, 2,000 mg). The first monthly infusion was administered 5 weeks after the last weekly infusion and the following three monthly infusions were administered every 4 weeks. Thus, the first dose was administered at week 0 (visit 2) and the last dose was administered at week 24 (visit 14).
The primary efficacy endpoint of this study was the response rate (RR) measured over a 24-week period from the start of treatment, as determined by an independent review committee (IRC). The response definition was based on the NCI Working Group criteria [9].
Patients were classified as responders or nonresponders as follows: patients with a complete remission (CR), nodular partial remission (nPR), or partial remission (PR) were classified as responders, whereas those with stable disease (SD) or progressive disease (PD) were classified as nonresponders. Responses were required to be maintained for at least 2 months (56 days).
The secondary efficacy endpoints included: duration of response, progression-free survival (PFS) time, overall survival time, progress of constitutional symptoms (night sweats, weight loss, fever and extreme fatigue), resolution of lymphadenopathy and organomegaly, improvement in Eastern Cooperative Oncology Group (ECOG) performance status score, hemoglobin, thrombocytopenia, and neutropenia.
Only patients with the potential of having primary endpoint data at cutoff were evaluated (i.e., patients with a planned or completed visit 2). This included 198 screened patients, of whom 154 were allocated and exposed to ofatumumab. Patient assignment based on the IRC assessment included 59 exposed DR patients, 79 exposed BFR patients, and 16 exposed CLL patients assigned as “other.” Their median age was 63 years and the majority of patients were male (72%). Patients were almost exclusively of white origin (97%).
Nearly all patients had received more than two previous treatment regimens for CLL (median, five in DR patients and four in BFR patients), most frequently alkylating agents (94%), purine analog combinations (81%), single-agent cytotoxics (68%), and mAbs (60%). Some patients had been treated with investigational agents as well. The majority of patients in all groups also received prior treatment with rituximab, either as monotherapy or as part of combination therapy.
Efficacy results for study Hx-CD20–406 are presented for the DR, BFR, and combined DR + BFR patients (combined group), because these represent the fludarabine-refractory CLL population (see Table 2).
Table 2.
Summary of response assessed by IRC, study Hx-CD20–406
Abbreviations: BFR, bulky lymphadenopathy-fludarabine refractory; CI, confidence interval; CR, complete response; DR, double refractory; IRC, independent review committee; NE, nonevaluable; nPR, nodular partial response; PD, progressive disease; PR, partial response; SD, stable disease.
Duration of response was defined as the time from initial response to disease progression or death, and was defined only for patients who responded (CR, nPR, or PR) during the 24-week period from the start of treatment. The median duration of response was 7.1 months (95% confidence interval [CI], 3.7–7.6 months) in the DR group, 5.6 months (95% CI, 3.6–7.0 months) in the BFR group, and 5.6 months (95% CI, 3.8–7.1 months) in the combined DR + BFR group, indicating that the median duration of response was nearly as long as the intended treatment duration with ofatumumab (24 weeks or 6 months).
The PFS interval was defined as the time from baseline (allocation to treatment) until progression of CLL or death. Progression events were defined in the protocol, and progression dates were verified by the IRC. The median PFS interval was 5.7 months (95% CI, 0.5–8.0 months) in the DR group, 5.9 months (95% CI, 4.9–6.4 months) in the BFR group, and 5.7 months (95% CI, 5.5–6.4 months) for the 102 patients (DR, 40; BFR, 62) with an observed progression of CLL or death.
At the time of the interim analysis, 27 DR and 31 BFR patients (58 combined group) had died. The median overall survival time was similar across groups—13.7 months (95% CI, 9.4 months to not reached) in the DR group, 15.4 months (95% CI, 10.2–20.2 months) in the BFR group, and 15.4 months (95% CI, 10.2–20.2 months) in the combined group.
More than three quarters of patients with baseline constitutional symptoms experienced complete resolution of all symptoms at some point during the study (79%, 61 of 77). All but one of those patients experienced complete resolution during the treatment period. Nearly all responders with baseline constitutional symptoms experienced complete resolution (93%, 40 of 43). In addition, all patients without constitutional symptoms at baseline remained symptom free during the study.
One fifth of the combined group patients with baseline lymphadenopathy had complete resolution at some point during the study (20%, 25 of 128); the majority had some persistent lymphadenopathy during study treatment. There were no patients without baseline lymphadenopathy who developed new lymphadenopathy during the study.
More than half of the patients in the combined group experienced complete resolution of splenomegaly some time during the study (60%, 42 of 70), and all occurred during the treatment period. Of these, 34 patients experienced complete resolution of splenomegaly during response. More than two thirds of the patients in the combined group experienced complete resolution of hepatomegaly some time during the study (71%, 24 of 34), and all occurred during the treatment period. Of these, 17 patients experienced complete resolution of hepatomegaly during response. The absence of organomegaly was maintained in the majority of patients without baseline organomegaly; two patients had worsening of splenomegaly and one patient had worsening of hepatomegaly as their best response during the study period.
Nearly half of the patients with the opportunity to improve their ECOG performance status score from baseline did improve (48%, 41 of 86), with the improvement occurring during the treatment period in 39 of 41 patients. Most improvements in responders occurred during the time of response to ofatumumab (64%, 27 of 42). More than one third of the patients with the opportunity to improve maintained their ECOG performance status score (41%, 35 of 86), and very few patients declined (5%, four of 86). Nearly all patients with an ECOG performance status score of 0 did not worsen during the study period (96%, 50 of 52).
Hemoglobin values increased steadily from baseline through the treatment period and into the follow-up period. Almost half of the patients with low platelet counts at baseline had a documented improvement in their platelet level at some time during the study (46%, 46 of 100). The median neutrophil counts remained above the lower limit of normal from baseline through the treatment period and into the follow-up period.
Clinical Safety
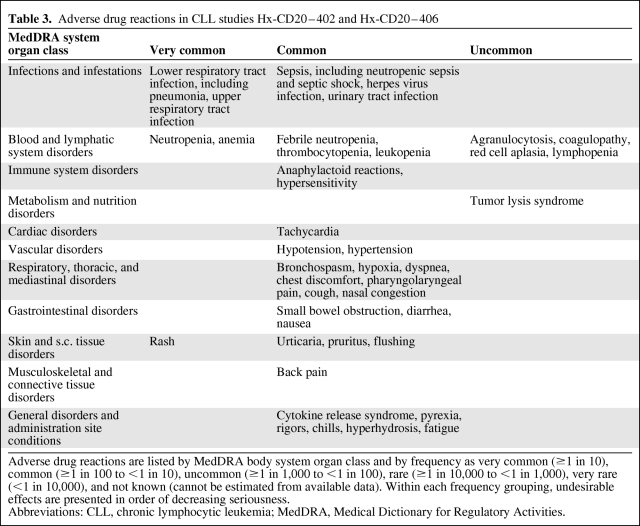
The safety database included patients who were exposed to at least one dose of ofatumumab. In total, 648 patients were exposed to ofatumumab in 12 studies across oncology and nononcology indications as of the cutoff date of June 20, 2008. Because patients with other indications received significantly lower doses of ofatumumab in the course of their treatment, the following analysis of safety focuses only on CLL patients. AEs considered related to treatment by the investigator (adverse drug reactions) in both CLL studies (Hx-CD20–402 and Hx-CD20–406) are shown in Table 3.
Table 3.
Adverse drug reactions in CLL studies Hx-CD20–402 and Hx-CD20–406
Adverse drug reactions are listed by MedDRA body system organ class and by frequency as very common (≥1 in 10), common (≥1 in 100 to <1 in 10), uncommon (≥1 in 1,000 to <1 in 100), rare (≥1 in 10,000 to <1 in 1,000), very rare (<1 in 10,000), and not known (cannot be estimated from available data). Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Abbreviations: CLL, chronic lymphocytic leukemia; MedDRA, Medical Dictionary for Regulatory Activities.
Most patients in study Hx-CD20–406 (90%, 139 patients) received all eight weekly infusions; 69% (107 patients) received at least 10 infusions and 55% (85 patients) received all 12 infusions (completed 24 weeks of treatment). In supportive study 402, most patients in the 2,000-mg group received the intended four infusions (25 of 27).
Ofatumumab was associated with infusion reactions leading to temporary interruption of treatment or withdrawal of treatment. Infusion reactions occurred more frequently on the first day of infusion and tended to decrease with subsequent infusions. In the pivotal study, 41% of patients had infusion reaction AEs following the first infusion, declining to 6% for the last infusion. The events were usually mild (grade ≤2). Seven patients had infusion reactions classified as SAEs. There were no deaths resulting from infusion reactions. Anaphylactoid events included five patients with cytokine release syndrome; all but one patient had cytokine release syndrome during their first infusion. Symptoms that characterize infusion reactions were considered drug related in 47% of the patients.
Premedications attenuate infusion reactions but these may still occur, predominantly during the first infusion. Infusion reactions may include anaphylactoid events, cardiac events, chills/rigors, cough, cytokine release syndrome, diarrhea, dyspnea, fatigue, flushing, hypertension, hypotension, nausea, pain, pyrexia, rash, and urticaria. Even with premedication, severe reactions, including cytokine release syndrome, were reported. Patients with a history of decreased pulmonary function may be at a greater risk for pulmonary complications from severe reactions and should be monitored closely during infusion. In cases of severe infusion reaction, the infusion must be interrupted immediately and symptomatic treatment instituted. Ofatumumab should be administered under the supervision of a physician experienced in the use of cancer therapy and in an environment where full resuscitation facilities are immediately available.
In total, 21% of patients in study Hx-CD20–406 had infection and infestation AEs that were considered related to treatment. Regardless of causality, infections reported in the pivotal study (Hx-CD20–406) were of grade 1 or 2 in severity in 91 of 154 patients (59%). In total, 31 (20%) patients had 39 grade 3 infections (in 27 patients, these events were also reported as SAEs). Eight of 154 patients (5%) had grade 4 infections, all of which were also reported as SAEs. The majority of these infections (six events in six patients) were respiratory tract infections, with pneumonias being the most common event (four patients, four events). Grade 5 (fatal) infections occurred in 16 patients (10%) during treatment or follow-up. The most common infections were septic complications (15 patients) and pneumonia (10 patients). Factors associated with a higher frequency or greater severity of infection included advanced stage of disease, high number of prior treatments, and baseline neutropenia (especially severe). In the dose-escalation supportive study (Hx-CD20–402), 16 patients (48%) had infections reported as AEs. Respiratory tract infections were the most common events, occurring in 10 of 33 patients (30%). Three patients reported infectious SAEs: grade 3 herpes zoster, grade 3 pneumonia, and grade 2 of sinusitis. Three patients had grade 5 (i.e., fatal) infections.
In addition to infections and infusion reactions, common AEs (experienced by ≥10% of patients) were: pyrexia (31 patients, 20%), cough (30 patients, 19%), diarrhea (28 patients, 18%), pneumonia (25 patients, 16%), neutropenia (25 patients, 16%), anemia (25 patients, 16%), fatigue (23 patients, 15%), and dyspnea (22 patients, 14%). Most of the AEs were seen in patients on treatment (81%). Infusion reactions occurred with the greatest incidence (44%) on the first infusion day (300 mg or 500 mg), decreased to 26% with the second infusion (2,000 mg), and declined further during subsequent infusions (2,000 mg).
There were no reports of the development of human–antihuman antibodies in any of the CLL studies.
In total, 61 patients (clinical cutoff, May 19, 2008) died during the pivotal study (Hx-CD20–406)—24 during treatment or follow-up and 37 during extended follow-up (seven before and 30 after initiation of new CLL treatment). Most patients died >60 days after the last dose of ofatumumab. Six patients died within 8 weeks after the start of treatment (i.e., early death); none of these early deaths were considered related to treatment by the investigator. Sixteen of the 24 deaths during treatment and follow-up were a result of infections. The remaining eight deaths were caused by disease progression, including CLL transformation and hemiparesis in six patients and cardiac events in two patients during treatment or follow-up.
Pharmacovigilance
The risk management plan agreed upon at the time of approval included identified risks, namely, infusion reactions, including cytokine release syndrome, tumor lysis syndrome, bowel obstruction, and cardiovascular events. Potential risks included cytopenia, infection, progressive multifocal leukoencephalopathy (PML), hepatitis B virus reactivation, an effect on immunizations (including interactions with live vaccines), immunogenicity, and an effect of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors on ofatumumab response. Important missing information includes limited data in pregnant and lactating females and limited experience in patients with relevant comorbidities. For all these risks and limitations, the applicant company agreed to conduct routine pharmacovigilance activities and evaluate data reported from ongoing and all other future clinical studies. In addition, for bowel obstruction and PML, further data will be collected using a targeted follow-up questionnaire. Detailed warnings about these risks are available in the product information.
Overall Conclusions, Risk–Benefit Assessment, and CHMP Recommendation
The CHMP initially considered that the uncontrolled design of the pivotal trial (Hx-CD20–406) was a major deficiency for a convincing demonstration of efficacy. An oncology Scientific Advisory Group (SAG) was convened by the CHMP to provide their clinical expert opinion on these issues. The SAG acknowledged that treatment options are limited for patients with “bulky fludarabine-refractory” disease that is refractory to at least two regimens (at least one of which included fludarabine) and for patients with “double-refractory” disease, and that the choice of a control arm was problematic. In study Hx-CD20–406, the RR observed in patients whose disease was refractory to fludarabine and alemtuzumab was high, the onset of the response was rapid, and the duration of the response was long lasting. Similar effects were observed for patients with “bulky fludarabine-refractory” disease that is refractory to at least two regimens (at least one of which included fludarabine). It is reasonable to assume that these effects will lead to some improvement in disease-related symptoms and that this is expected to be of clinical relevance. The clinical improvements may in principle allow further clinically relevant benefits, for example, allowing further salvage treatments (or allogeneic stem cell transplantation) in general not doable in such patients. Treatment with ofatumumab was associated with AEs that did not give rise to particular concerns. The SAG also recommended that further studies should address the basis for stopping treatment after 24 weeks, because treatment discontinuation was associated with loss of response.
In conclusion, the CHMP judged that the benefit–risk assessment of ofatumumab was positive for the DR (fludarabine and alemtuzumab) population. However, the CHMP considered that there was a need to further confirm the positive benefit–risk ratio in this population through the conduct of controlled trials in CLL disease settings in which such trials are feasible (fludarabine refractory, bulky lymphadenopathy population and earlier lines of therapy). Thus, the CHMP recommended the granting of a “conditional” marketing authorization. This means that further evidence on this medicinal product is awaited. The applicant company has committed to provide comprehensive clinical data from ongoing phase III randomized, controlled clinical studies in earlier disease settings. In addition, the CHMP considered that it is important to further confirm the high RR and control of disease in the refractory setting through controlled trials and extended ofatumumab treatment. As a specific obligation with the aim to satisfy the conditions of the conditional marketing authorization, the applicant company will conduct a controlled trial comparing ofatumumab with the physician's choice in fludarabine-refractory, bulky lymphadenopathy patients. After 24 weeks of treatment, patients in the ofatumumab arm will be further randomized to either extended ofatumumab treatment or observation alone. This should further address the optimum treatment duration.
The EMA will review new information on a yearly basis until all specific obligations are fulfilled. Detailed information on this medicinal product is available on the website of the EMA (http://www.ema.europa.eu/).
Acknowledgments
The CHMP scientific assessment as summarized in this report is based on the application submitted and on important contributions from, among others, the rapporteur and corapporteur assessment teams, the Oncology Scientific Advisory Group members, and additional experts.
This publication is a summary of the European Public Assessment Report (EPAR) available in the public domain, together with the summary of product characteristics (SmPC) and other product information on the EMA website (http://www.ema.europa.eu). The authors remain solely responsible for the opinions expressed therein. This is the first in a series of similar reports that will be published for cancer drugs approved as of 2010.
Author Contributions
Data analysis and interpretation: Jens Ersbøll, Eva Skovlund, Eric Abadie, Michel Marty, Francesco Pignatti
Manuscript writing: Iordanis Gravanis, Francesco Pignatti
References
- 1.Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343:1750–1757. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- 2.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukemia (the LRF CLL4 Trial): A randomised controlled trial. Lancet. 2007;370:230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 3.Wierda W, O'Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophoshamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–4078. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 4.Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 5.van der Kolk LE, Grillo-López AJ, Baars JW, et al. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol. 2001;115:807–811. doi: 10.1046/j.1365-2141.2001.03166.x. [DOI] [PubMed] [Google Scholar]
- 6.Deans JP, Robbins SM, Polyak MJ, et al. Rapid redistribution of CD20 to a low density detergent-insoluble membrane compartment. J Biol Chem. 1998;273:344–348. doi: 10.1074/jbc.273.1.344. [DOI] [PubMed] [Google Scholar]
- 7.Teeling JL, French RR, Cragg MS, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104:1793–1800. doi: 10.1182/blood-2004-01-0039. [DOI] [PubMed] [Google Scholar]
- 8.Coiffier B, Lepretre S, Pedersen LM, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: A phase 1–2 study. Blood. 2008;111:1094–1100. doi: 10.1182/blood-2007-09-111781. [DOI] [PubMed] [Google Scholar]
- 9.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-Sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 10.Wierda WG, Kipps TJ, Mayer J, et al. Hx-CD20–406 Study Investigators. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]