The impact of regulatory restrictions of erythropoiesis-stimulating agents (ESAs) on their use and on RBC transfusions was examined. A multivariate logistic regression showed a significant decline in ESA use without an increase in RBC transfusions.
Keywords: Erythropoiesis-stimulating agents, RBC transfusions, Anemia, Regulatory restrictions
Abstract
Purpose.
Safety concerns raised in the recent oncology trials with erythropoiesis-stimulating agents (ESAs) have led to regulatory restrictions on their use. We wished to determine the impact of these changes on the use of ESAs and RBC transfusions.
Methods.
In a retrospective observational study of patients treated at a comprehensive cancer center in 2006–2008, data on all ESA doses dispensed, RBCs transfused, and hemoglobin levels on the days of transfusions and ESA initiations were analyzed.
Results.
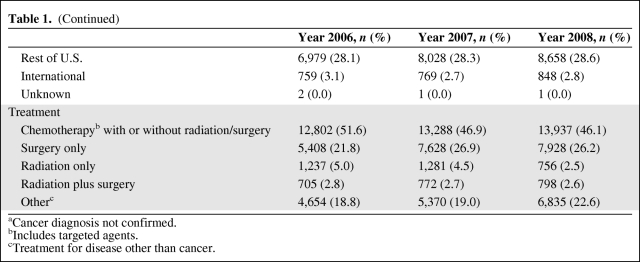
Compared with 2006, the total patients treated was 14% higher (28,339 versus 24,806) in 2007 and 22% higher (30,254) in 2008. Patients receiving ESAs decreased by 26% and 61%, and ESA units dispensed decreased by 29% (from 30,206 units to 21,409 units) and 80% (6,102 units) in 2007 and 2008, respectively. However, RBC transfusions increased by only 2% (from 38,218 units to 38,948 units) in 2007 and by 8% (41,438) in 2008. The mean hemoglobin on the day of transfusion was the same for each year (8.4 g/dl); however, an increasing proportion of patients initiated ESAs at lower hemoglobin (<10 g/dl) levels. After adjusting for demographics and diagnostic variables for 3 years (n = 83,399), a multivariate logistic regression showed a significant decline in ESA use (p < .0001) without an increase in RBC transfusions.
Conclusions.
Recent ESA safety concerns and regulatory restrictions have significantly decreased ESA use. The lack of a significant impact on transfusions may be related to a lower hemoglobin threshold used to initiate ESAs or treatment of patients less likely to respond.
Introduction
Anemia is a frequent side effect in cancer patients receiving chemotherapy [1, 2]. Erythropoiesis-stimulating agents (ESAs) have been approved for the treatment of anemia in patients with nonmyeloid malignancies undergoing chemotherapy, based on their ability to significantly reduce the proportion of patients requiring RBC transfusions [3, 4]. Since the approval of two preparations in the U.S. (epoetin alfa in 1993 and darbepoetin alfa in 2002) and several preparations in other parts of world, ESAs have been widely used to improve hemoglobin levels, reduce transfusions, and reduce symptoms of anemia. Several studies have shown the ability of ESAs to increase hemoglobin levels and decrease the need for transfusions; some of these studies suggested an improvement in quality of life (QOL) [5–11]. However, the U.S. Food and Drug Administration (FDA) has not felt that the studies support a claim of an improvement in QOL; thus, an impact from ESAs on QOL remains, at best, controversial.
Recently, several studies have reported shorter survival, tumor progression, and/or a higher risk for thromboembolic events in cancer patients receiving ESAs [12–21]. In these studies, ESA dosing was targeted to achieve and maintain a hemoglobin level ≥12 g/dl in an attempt to have a positive treatment outcome. Although the mechanisms for possible adverse effect of ESAs on tumor progression and survival and their relationship to hemoglobin concentration have not been established, emerging safety concerns with ESAs led to various regulatory restrictions in 2007, including national coverage determination by the Centers for Medicare and Medicaid Services (CMS) and FDA black box warnings [22–26].
In March 2007, the FDA modified the label to include a black box warning describing the thromboembolic and mortality risks associated with ESA use targeted to hemoglobin levels ≥12 g/dl [22, 23]. Physicians were advised to administer the lowest ESA dose sufficient to avoid transfusions. In August 2007, the CMS implemented cancer-related national coverage determination, restricting the initiation of ESAs to a hemoglobin level <10 g/dl, target hemoglobin of 10 g/dl, and treatment duration ≤8 weeks after chemotherapy completion [24]. The black box warning was updated in Nov 2007 to indicate that ESAs shortened overall survival and/or time to tumor progression in clinical studies in breast, non-small cell lung, head and neck, lymphoid, and cervical cancers when dosed to a target hemoglobin level ≥12 g/dl [25–27]. The black box warning also indicated that the risks for shorter survival and cancer progression are not excluded with ESAs dosed to a target hemoglobin level <12 g/dl. In July 2008, the ESA label was revised to indicate that ESAs should not be administered until hemoglobin levels are <10 g/dl and are not indicated when the anticipated outcome of therapy is cure [26–28, 29].
These regulatory changes have raised concerns among clinicians that restricting ESA use would increase the need for blood transfusions, strain the nation's blood supply, and lower the QOL of cancer patients [30–33]. Using a simulation model, a recent study predicted that limiting ESA use for chemotherapy-induced anemia would impose considerable pressure on the available blood supply margin [30]. We, therefore, sought to determine the impact of recent safety concerns and regulatory changes on ESA use and RBC transfusions at our comprehensive cancer center during 2006, prior to regulatory changes, and during 2007 and 2008, when the product labels were revised and the ESA reimbursement policy became more restrictive. We report here the usage patterns of ESAs, RBC transfusions, and threshold hemoglobin levels for transfusions and ESA initiation during these periods.
Methods
Patients and Methods
We reviewed the data from patients treated at our cancer center between January 2006 and December 2008. Patients on active treatment were defined as patients who received any of the following: in-patient admission, emergency center visit, blood transfusion, surgery, chemotherapy, radiation therapy, and other therapy for cancer. Hyperion® Interactive Reporting Studio Version 9.3.1.0.0.248 Windows XP (Oracle Corporation, Redwood Shores, CA) was used to retrieve data regarding demographics, cancer diagnosis, the patient's primary hospital service, insurance, distance from the hospital, and treatment dates. Tables were created to determine the number of unique patients (each patient counted once) treated in each year and in each month.
To determine the total RBC units transfused and ESA units dispensed, all treatments given with ESAs and transfusions were analyzed. The ESA usage data for both darbepoetin alfa and epoetin alfa were collected for all doses dispensed in in-patient units, the ambulatory treatment center, and out-patient pharmacy areas. The doses of both drugs were standardized into a single unit, whereby one ESA unit is equivalent to 40,000 units of epoetin alfa or 100 μg of darbepoetin alfa. The acquisition cost of each prescription at the time of dispensing was extracted and adjusted to January 2008 dollar values using the producer price index adjustment factor for pharmaceutical products from the U.S. Bureau of Labor Statistics. Information on RBC transfusions administered during this study period was obtained from the blood bank database. Hemoglobin values for patients receiving transfusions and ESAs were obtained from the laboratory medicine database. The research protocol was approved by the institutional review board.
Statistical Methods
Patient characteristics were summarized using the mean (± standard deviation) for continuous variable and frequency (percentage) for categorical variables. χ2 tests were used to assess differences in the proportion of patients receiving chemotherapy, the proportion of patients receiving ESAs, and the proportion of patients receiving RBCs among the 3 years 2006–2008. Piecewise linear models were fitted to assess whether or not there was any change point during the 36-month period for the variables of interest, which included the total adjusted ESA units dispensed, total number of RBC transfusions, total number of patients treated during the period, mean hemoglobin level on the day of transfusion, proportion of ESA-receiving patients requiring RBC transfusions, and proportion of RBC-transfused patients receiving ESAs [34]. When fitting a piecewise linear model to each of the variables, the change point was treated as a random quantity and was estimated based on the fitted model, along with the intercepts and slopes for the two pieces of straight lines before and after the change point. Ninety-five percent confidence intervals (CIs) were also provided for the two slopes and the Wald test was used to assess the statistical significance of the change in slope. Multiple logistic regression models were fit for the indicators of ESA use or RBC transfusion use in order to assess change in these usages over time while adjusting for other patient demographics and clinical characteristics. All statistical analyses were carried out in SAS (version 9.1.3, SAS Institute, Cary, NC), and all tests were two-sided.
Results
Number of Unique Patients Treated and Patient Characteristics
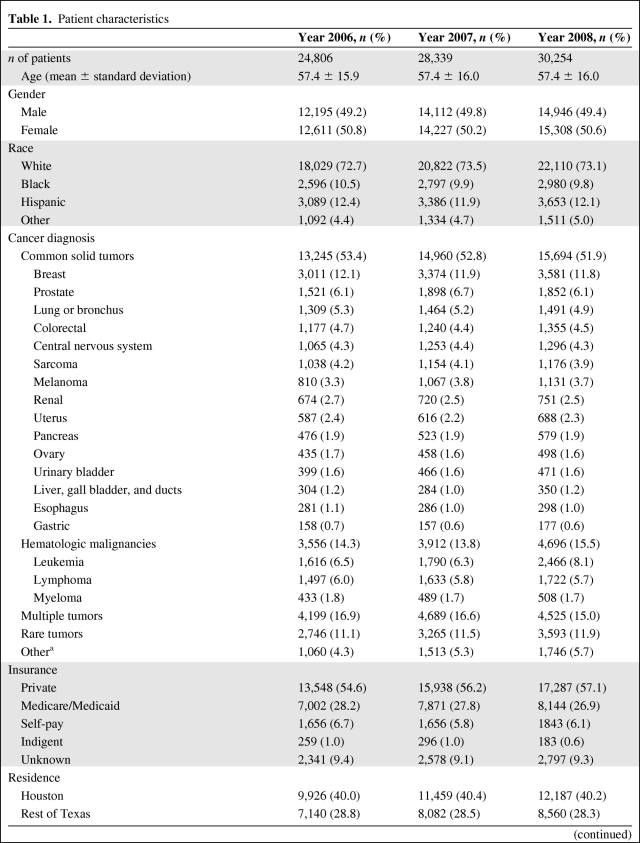
During the 3-year time period January 2006 to December 2008, we recorded the total number of unique patients treated in each year at this institution, including chemotherapy, radiation treatment, surgery, transfusions, and other treatments (Table 1). Patient characteristics, including age, gender, race, insurance type, distance from the hospital, and type of diagnosis, were similar over the 3-year period. The total number of unique patients who received treatment at the MD Anderson Cancer Center increased by 14% in 2007 and 22% in 2008, from 2006 (24,806 patients in 2006, 28,339 patients in 2007, and 30,254 patients in 2008) (Table 2).
Table 1.
Patient characteristics
Table 1.
(Continued)
aCancer diagnosis not confirmed.
bIncludes targeted agents.
cTreatment for disease other than cancer.
Table 2.
Use of erythropoiesis-stimulating agents (ESAs) and RBC transfusions during 2006–2008
aIncrease (↑) or decrease (↓).
bESA units were calculated by the conversion ratio of 400:1 for epoetin alfa:darbepoetin alfa, such that one ESA unit was equivalent to 40,000 units of epoetin alfa or 100 μg of darbepoetin alfa.
ESA Use During 2006–2008
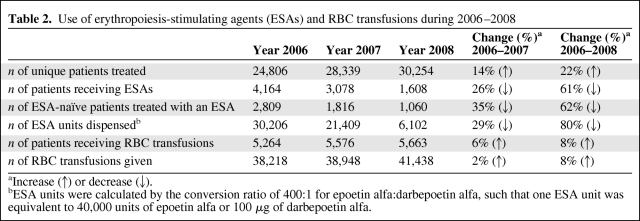
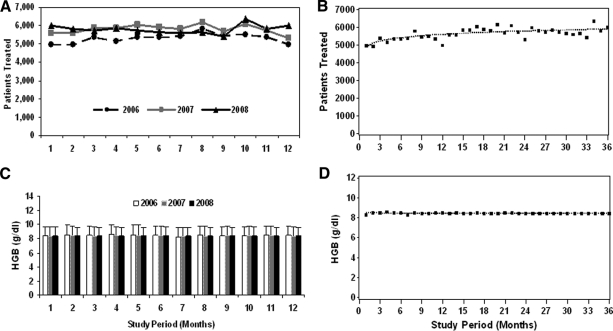
Compared with 2006, the number of patients who received ESAs decreased by 26% in 2007 (from 4,164 to 3,078 patients) and decreased by 61% in 2008 (1,608 patients). The total number of standardized ESA units dispensed decreased significantly, by 29% in 2007 and by 80% in 2008 (from 30,206 units in 2006 to 21,409 units in 2007 and to 6,102 units in 2008) (Table 2). The fitted piecewise linear model suggested a change point at 9.8 months (October 2006), after which the ESA units dispensed decreased dramatically. The slopes before and after the change point were 31.58 ESA units/month (95% CI, −19.75 to 82.92) and −91.38 ESA units/month (95% CI, −101.2 to −81.55), respectively (Fig. 1). The difference between these two slopes was statistically significant (p < .0001). Monthly usage of ESAs decreased by 77%, from 2,398 units dispensed in January 2006 to 549 units dispensed in December 2008. The estimated acquisition cost of ESAs also was proportionately lower in 2007 and 2008, compared with 2006 (Fig. 1), with a decrease of 78% from $ 1,131,552 in January 2006 to $254,486 in December 2008.
Figure 1.
Monthly use of erythropoiesis-stimulating agents (ESAs) and RBC transfusions for 2006–2008. On the left, the ESA units dispensed and the cost of ESAs (A) as well as the total number of RBC units transfused (C) are shown. On the right, results of the corresponding piecewise linear regression model for ESA units (B) and RBC transfusions (D) are shown.
Transfusions Administered During 2006–2008
The total number of RBC transfusions increased by 2% in 2007 and by 8% in 2008 (38,218 units in 2006, 38,948 units in 2007, and 41,438 units in 2008). The number of patients who received RBC transfusions increased by 6% in 2007 and by 8% in 2008 (5,264 patients in 2006, 5,576 patients in 2007, and 5,663 patients in 2008) (Table 2). Although there was a linear increase over time (p = .003), the piecewise linear model did not show any change point during the 3-year study period.
ESA Use and Transfusions in Patients Receiving Chemotherapy
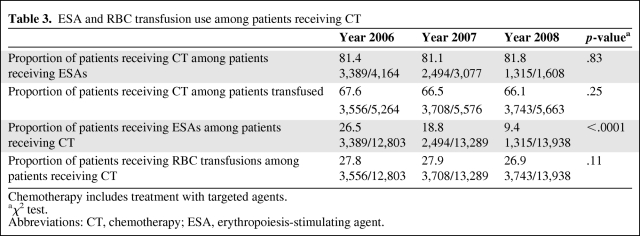
We further investigated the proportions of patients receiving ESAs among patients who received systemic cancer treatment, because >80% of the ESA use was among these patients (Table 3). The proportion of patients receiving ESAs among these patients decreased significantly over 3-year period (26.5% in 2006, 18.8% in 2007, and 9.4% in 2008; p < .0001). However, the proportion of patients receiving RBC transfusions did not increase over the 3-year period (27.8% in 2006, 27.9% in 2007, and 26.9% in 2008; p = .11) (Table 3). In addition, the proportion of patients receiving chemotherapy remained the same over the 3-year period among ESA-receiving patients (81.4% in 2006, 81.1% in 2007, and 81.8% in 2008; p = .83) and among RBC-transfused patients (67.6% in 2006, 66.5% in 2007, and 66.1% in 2008; p = .25).
Table 3.
ESA and RBC transfusion use among patients receiving CT
Chemotherapy includes treatment with targeted agents.
aχ2 test.
Abbreviations: CT, chemotherapy; ESA, erythropoiesis-stimulating agent.
Hemoglobin Value on the Day of Transfusion
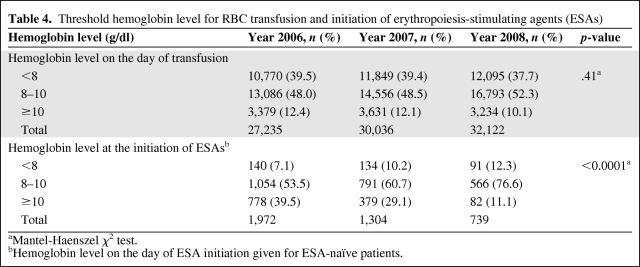
To determine whether the transfusion threshold changed during this 3-year period, we examined hemoglobin levels on the day of transfusion. The piecewise linear model indicated that there was no significant change in the hemoglobin level on the day of transfusion during the 3-year period (Fig. 2). The proportions of patients with a hemoglobin level <10g/dl on the day of transfusion were 87.6%, 87.9%, and 89.9% in 2006, 2007, and 2008, respectively (p = .41) (Table 4).
Figure 2.
Monthly number of patients treated and hemoglobin (HGB) level for 2006–2008. On the left, the number of unique patients treated monthly (A) and the HGB value (mean ± standard deviation) on the day of transfusion (C) are shown. On the right, results of the corresponding piecewise linear regression model for the number of patients treated (B) and HGB value on the day of transfusion (D) are shown.
Table 4.
Threshold hemoglobin level for RBC transfusion and initiation of erythropoiesis-stimulating agents (ESAs)
aMantel-Haenszel χ2 test.
bHemoglobin level on the day of ESA initiation given for ESA-naïve patients.
Hemoglobin Value at the Initiation of ESAs in ESA-Naïve Patients
To determine whether the threshold for initiating ESAs changed during this period, we examined hemoglobin values prior to the initiation of ESAs in the ESA-naïve population (Table 4). The proportion of patients who started ESAs at a hemoglobin level ≤10 g/dl increased from 60.6% in 2006 to 71% in 2007 and to 88.9% in 2008 (p < .0001) (Table 4). During the 3 years, an increasing proportion of ESA-naïve patients received RBC transfusions before the initiation of ESAs (26.4% in 2006, 33.7% in 2007, and 40.7% in 2008).
ESA Use in the RBC-Transfusion Receiving Patients
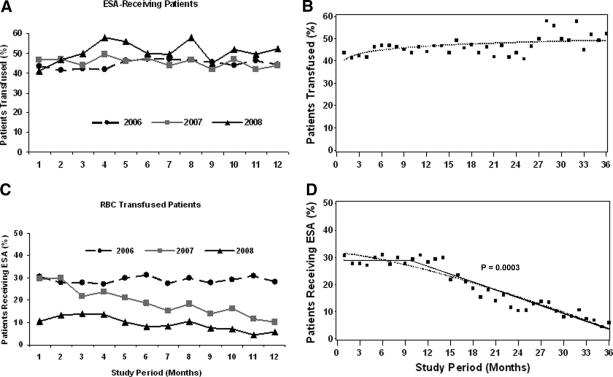
ESA use in patients receiving RBC transfusions decreased significantly, from around 30.7% in January 2006 to 5.9% in December 2008 (Fig. 3). Based on the regression model, there was a change point at 9.9 months (October 2006), with estimated slopes before and after the change point of −0.02 percent/month (95% CI, −0.69 to 0.64) and −0.97 percent/month (95% CI, −1.1 to −0.84), respectively. The difference between the two slopes was statistically significant (p = .0003). These results indicate that, from January 2006 to October 2006, there was no significant difference in the proportion of ESA-receiving patients among RBC-transfused patients, and after October 2006, there was a significant decrease in the ESA use in this population (Fig. 3).
Figure 3.
Monthly use of RBC transfusions in erythropoiesis-stimulating agent (ESA)-receiving patients and ESAs in RBC-transfused patients for 2006–2008. On the left, the proportion of ESA-receiving patients who required RBC transfusions (A) and the proportion of RBC-transfused patients who received ESAs (C) are shown. On the right, results of the corresponding piecewise linear regression model for the proportion of patients transfused (B) and patients receiving ESAs (D) are shown.
Transfusion Requirement in ESA-Receiving Patients
We examined whether the RBC transfusion use in patients receiving ESAs changed during this period. As shown in Figure 3, there was no decrease in the proportion of patients receiving transfusions—43.7% of ESA-receiving patients required transfusions in January 2006 and 52.3% of ESA-receiving patients required transfusions in December 2008. Based on the regression model, there was no change point over the 3-year period.
Patient Population Receiving ESAs
The greatest ESA use was by the hematologic services (leukemia, lymphoma/myeloma, and stem cell transplantation). The combined use of ESAs by the hematologic services was 28% over the 3-year period (31% in 2006, 29% in 2007, and 20% in 2008). Among solid tumor patients, the genitourinary and gastrointestinal medical oncology services were the lead users. A decrease in ESA use was seen across all top 10 services.
Multiple Logistic Regression
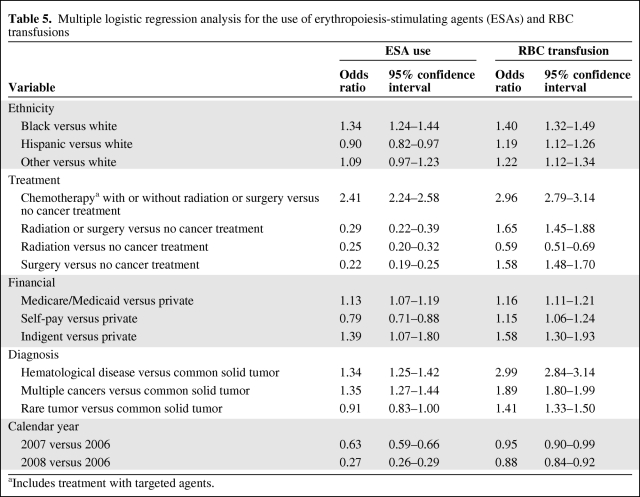
Multiple logistic regression models were fit for the indicators of ESA use and RBC transfusions in order to assess the change of ESA usage or RBC transfusion over time while adjusting for other patient and clinical characteristics (ethnicity, financial status, diagnosis, and treatment). Age, gender, and distance from the cancer center were not significant predictors of either outcome variable in the univariate analysis. The multivariate analysis indicated that, after accounting for other patient characteristics, ESA use in 2007 and 2008 was significantly lower than in 2006 (Table 5). However, the use of RBC transfusions was not higher in 2007 and 2008 than in 2006.
Table 5.
Multiple logistic regression analysis for the use of erythropoiesis-stimulating agents (ESAs) and RBC transfusions
aIncludes treatment with targeted agents.
Discussion
Recent safety concerns and regulatory restrictions on ESA use have caused controversy and uncertainties surrounding ESA use in the oncology community. As ESA policies have become more restrictive, there has been major concern of a potential negative impact on transfusion patterns [30–33]. The purpose of this study was to determine the impact of these changes on the use of ESAs and blood transfusions at a comprehensive cancer center. Our findings indicate that, compared with 2006, ESA use dramatically declined in 2007 and 2008, when the FDA revised the ESA label with black box warnings and the reimbursement of ESAs by the CMS became more restrictive [23–27]. Surprisingly, however, a marked decline in ESA use, by 29% in 2007 and by 80% in 2008, compared with 2006, did not result in a concomitant increase in RBC transfusion.
Recently, there has been growing concern that limiting ESA use will lead to an increase in blood transfusions and burden the nation's blood supply. Using a modeling simulation technique, a recent study estimated that a 25% reduction in ESA use would lead to 118,602 units of incremental RBC transfusions that would require 18% of the marginal U.S. blood supply, and the total cessation of ESA use would exceed the U.S. blood supply [31]. The reasons for the lack of impact of a significant reduction in ESA use on transfusions at our center are not well understood. It was not a result of a decrease in the number of patients treated, because the number of patients referred and treated was 22% higher during 2008 than during 2006. Transfusion practice did not change during this period, as evidenced by the similar monthly mean hemoglobin values on the day of transfusion during all 3 years. Furthermore, the proportion of patients transfused with different degrees of anemia was also not significantly different, suggesting that the hemoglobin threshold for transfusion was not the reason for a lack of increase in RBC transfusions.
To determine whether changes in patient demographics or the diagnoses of patients treated at our cancer center were responsible for the decline in ESA use or the lack of impact on transfusions, multiple logistic regression models were fit. Although there were minor changes in patient demographics during the study period, the multiple logistic regression analysis after accounting for patient demographics still showed a significant decline in ESA use without any impact on transfusion use in the 3-year period.
One possible explanation for the lack of impact of a lower ESA use on transfusions is the ESA practice pattern, that is, the use of ESAs in patients with more significant anemia and compromised bone marrow. Several observations support this. First, 28% of the ESA-receiving population was from hematologic services, wherein compromised bone marrow may be responsible for the anemia. Second, the majority of ESA-naïve patients initiated ESAs at a hemoglobin level <10 g/dl (60.6%, 70.7%, and 88.9% in 2006, 2007, and 2008, respectively), with a higher proportion of patients being more anemic (hemoglobin <9 g/dl: 29% in 2006, 37.7% in 2007, and 46.7% in 2008). Third, and more importantly, about one third of the ESA-naïve patients had already received prior transfusions.
Because there is a time lag for the production of mature RBCs from the effect of ESAs on progenitor cells, ESAs may not be effective in avoiding transfusion when delayed until patients become significantly anemic, especially with ongoing injury to bone marrow. Prior studies have shown the importance of baseline hemoglobin and prior transfusion status [35–37]. Blood use was greater when ESA therapy was initiated in patients with a baseline hemoglobin level <10 g/dl than when it was initiated in patients with a hemoglobin level of 10–11 g/dl. A meta-analysis confirmed that patients who start ESAs with a hemoglobin level <10 g/dl are more likely to require transfusions [37]. In that analysis, patients transfused prior to ESA initiation were approximately twice as likely to be transfused than those not pretransfused. Taken together, these findings suggest that restriction to a lower hemoglobin threshold, as currently recommended, may compromise ESA efficacy. Considering the time lag for their effect, ESAs are more effective as preventive agents rather than therapeutic agents for anemia.
One limitation of our study is that it is a retrospective observational study and the data are reflective of only those patients treated at our cancer center. Nevertheless, the reduction in ESA use at our center is in line with trends in the country. Furthermore, subsequent to the presentation of our findings [38], data reported in abstract form by another cancer center [39] support our finding that, despite a decline in ESA use, there is no significant impact on transfusions.
In conclusion, our findings indicate that recent safety concerns and regulatory restrictions related to ESA use have significantly impacted the management of anemia in cancer patients and reduced ESA use markedly. Surprisingly, the decline in ESA use is not associated with an increase in RBC transfusion use at our center.
Acknowledgments
We thank Teofila Spear for her assistance in the preparation of the manuscript; Priya Patel, Wanda Toole, Victoria Hawkins, and James Fleming for collection of the data; and Rebecca Arbuckle for helpful discussions.
Author Contributions
Conception/Design: Saroj Vadhan-Raj
Administrative support: Saroj Vadhan-Raj, Benjamin Lichtiger
Provision of study material or patients: Saroj Vadhan-Raj, Lincy Lal
Collection and/or assembly of data: Saroj Vadhan-Raj, Xiao Zhou, Kurt C. Sizer, Lincy Lal, Joyce Roquemore, Weiming Shi, Benjamin Lichtiger
Data analysis and interpretation: Saroj Vadhan-Raj, Xiao Zhou, Xuemei Wang
Manuscript writing: Saroj Vadhan-Raj, Xiao Zhou, Robert Benjamin
Final approval of manuscript: Saroj Vadhan-Raj, Lincy Lal, Joyce Roquemore, Weiming Shi, Robert Benjamin, Benjamin Lichtiger
References
- 1.Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS): A large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004;40:2293–2306. doi: 10.1016/j.ejca.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: Incidence and treatment. J Natl Cancer Inst. 1999;91:1616–1634. doi: 10.1093/jnci/91.19.1616. [DOI] [PubMed] [Google Scholar]
- 3.Henry DH, Brooks BJ, Jr, Case DC, Jr, et al. Recombinant human erythropoietin therapy for anemic cancer patients receiving cisplatin chemotherapy. Cancer J Sci Am. 1995;1:252–260. [PubMed] [Google Scholar]
- 4.Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94:1211–1220. doi: 10.1093/jnci/94.16.1211. [DOI] [PubMed] [Google Scholar]
- 5.Glaspy J, Bukowski R, Steinberg D, et al. Impact of therapy with epoetin alfa on clinical outcomes in patients with nonmyeloid malignancies during cancer chemotherapy in community oncology practice. J Clin Oncol. 1997;15:1218–1234. doi: 10.1200/JCO.1997.15.3.1218. [DOI] [PubMed] [Google Scholar]
- 6.Demetri GD, Kris M, Wade J, et al. Quality-of-life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: Results from a prospective community oncology study. Procrit Study Group. J Clin Oncol. 1998;16:3412–3425. doi: 10.1200/JCO.1998.16.10.3412. [DOI] [PubMed] [Google Scholar]
- 7.Littlewood TJ, Bajetta E, Nortier JW, et al. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: Results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2001;19:2865–2874. doi: 10.1200/JCO.2001.19.11.2865. [DOI] [PubMed] [Google Scholar]
- 8.Glaspy J, Vadhan-Raj S, Patel R, et al. Randomized comparison of every-2 -week darbepoetin alfa and weekly epoetin alfa for the treatment of chemotherapy-induced anemia: The 200030125 Study Group Trial. J Clin Oncol. 2006;24:2290–2297. doi: 10.1200/JCO.2005.03.8570. [DOI] [PubMed] [Google Scholar]
- 9.Vadhan-Raj S, Mirtsching B, Charu V, et al. Assessment of hematologic effects and fatigue in cancer patients with chemotherapy-induced anemia given darbepoetin alfa every two weeks. J Support Oncol. 2003;1:131–138. [PubMed] [Google Scholar]
- 10.Gabrilove JL, Cleeland CS, Livingston RB, et al. Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: Improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J Clin Oncol. 2001;19:2875–2882. doi: 10.1200/JCO.2001.19.11.2875. [DOI] [PubMed] [Google Scholar]
- 11.Shasta D, George MJ, Harrison LB. Once-weekly dosing of epoetin-alpha increases hemoglobin and improves quality of life in anemic cancer patients receiving radiation therapy either concomitantly or sequentially with chemotherapy. Cancer. 2003;98:1072–1079. doi: 10.1002/cncr.11616. [DOI] [PubMed] [Google Scholar]
- 12.Henke M, Laszig R, Rübe C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomized, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 13.Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: A survival study. J Clin Oncol. 2005;23:5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 14.Wright JR, Ung YC, Julian JA, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol. 2007;25:1027–1032. doi: 10.1200/JCO.2006.07.1514. [DOI] [PubMed] [Google Scholar]
- 15.Thomas G, Ali S, Hoebers FJ, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dl with erythropoietin vs above 10.0 g/dl without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008;108:317–325. doi: 10.1016/j.ygyno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg P. Danish researchers post long-awaited Aranesp results—ever so discreetly. Cancer Lett. 2007;33:1–6. [Google Scholar]
- 17.Overgaard J, Hoff C, Sand Hansen H, et al. Randomized study of the importance of novel erythropoiesis stimulating protein (Aranesp) for the effect of radiotherapy in patients with primary squamous cell carcinoma of the head and neck (HNSCC): The Danish Head and Neck Cancer Group DAHANCA 10 rand [abstract] Eur J Cancer. 2007;5:7. [Google Scholar]
- 18.Vadhan-Raj S, Crane C, Bueso-Ramos CE, et al. Randomized, double-blind, placebo-controlled trial of epoetin alfa (Procrit) in patients with rectal and gastric cancer undergoing chemo-radiotherapy (CT/RT) followed by surgery: Early termination of the trial due to increased incidence of thrombo-embolic events (TEE) Blood. 104:797a. [Google Scholar]
- 19.Hedenus M, Adriansson M, San Miguel J, et al. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: A randomized, double-blind, placebo-controlled study. Br J Haematol. 2003;122:394–403. doi: 10.1046/j.1365-2141.2003.04448.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith RE, Aapro MS, Ludwig H, et al. Darbepoetin alfa for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: Results of a phase 3, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol. 2008;26:1040–1050. doi: 10.1200/JCO.2007.14.2885. [DOI] [PubMed] [Google Scholar]
- 21.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 22.Juneja V, Keegan P, Gootenberg JE, et al. Continuing reassessment of the risks of erythropoiesis-stimulating agents in patients with cancer. Clin Cancer Res. 2008;14:3242–3247. doi: 10.1158/1078-0432.CCR-07-1872. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Food and Drug Administration. Drugs at FDA: Pocrit and Epogen label. March 2007 label. [accessed March 11, 2009]. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/103234s5122lbl.pdf.
- 24.Centers for Medicare & Medicaid Services. Washington, DC: Department of Health & Human Services; 2007. [accessed November 17, 2010]. Decision Memo for Erythropoiesis Stimulating Agents (ESAs) for Non-Renal Disease Indications (CAG-00383N) Available at https://www.cms.gov/mcd/viewdecisionmemo.asp?id=203. [Google Scholar]
- 25.U.S. Food and Drug Administration. Drugs at FDA: Procrit and Epogen Label. November 2007 label. [accessed March 11, 2009]. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/103234s5158lbl.pdf.
- 26.U.S. Food and Drug Administration. Drugs at FDA: Aranesp® (Darbepoetin Alfa) For Injection. November 2007 label. [accessed March 11, 2009]. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/103951s5164lbl.pdf.
- 27.Hagerty K. Continued regulatory actions affecting the use of erythropoiesis-stimulating Agents. J Oncol Pract. 2008;4:267–270. doi: 10.1200/JOP.0863501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Food and Drug Administration. FDA Drug Safety Communication: Erythropoiesis-Stimulating Agents (ESAs): Procrit, Epogen and Aranesp. [accessed November 18, 2010]. Available at http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm200297-REMS.
- 29.Rizzo JD, Brouwers M, Hurley P, et al. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood. 2010;116:4045–4059. doi: 10.1182/blood-2010-08-300541. [DOI] [PubMed] [Google Scholar]
- 30.Samaras AT, Bennett CL, Lai SY. Transfusion in patients with chemotherapy-induced anemia. Johns Hopkins Adv Stud Medicine. 2008;8:352–356. [Google Scholar]
- 31.Vekeman F, Bookhart BK, Duh MS, et al. Impact of limiting erythropoiesis-stimulating agent use for chemotherapy induced anemia on the United States blood supply margin. Transfusion. 2009;49:895–902. doi: 10.1111/j.1537-2995.2008.02072.x. [DOI] [PubMed] [Google Scholar]
- 32.Larholt K, Pashos CL, Wang Q, et al. Dosing and Outcomes Study of Erythropoiesis-Stimulating Therapies ((DOSE): A registry for characterizing anaemia management and outcomes in oncology patients. Clin Drug Investig. 2008;28:159–167. doi: 10.2165/00044011-200828030-00003. [DOI] [PubMed] [Google Scholar]
- 33.Nailm A, Glaspy J. Changes in RBC supportive medications and transfusions in cancer patients undergoing chemotherapy before and after FDA and Medicare actions in 2007 [abstract 20595] J Clin Oncol. 2008;26(15 suppl):732s. [Google Scholar]
- 34.Neter J, Kutner M, Nachtsheim C, et al. Applied Linear Statistical Models. Boston: McGraw-Hill Irwin; 2004. pp. 1–1396. [Google Scholar]
- 35.Quirt I, Kovacs M, Couture F, et al. Patients previously transfused or treated with epoetin alfa at low baseline hemoglobin are at higher risk for subsequent transfusion: An integrated analysis of the Canadian experience. The Oncologist. 2006;11:73–82. doi: 10.1634/theoncologist.11-1-73. [DOI] [PubMed] [Google Scholar]
- 36.Spano JP, Khayat D. Treatment options for anemia, taking risks into consideration: Erythropoiesis-stimulating agents versus transfusions. The Oncologist. 2008;13(suppl 3):27–32. doi: 10.1634/theoncologist.13-S3-27. [DOI] [PubMed] [Google Scholar]
- 37.Couture F, Turner AR, Melosky B, et al. Prior red blood cell transfusions in cancer patients increase the risk of subsequent transfusions with or without recombinant human erythropoietin management. The Oncologist. 2005;10:63–71. doi: 10.1634/theoncologist.10-1-63. [DOI] [PubMed] [Google Scholar]
- 38.Vadhan-Raj S, Hawkins V, Zhou X, et al. Impact of safety concerns of erythropoiesis-stimulating agents (ESAs) and regulatory changes on the use of ESAs and red blood cell (RBC) transfusions at a comprehensive cancer center [abstract 1300] Blood. 2008;112:470. [Google Scholar]
- 39.Shapira I, Raftopoulos H, Gralla RJ, et al. The impact of randomized trial results and altered regulatory policies on ESA use, transfusions and thrombosis: A longitudinal analysis over a 3-year period of resource utilization data from a large comprehensive program [abstract 6611] J Clin Oncol. 2009;27(15 suppl):350s. [Google Scholar]