This review summarizes the current data on the epidemiology, pathology, molecular biology, clinical management, and outcome of patients with pancreatic intraductal papillary mucinous neoplasms.
Keywords: Intraductal papillary mucinous neoplasm, IPMN, Cystic pancreatic tumors, Surgery, Subtypes, Surveillance, Treatment
Abstract
Pancreatic intraductal papillary mucinous neoplasms (IPMNs) rank among the most common cystic tumors of the pancreas. For a long time they were misdiagnosed as mucinous cystadenocarcinoma, ductal adenocarcinoma in situ, or chronic pancreatitis. Only in recent years have IPMNs been fully recognized as clinical and pathological entities, although their origin and molecular pathogenesis remain poorly understood. IPMNs are precursors of invasive carcinomas. When resected in a preinvasive state patient prognosis is excellent, and even when they are already invasive, patient prognosis is more favorable than with ductal adenocarcinomas. Subdivision into macroscopic and microscopic subtypes facilitates further patient risk stratification and directly impacts treatment. There are main duct and branch duct IPMNs, with the main duct type including the intestinal, pancreatobiliary, and oncocytic types and the branch duct type solely harboring the gastric type. Whereas main duct IPMNs have a high risk for malignant progression, demanding their resection, branch duct IPMNs have a much lower risk for harboring malignancy. Patients with small branch duct/gastric-type IPMNs (<2 cm) without symptoms or mural nodules can be managed by periodic surveillance.
Introduction
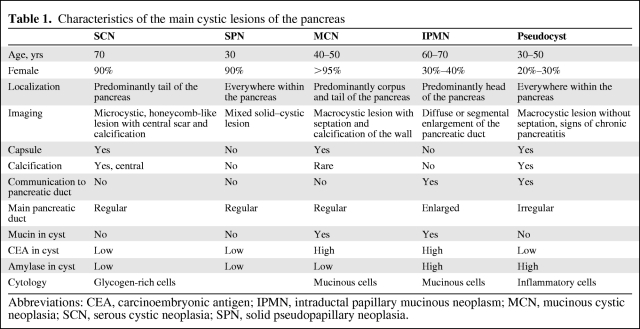
Pancreatic intraductal papillary mucinous neoplasms (IPMNs) have been increasingly recognized in clinical practice. IPMNs belong to the heterogeneous cystic lesions of the pancreas. Cystic lesions of the pancreas can be either inflammatory or proliferative in nature. Pseudocysts make up the majority of all cystic lesions of the pancreas, the remainder comprising cystic tumors and true cysts; the latter are very rare. Pancreatic cystic tumors fall into one of three major groups: serous tumors (including serous cystadenoma and cystadenocarcinoma), mucinous tumors (including mucinous cystic neoplasia and intraductal papillary mucinous neoplasia), and solid pseudopapillary tumors [1]. They appear different in clinical and radiologic characteristics (Table 1). The increasing frequency of identified asymptomatic cystic tumors of the pancreas has resulted in a dramatic increase in the diagnosis of IPMNs at specialized centers. Consequently, knowledge of the clinicopathologic characteristics and natural history of specific subtypes of IPMN has become critical.
Table 1.
Characteristics of the main cystic lesions of the pancreas
Abbreviations: CEA, carcinoembryonic antigen; IPMN, intraductal papillary mucinous neoplasm; MCN, mucinous cystic neoplasia; SCN, serous cystic neoplasia; SPN, solid pseudopapillary neoplasia.
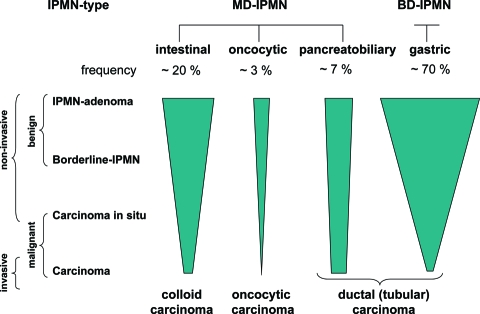
The first cases of IPMNs were described in the 1970s and 1980s and reported under various names [2, 3]. In the 1990s, the term IPMN was coined, and these tumors were established as a specific entity among pancreatic neoplasms in the 2000 World Health Organization classification [4]. In centers of pancreas surgery, they account nowadays for approximately 25% of cases of resected pancreatic neoplasms [5]. IPMN is an intraductal tumor whose papillary epithelial proliferation and mucin production leads to cystic dilatation of the involved ducts. IPMNs are therefore included among the cystic tumors of the pancreas [6]. IPMNs are precursors of invasive carcinomas and therefore provide models of neoplastic progression from a benign intraductal tumor through increasing grades of dysplasia to invasive adenocarcinoma. In addition, they are distinguished according to their site of origin into main duct and branch duct types and according to their histological appearance into intestinal, pancreatobiliary, oncocytic, and gastric types (Fig. 1).
Figure 1.
Types and biology of IPMN.
Abbreviations: BD-IPMN, branch duct IPMN; IPMN, intraductal papillary mucinous neoplasm; MD-IPMN, main duct IPMN.
The clinical diagnosis of IPMN may be difficult, especially if the lesion is small. The indication for surgery and the postoperative prognosis depend on the stage of the disease and the IPMN subtype.
The objectives of this review are to summarize the current data on the epidemiology, pathology, molecular biology, clinical management, and outcome for patients with pancreatic IPMNs.
Incidence and Pathology
Since 2000, the number of case reports of IPMN has been significantly increasing. This increase, however, is not a true reflection of an alteration in IPMN incidence but is mostly a result of improved IPMN diagnosis. One explanation for this is that the distinction between IPMNs and mucinous cystic neoplasms (MCNs) had not been clarified until 1999; therefore, many IPMNs had been misdiagnosed as MCNs [7]. Another reason is the improvement in modern imaging technology that enables a more precise identification of cystic lesions, even if they are small and asymptomatic.
Currently, IPMNs account for 1%–3% of all exocrine pancreatic neoplasms and for 20%–50% of all cystic neoplasms of the pancreas [5, 8, 9]. The exact incidence of IPMN, however, is not known because many of them are small and asymptomatic. Imaging studies revealed that asymptomatic cysts of the pancreas that presumably contain predominantly small IPMNs were found in 2.8% of 2,832 consecutive computed tomography (CT) scans performed in one facility at a single institution, and this figure rose to 8.7% in individuals aged >80 years [10].
There are no well-established etiological factors. In one series, most IPMN patients were cigarette smokers [11]. IPMNs have been reported in patients with Peutz-Jeghers syndrome and in patients with familial adenomatous polyposis [12, 13]. Some studies have suggested that IPMNs may be particularly common among the neoplasms arising in patients with a history of familial pancreatic carcinoma (FPC) [14, 15].
IPMN is defined as a grossly visible (>1.0 cm) intraductal epithelial neoplasm composed of mucin-producing columnar cells showing papillary proliferation, cyst formation, and variable degrees of cellular atypia, even within an individual neoplasm [16, 17]. Noninvasive IPMN is graded according to the most atypical area as IPMN with low-grade dysplasia (adenoma), IPMN with moderate dysplasia (borderline), and IPMN with high-grade dysplasia (carcinoma in situ) (Fig. 1). If an invasive component is present, which occurs in 30%–50% of IPMN cases, the tumor is called an IPMN with an associated invasive carcinoma [5, 18–20]. Progression from adenoma to carcinoma is estimated to occur within about 5–6 years [19, 20]. Therefore, IPMN provides a model of neoplastic progression from a benign intraductal neoplasm to an invasive carcinoma of the pancreas through increasing grades of dysplasia (Fig. 1).
Early reports have suggested that IPMN is comprised of a group of heterogeneous neoplasms [21, 22]. A further argument for the heterogeneity of IPMN was the detection of IPMN not only in the main duct but also in the branch ducts. Finally, it was recognized that IPMNs differ in their histological and cytological features and in their mucin profiles [23–28]. Currently, four subtypes of IPMN have been characterized (Fig. 1, Table 1)—intestinal, pancreatobiliary, oncocytic, and gastric types [25]. The first three types originate from the main duct, whereas the last type, the gastric type, typically occurs in the secondary ducts, the branch ducts.
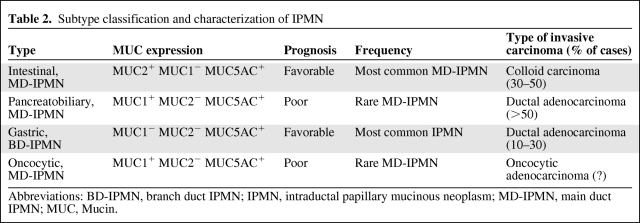
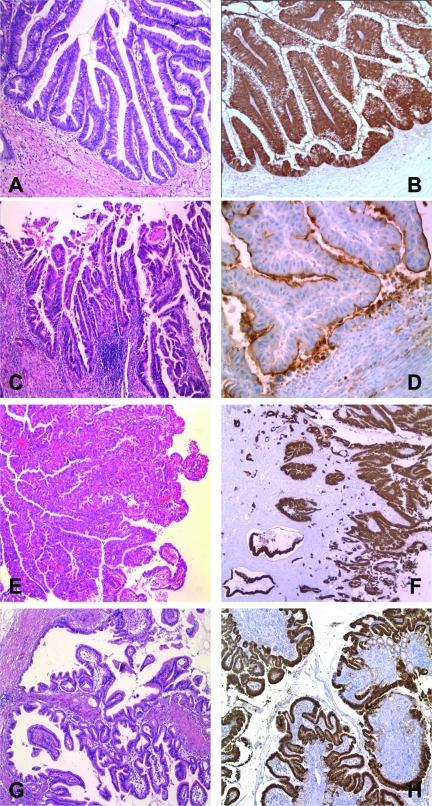
Of the main duct (MD)-IPMNs, the intestinal type is most common (Table 2). It usually occurs in the pancreatic head but may also involve the entire main duct, including the ampulla of Vater [29]. It shows a villous growth pattern similar to that of villous adenoma in the colon (Fig. 2A). It expresses Mucin-2 (MUC2), MUC5, and caudal type homeobox 2 (CDX2), but not MUC1 (Fig. 2B). For invasive intestinal IPMN, the invasive component corresponds to mucinous (colloid) carcinoma and is characterized by extensive stromal pools of extraluminal mucin, containing single cells or strands of neoplastic glandular epithelium or even a small component of signet ring cells [24, 26, 30]. Patients with these colloid carcinomas have a higher 5-year survival rate than those with ductal (tubular) adenocarcinomas (57% versus 37%) [31].
Table 2.
Subtype classification and characterization of IPMN
Abbreviations: BD-IPMN, branch duct IPMN; IPMN, intraductal papillary mucinous neoplasm; MD-IPMN, main duct IPMN; MUC, Mucin.
Figure 2.
Histopathological subtypes of intraductal papillary mucinous neoplasm (IPMN) of the pancreas and their typical mucin patterns. (A, B): IPMN of the intestinal type positive for MUC2. (C, D): IPMN of the pancreatobiliary type positive for MUC1. (E, F): IPMN of the oncocytic type showing positivity for MUC5. (G, H): IPMN of the gastric type, positive for MUC5.
The pancreatobiliary type also predominantly occurs in the main duct of the pancreas head, but it is much rarer than intestinal-type IPMN (Table 2). It shows complex arborizing papillae and only expresses MUC1 and MUC5 (Fig. 2C, 2D). When it becomes invasive, the invasive component usually corresponds to a conventional ductal (tubular) adenocarcinoma (Fig. 1).
The oncocytic type, also known as intraductal oncocytic papillary neoplasm, often forms large tissue nodules in the main pancreatic duct, with only little mucin production [32]. It shows the same complex papillae as the pancreatobiliary type, but the lining cells reveal strong eosinophilic cytoplasm (Fig. 2E). In addition, there are often numerous goblet cells. The tumor cells focally and inconsistently express MUC1, MUC2, and MUC5AC (Fig. 2F).
The gastric type corresponds to branch duct (BD)-IPMN. It is probably the most frequent IPMN and is usually found in the periphery of the pancreatic parenchyma, most often in the uncinate process, where it presents as a multicystic lesion with cysts <3 cm [33]. Histologically, it exhibits papillary projections lined by epithelial cells resembling gastric foveolar cells and shows pyloric gland-like structures at the base of the papillae (Fig. 2G). These cells consistently express MUC5AC and MUC6 (Fig. 2H), whereas they are negative or only focally positive for MUC1 and/or MUC2.
When IPMN becomes invasive, two distinct types of invasive carcinoma commonly occur in association with IPMN—the tubular type and the colloid (mucinous noncystic) type [24] (Fig. 1). The tubular type typically arises from pancreatobiliary-type IPMN and resembles standard pancreatic adenocarcinoma. The colloid type typically arises from intestinal-type IPMN and is characterized by extensive stromal pools of extraluminal mucin [24]. Distinguishing these two types of IPMN has prognostic relevance because patients with colloid carcinomas have a higher 5-year survival rate than those with tubular carcinomas (57% versus 37%) [31].
Origin and Biology of IPMN
IPMNs originate from stem cells that are located in the epithelium of the large and small pancreatic ducts and are able to give rise to tumors with different phenotypes. The most predominant subtype is the intestinal type that shows complete intestinal differentiation, producing lots of MUC2+ intestinal mucin and expressing the intestinal transcription factor CDX2. Additionally, the invasive component corresponds to a colloid carcinoma, a slowly growing adenocarcinoma that is much less aggressive than an ordinary pancreatic ductal adenocarcinoma (PDAC) [30, 31].
The pancreatobiliary type shows the phenotype of large pancreatic and biliary duct cells. It produces less mucus than the intestinal type and expresses MUC1 in addition to MUC5AC. If invasive, it resembles, also prognostically, PDAC [24].
The oncocytic type has a phenotype that does not match any of the cells in the gastroenteropancreatic epithelial system. It usually presents with severe cellular atypia and is diagnosed as carcinoma. Some of these tumors have an invasive component or even distant metastases [34]. Because the follow-up of this patient group is very short, no relevant data have been available yet on their survival and outcome.
The gastric type phenotypically resembles gastric antral mucosa, the foveolar cells of which also express MUC5AC and MUC6 as mucin markers. Although it seems to be less aggressive, that is, less invasive, than the other IPMN subtypes [28, 29, 35, 36], it may also show severe cellular atypia and invasion (in up to 27% cases) [36]. Interestingly, gastric-type IPMN usually occurs in association with lesions of pancreatic intraepithelial neoplasia (PanIN)-1 (also positively staining for MUC5AC), from which they are distinguished only by size and cystic dilatation. In addition, there may also be some foci of lobular fibrosis containing PanIN-1B lesions. Similar, if not identical, changes have been described in the pancreata from otherwise healthy subjects [37] and in the pancreata removed from patients with a strong history of FPC [38, 39]. These observations raise the question of whether gastric-type IPMNs are components or facets (i.e., large PanIN-1 lesions) of diffuse PanIN-1 disease rather than individual disorders. Given the obviously common occurrence of low-grade PanIN lesions and the associated gastric-type IPMN in the pancreata of patients with FPC, it is conceivable that these pancreatic changes (“PanIN disease”) could predispose to the development of PDAC.
Molecular Biology of IPMN
Despite the fact that IPMNs are the second most common neoplasms of the exocrine pancreas, their molecular biology is relatively less investigated than that of PDAC. However, in recent years, a number of studies have identified molecular events that may play a role in the development of IPMN. Together with investigations on global genomic analysis, molecular genetic changes have been defined for IPMN that are distinct from those of PDAC [40]. However, detailed analyses of the molecular profiles of the four IPMN subgroups are missing.
Oncogenes
Analyses of oncogenes in IPMN have mainly been focused on KRAS. KRAS is a GTP-binding protein that mediates the early signal transduction from growth factor receptors to the mitogen-activated protein kinase (MAPK) pathway. Mutations in KRAS typically lead to KRAS activation and, therefore, trigger constitutive activation of MAPK signaling. It was shown, in studies on colon cancer, that KRAS activation leads to a desensitization of cancer cells to growth factor withdrawal and, therefore, leads to the cancer hallmark of “self-sufficiency in growth signals.” Thus, KRAS mutations impair the efficacy of therapies affecting growth factor receptors [41, 42]. Whereas mutations in codons 12, 13, and 61 of KRAS are observed in nearly 100% of PDAC cases, the frequency of KRAS mutations in IPMN is somewhere in the range of 31%–86%. Recent studies indicated that the mutation rate might be around 50%, which is in line with that of other solid cancers [43]. The variability is most likely a result of the improvement in the definition and subclassification of IPMN. Further analysis is therefore needed to determine the KRAS mutation rate in different subtypes of IPMNs.
Tumor Suppressor Genes
The investigation of tumor suppressor genes in IPMN is still at an early stage. It has been reported that CDKN2a is nearly as often aberrantly expressed in IPMN as in PDAC [44]. However, this seems not to be the case for p53 and SMAD4. In IPMN, in contrast to PDAC, only a minor fraction of tumors display aberrant expression of these genes [44]. Interestingly, in a subset of patients with Peutz-Jeghers syndrome harboring mutations of STK11, which are known to be prone to pancreatic cancer, IPMNs have been identified, indicating that STK11 mutations might contribute to sporadic IPMNs [12, 13].
Epigenetic Changes
Hypermethylation
Several studies have been performed to identify epigenetically silenced genes in IPMN [45, 46]. Those studies have also tried to delineate crucial changes among different forms of IPMN, and it has been shown that the rate of analyzed methylation events is far lower in IPMNs that are not associated with PDAC [45]. Interestingly, CDKN2a is hypermethylated in IPMN at a similar rate as in PDAC [47]. Because other genes have been found to be hypermethylated in IPMN at various rates (cyclin D2 in approximately 50%, SOCS in 6%), it is noteworthy that the methylation of certain genes may correlate with clinical stage of IPMN, as shown in the case of TFPI-2. This gene is more frequently hypermethylated in high-grade IPMNs than in low-grade IPMN (85% versus 17%, respectively; p = .0002) [48]. Methylation analysis has also been used for further subclassification of these tumors. However, despite the use of seven different methylation markers, it was impossible to correlate the findings with different histological subtypes of IPMN [46].
Gene Expression
Recently, it was shown that IPMNs differ in their gene expression profiles from other types of pancreatic tumors, but that they share many gene expression alterations with serous cystic neoplasms [49]. In cystic tumors with ULK2 overexpression, a kinase associated with the mammalian target of rapamycin (mTOR) signaling pathway was shown to be upregulated, which might lead to the repression of autophagy during the development of those tumors [50]. Recently, two micro (mi)RNAs, miR-155 and miR-21, were reported to be overexpressed in IPMNs, compared with their expression in normal ducts, indicating crucial changes in IPMNs [51]. Moreover, miR-155 is able to bind to the mRNA of the rapamycin-insensitive companion of mTOR (RICTOR), another member of the mTOR signaling pathway, indicating that alterations in this pathway might be essential for the development of IPMN.
These differences might explain the fate of cells developing into IPMNs but there are many expression changes in IPMN that they share with PDACs. Both demonstrate overexpression of sonic hedgehog, a member of the hedgehog signaling pathway, compared with normal pancreatic tissue, which was usable do differentiate IPMNs from pancreatitis in an assay based on pancreatic juice [52]. Analysis of the hedgehog pathway in IPMN tissue and xenografts showed that activation of hedgehog is an important step during the development of IPMN [53, 54].
Animal Models
The use of genetically engineered mice has revived the field of cancer research. Recently, two different models that induce cystic neoplasms comparable with those in humans have been established. Both models use the activation of KRAS, emphasizing the importance of mutations in this gene in the development of pancreatic carcinomas. In one model, cystic neoplasms in the pancreas were induced by activating transforming growth factor α, and the other was generated by inactivating SMAD4, demonstrating that there may be different molecular pathways that result in the development of cystic neoplasms in the pancreas, including IPMNs [55, 56].
Prognosis of Patients with IPMN
Based on the results of an analysis of the California Cancer Registry (U.S.) during the years 2000–2007, malignant IPMN has a more favorable prognosis than other pancreatic malignancies, such as PDAC, or neuroendocrine tumors [57]. IPMNs are subdivided as MD-IPMN (20%), BD-IPMN (40%), and mixed-type IPMN (40%) by imaging [58]. MD-IPMNs and BD-IPMNs display different biological behaviors. Mixed-type IPMNs belong to MD-IPMNs in terms of their biological behavior. Whereas malignant tumor is found in 57%–92% of MD-IPMN cases, in BD-IPMN a much lower malignancy rate of 6%–46% is observed [59]. MD-IPMNs therefore have a worse prognosis than BD-IPMNs in most clinical series [60]. Only one large retrospective study with 136 resected IPMN samples from Johns Hopkins Hospital showed no prognostic relevance among different subtypes [20]. The strongest adverse predictor of survival in IPMN is the presence of invasive carcinoma [61–65].
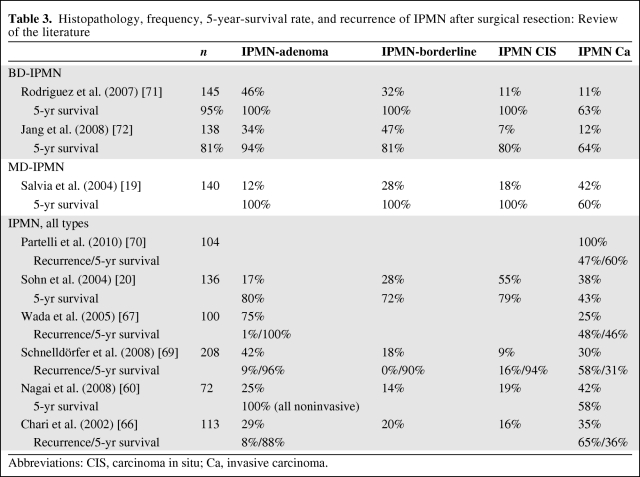
Several other factors have been associated with the prognosis of IPMN patients. The prognosis for patients with IPMN without an invasive component is much better than that of PDAC patients. It has been reported that the prognosis for patients with resected noninvasive IPMNs is much better than that of patients with invasive IPMNs, as shown by the 5-year survival rates of 80%–100% and 40%–60%, respectively [20, 31, 66, 67] (Table 3). This outcome is distinctly favorable compared with that of PDAC patients, which shows a 5-year survival rate of 10%–25% according to previous reports. The reason for the prognostic advantage in invasive IPMN seems to be that the diagnosis and therapy occur at an earlier stage. Only the prognosis of advanced forms of invasive IPMN is as poor as that of PDAC [31, 68]. Poultsides et al. [31] reported that the favorable biologic behavior of IPMN carcinoma compared with that of PDAC is based on its lower rates of advanced T stage, lymph node metastasis, high tumor grade, positive resection margin, perineural invasion, and vascular invasion. There have also been reports of recurrences in a small percentage of cases of noninvasive IPMN [66, 69]. Partelli et al. [70] demonstrated that the lymph node ratio is a strong predictor of survival after resection of IPMN.
Table 3.
Histopathology, frequency, 5-year-survival rate, and recurrence of IPMN after surgical resection: Review of the literature
Abbreviations: CIS, carcinoma in situ; Ca, invasive carcinoma.
Clinical Definition of MD-IPMN and BD-IPMN.
MD-IPMN: cystic tumor and dilatation of the main pancreatic duct.
BD-IPMN: cystic tumor within the side braches of the main pancreatic duct with communication to the main duct; <6-mm diameter of the main pancreatic duct.
Mixed-duct IPMN: involvement of both main duct and side branches.
Clinical Management: Diagnosis and Therapy
Clinical Presentation of Patients with IPMN
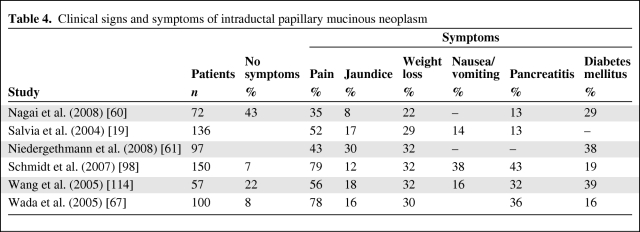
Even if there are no typical clinical signs of IPMN, the medical history of a patient plays an important role in the diagnosis of IPMN. Approximately 80% of IPMN patients have only nonspecific clinical signs such as malaise, nausea and vomiting, abdominal or back pain, and weight loss (Table 4). Some patients have pancreatitis-like symptoms. They suffer from episodes of abdominal or back pain and slowly develop exocrine and endocrine pancreatic insufficiency. This phenomenon is related to obstruction of the main pancreatic duct by mucin [73]. In contrast to chronic pancreatitis (CP), patients with IPMN are more often female, older, and less likely to have a history of alcohol intake [73]. The risk for malignancy in IPMN seems to be higher in symptomatic patients than in others [9]. A history of pancreatitis can cause misdiagnosis of IPMN as CP.
Table 4.
Clinical signs and symptoms of intraductal papillary mucinous neoplasm
Imaging of IPMN
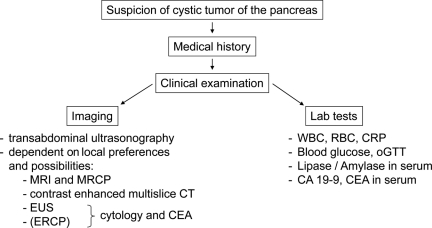
The aim of imaging is to detect IPMN, to exclude other cystic tumors of the pancreas, to distinguish between MD-IPMN and BD-IPMN, to evaluate the risk for malignancy, and to predict resectability. Transabdominal ultrasonography (US) serves as a basic imaging examination. In experienced hands, it is possible to predict resectability with high accuracy using US [74]. Using contrast-enhanced transabdominal US, further characterization of IPMN is feasible, but this is not yet prevalent [75, 76].
Endoscopic ultrasonography (EUS) is a useful diagnostic method, especially in small cystic tumors. It has high resolution to demonstrate mural nodules or irregularities of the pancreatic duct and enables fine-needle aspiration (FNA) of cystic fluid for cytology analysis (tumor or atypical cells) and laboratory tests (amylase, carcinoembryonic antigen [CEA] level). The accuracy of discrimination between benign and malignant IPMN using EUS varies from 40% to >90% [77–81].
IPMN also can be visualized by endoscopic retrograde cholangiopancreatography (ERCP) [6]. The appearance of a mucin extrusion from a widely patent ampulla is pathognomic of IPMN. Injecting contrast material in the main pancreatic duct will enhance the characteristic phenotypes of mucinous filling defects, ductal dilatation, and cystic dilatation of side branches. However, ERCP has been mostly replaced by magnetic resonance cholangiopancreatography (MRCP) because it is much less invasive than ERCP [82].
Both CT and magnetic resonance imaging (MRI) with MRCP are useful for the diagnosis and characterization of IPMN [83, 84] (see examples in Fig. 3). Both modalities provide an accurate assessment of a tumor and its relationship with surrounding organs and vessels [83, 85]. Moreover, mural nodules as a sign of possible malignancy can be recognized by both modalities [84, 86]. Evaluation of communication between the dilated branch ducts and the main pancreatic duct is important for distinguishing IPMNs from other cystic lesions, particularly MCNs, which rarely communicate with the main pancreatic duct. In an analysis of 30 resected IPMNs, MRCP was superior to CT in detecting a ductal connection, estimating main duct involvement, and identifying small branch duct cysts [87]. Preliminary investigation suggests that 18F-fluorodeoxyglucose positron emission tomography may be useful for malignancy detection in IPMN [88].
Figure 3.
Diagnostic algorithm in suspicious IPMN.
In summary, MRI with MRCP might be the primary options for optimal management of IPMN patients [82]. A diagnostic algorithm is shown in Figure 3.
Cytology and Laboratory Markers
Theoretically, the cytology of cysts obtained by EUS FNA could be an ideal tool for surgical decision making, because it may detect malignant cells even in small cysts. However, 50%–60% of these samples are either nondiagnostic or acellular, and the discrimination between serous and mucinous lesions is unreliable, evidenced by the fact that 67% of negative and 92% of nondiagnostic specimens have been associated with malignant or premalignant pathology [89, 90].
Different studies have investigated the role of tumor markers in the cyst fluid of IPMN. Maire et al. [91] showed, in 41 patients with IPMN, that a CEA level >200 ng/ml has a sensitivity, specificity, positive predictive value, and negative predictive value for the diagnosis of malignant IPMN of 90%, 71%, 50%, and 96%, respectively. However, another study showed that neither CEA nor cancer antigen 19–9 were useful to distinguish malignant IPMN from benign IPMN (n = 75) [92]. In summary, the role of tumor markers is still not clear, but if any, CEA seems to be the first choice.
The identification of atypical cells by cytology in combination with a high CEA level in the cyst fluid (>2,500 ng/ml) was found to be more sensitive than the detection of malignant cells alone [93]. Atypical cells were found by cytology in five of the six malignant BD-IPMN patients whose cytologic examination revealed no malignant cells, suggesting that the absence of atypical or malignant cells does not exclude the possibility of malignant or invasive IPMN [89]. Therefore, the decision to proceed with nonoperative management should not be solely based on negative cytologic features [89, 94].
The application and benefit of FNA remains controversial. In the international consensus statement, cytology was discussed but not included in the algorithm for the management of BD-IPMN [59].
Prediction of Malignancy in IPMN
International guidelines have defined clinical symptoms, IPMN size >3 cm, and mural nodules as important criteria for malignancy [59]. A review of studies on BD-IPMN suggests that the prevalence of invasive cancer may be as high as 30% in symptomatic cases and as low as 0%–5% in patients with asymptomatic BD-IPMN [95]. BD-IPMN >3 cm should be resected. The treatment for asymptomatic, radiologically unsuspicious lesions ≤3 cm is, however, debatable. The cutoff point of a diameter of 3 cm has been widely accepted for the prediction of malignancy in BD-IPMN [59, 96]. However, there is a 10%–25% malignancy risk in BD-IPMN of 2–3 cm according to a multi-institutional study from South Korea on 138 BD-IPMN cases, wherein the risk for malignancy in BD-IPMN of 2–3 cm was 25% [72]. In tumors with a diameter <2 cm, malignancy is rare in the absence of additional typical radiographic findings. In an attempt to stratify the indication for surgery in BD-IPMN by age and quality of life, Weinberg and coworkers conducted a Markov-based clinical nomogram for BD-IPMN of the pancreatic head [97]. They revealed that, for patients merely focusing on longest survival, regardless of quality of life, all BD-IPMNs >2 cm should be surgically removed. For patients aged 65–75 years with a main focus of optimizing both quantity and quality of life, either surveillance or “do nothing” was appropriate for lesions with a diameter <3 cm. Based on quality of life alone, in patients aged >85 years, no resection should be performed for lesions <3 cm [97].
Malignancy in BD-IPMNs ≤3 cm is mostly associated with older age, male gender, presence of symptoms (e.g., jaundice, weight loss, and pain), and presence of concerning radiographic features (solid component, main pancreatic and/or common bile duct dilation, and lymphadenopathy) [9]. Nagai et al. [60] demonstrated that four of 57 patients with BD-IPMNs <30 mm without mural nodules harbored malignancy. Moreover, two of these malignancy cases were asymptomatic IPMNs [60]. Another study showed that, in BD-IPMN patients, the size of a cystic lesion was not predictive of invasiveness or malignancy [98]. Interestingly, they found three invasive cancers among 11 asymptomatic cases, including one with a lesion <10 mm.
In contrast, in 2007, Tanno et al. [99] published a series of 82 BD-IPMN cases with a median follow-up of 61 months. IPMNs without mural nodules were unchanged during the follow-up, and none of the resected cases without mural nodules was invasive, as shown by histology. Of five cases with newly developed mural nodules, three adenomas and one carcinoma in situ were found.
In summary, there is no clear evidence indicating a recommended procedure in cases of asymptomatic BD-IPMN without mural nodules and with a size of 20–30 mm. The indications for resection of BD-IPMN remain controversial because preoperative identification of malignancy is often difficult. Until proven by further clear evidence, patients with BD-IPMN of 20–30 mm in size have to be treated individually. Many of them might be good candidates for resection to achieve excellent survival before the neoplasm progresses to an invasive tumor. Age and surgery risk as well as expected quality of life and lifetime should be taken into consideration.
Criteria for Malignant IPMN.
Main duct involvement (MD-IPMN and mixed-type IPMN).
Male gender.
Tumor size >2 cm (3 cm).
Solid changes within IPMN, “mural nodules.”
Clinical symptoms.
Positive cytology (ERCP, EUS), CEA in cyst fluid.
Therapy of IPMN
Indication for Surgery and Surgical Procedures
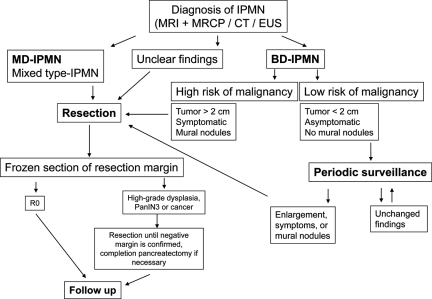
While MD-IPMNs generally should be resected in patients in appropriate clinical condition that allows major pancreatic resection, there is still controversy for BD-IPMN. The accepted indications for surgical resection of BD-IPMN are as follows: size >3 cm, symptoms, and suspicious radiological findings such as mural nodules, a solid component, main pancreatic duct dilation, common bile duct dilation, and lymphadenopathy. A therapeutic algorithm is shown in Figure 4.
Figure 4.
Therapeutic algorithm for IPMN.
Abbreviations: BD-IPMN, branch duct IPMN; CT, computed tomography; EUS, endoscopic ultrasound; IPMN, intraductal papillary mucinous neoplasm; MD-IPMN, main duct IPMN; MRCP, magnetic resonance cholangiopancreatography; MRI, magnetic resonance imaging.
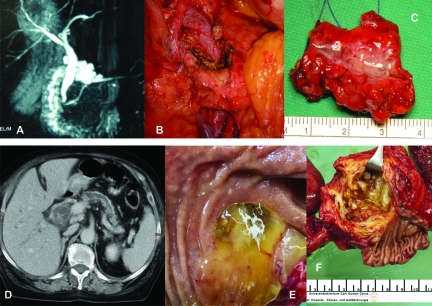
The most common surgical procedures performed for IPMN are pancreaticoduodenectomies (70%) and distal pancreatectomies (25%), because IPMNs are predominantly located in the head of the pancreas [19, 62]. Other surgical procedures include total pancreatectomies, segmental resections, enucleations, and duodenum-preserving resections [100, 101]. Clinical examples are shown in Figure 5.
Figure 5.
Clinical examples of IPMN. (A–C): BD-IPMN. (A): MRCP; (B): Intraoperative situs after enucleation; (C): Surgical specimen with BD-IPMN. (D–F): MD-IPMN. (D): CT with cystic head tumor and enlarged main pancreatic duct; (E): Mucin extrusion from a widely patent ampulla of Vater; (F): Surgical specimen with MD-IPMN.
Abbreviations: BD-IPMN, branch duct IPMN; CT, computed tomography; IPMN, intraductal papillary mucinous neoplasm; MD-IPMN, main duct IPMN; MRCP, magnetic resonance cholangiopancreatography.
Although some surgeons advocate total pancreatectomy because of potentially multicentric disease [102], it is generally accepted to not recommend total pancreatectomy. The postoperative problem of complete endocrine and exocrine pancreatic insufficiency is not the only reason. It has been reported that, for malignant IPMN, the recurrence rate (locoregional or metastatic) is similar after total or partial pancreatectomy [66]. Nevertheless, surgeons must try to achieve negative surgical margins (R0 resection). Therefore, frozen section examination is recommended, and when a surgical margin is positive, an additional subsegment (2-cm wide slice) should be resected until a negative margin is confirmed by dysplasia grade [103, 104].
Surveillance of Nonresected Lesions
The evidence for a positive effect on survival of surveillance is weak because of the lack of prospective randomized data. Whereas MD-IPMN represents a general indication for surgery, the clinical, morphological, and radiological findings are crucial for decision making in BD-IPMN cases. However, even if the risk for malignancy is low, especially for BD-IPMN, it should be considered that IPMN patients, in general, are at a higher risk for the development of PDAC than healthy individuals. A group from Japan showed that 8% (5 of 60) of patients with BD-IPMN developed PDAC distinct from IPMN during follow-up [105]. The 5-year rate of development of ductal carcinoma was 6.9% (95% confidence interval [CI], 0.4%–13.4%) and the incidence of ductal carcinoma was 1.1% (95% CI, 0.1%–2.2%) per year. Moreover, it was demonstrated by Yoon et al. [106] that patients with IPMN have a prevalence of extrapancreatic malignancies of up to 34%. Therefore, surveillance of patients with nonresected lesions seems to be important for early diagnosis of pancreatic and extrapancreatic malignancies.
Ideally, the imaging modality at baseline and follow-up should provide adequate information regarding the size of the lesion, its communication with the main duct, the size of the main duct, and the presence of mural nodules. These criteria can be sufficiently assessed using noninvasive imaging methods, such as CT or MRI with MRCP, or using more invasive examinations, such as EUS. Because of the progress in imaging technology, MRI/MRCP or CT has largely replaced ERCP [107]. Cysts and mural nodules can be sampled via EUS FNA. As reported by a group from Baltimore [108], the presence of an elevated CEA level is extremely suspicious for malignancy.
As long as there are no data available from prospective, randomized trials, yearly follow-up is recommended for lesions with a diameter <10 mm. For lesions of 10–20 mm, a 6- to 12-monthly follow-up is recommended. A 3- to 6-monthly follow-up is recommended for lesions of 20–30 mm [59]. Appearance of symptoms, presence of intramural nodules, cyst size >30 mm, and >6-mm dilation of the main pancreatic duct are, however, indications for surgery. The follow-up interval might be extended after 2 years of a steady state [59].
Adjuvant Therapy
There is no further evidence on adjuvant treatment for IPMN. Therefore, the role of adjuvant therapy in the management of IPMN remains unclear [109].
Follow-Up
Follow-Up After Resection
The evidence concerning follow-up after resection for IPMN is poor because there are no studies with an evidence level of 1, 2, or 3. A convincing statement for a follow-up program is confounded because most of the studies do not distinguish between MD-IPMN and BD-IPMN, or between invasive and noninvasive IPMN. There are some studies with an evidence level of 4 and a consensus article giving information regarding recurrence and follow-up [36, 59, 61, 66, 110, 111]. A cooperative series from the Massachusetts General Hospital and the University of Verona showed a 100% disease-free survival rate for both noninvasive MD-IPMN and BD-IPMN. The 5-year survival rates for invasive IPMN were 50% and 60% for the MD-IPMN and BD-IPMN types, respectively [111]. A Mayo Clinic series followed 113 patients for a mean duration of 3 years after resection. For the 40 invasive tumors, recurrence occurred in 65%, usually within 3 years, mostly observed as metastases. For the 60 cases of noninvasive IPMN, recurrence occurred in 8% [66]. For invasive IPMN, a recurrence rate of 50% was reported by Sho et al. [110], whereas a series from Terris et al. [36] reported a 26% recurrence rate, mainly at the pancreatic remnant. A combined series from Dresden and Mannheim (both Germany) with 68 invasive and 29 noninvasive resected IPMNs over a 10-year follow-up period revealed recurrence rates of 28% and 13% for invasive and noninvasive IPMNs, respectively [61]. Carcinoma in situ was included in the noninvasive IPMN group and was the main cause for local recurrence. Two of 19 patients with local recurrence of an invasive IPMN underwent resection again, whereas the others did not undergo resection because of distant metastases or reduced general condition. All patients with recurrence from invasive IPMN died as a result of their tumor burden within the 10-year follow-up period. In contrast, none of the patients with noninvasive IPMN died from the tumor [61]. In summary, recurrence occurs in 25%–60% of resected invasive BD-IPMN and MD-IPMN cases, whereas recurrence of noninvasive IPMN after resection is rare.
For resected noninvasive IPMN, therefore, a yearly follow-up with abdominal MRI/MRCP or CT seems appropriate. For resected invasive IPMN, re-evaluation is recommended every 6 months [59]. However, the benefit and effectiveness of this procedure have not been proven by evidence.
Surgery After Recurrence Following Resection for IPMN
After resection of invasive IPMN, recurrence occurs in 40%–65% of patients, and lymph node involvement, vascular invasion, surgical margin involvement, and the presence of jaundice are adverse prognostic factors [67, 112]. Several reports have demonstrated that, in terms of resectability, surgery is the only therapeutic option for recurrence, even for noninvasive IPMN [61, 66, 113]. Chari et al. [66] have described invasive cancer as recurrence following initially noninvasive IPMN, suggesting that recurrence in noninvasive IPMN might occur as a result of the presence of dysplastic tissue at the resection margin. It might also occur because of undetected multifocal disease or metachronous lesions developing in the remnant pancreas. Recurrence may become evident at a later time after surgery because IPMN is a slow-growing tumor [61]. In the Dresden and Mannheim series, none of the patients with noninvasive IPMN died from this disease. Four of 29 patients with noninvasive IPMN, however, had a recurrence, and two underwent a subsequent resection. Consequently, we suggest a lifetime follow-up program, because otherwise the recurrence rate might be underestimated. In patients with a good general condition, resection of recurrent disease is recommended.
Conclusion
IPMNs are a heterogeneous group of pancreatic cystic tumors that are increasingly diagnosed. Decisions concerning treatment should be individualized according to guidelines. In the future, a better understanding of the natural history of IPMN and its subtypes is necessary to refine the existing guidelines. This is especially true regarding the management of patients with BD-IPMN, to individualize treatment, to avoid unnecessary surgery, and to circumvent the development of PC during surveillance of the diagnosed patient.
Acknowledgment
Robert Grützmann and Marco Niedergethmann contributed equally.
Author Contributions
Conception/Design: Robert Grützmann, Marco Niedergethmann, Christian Pilarsky, Günter Klöppel, Hans-Detlev Saeger
Collection and/or assembly of data: Robert Grützmann, Marco Niedergethmann
Data analysis and interpretation: Günter Klöppel
Manuscript writing: Robert Grützmann, Marco Niedergethmann, Christian Pilarsky, Günter Klöppel, Hans-Detlev Saeger
Final approval of manuscript: Robert Grützmann, Marco Niedergethmann, Christian Pilarsky, Günter Klöppel, Hans-Detlev Saeger
References
- 1.Kosmahl M, Pauser U, Anlauf M, et al. [Cystic pancreas tumors and their classification: Features old and new] Pathologe. 2005;26:22–30. doi: 10.1007/s00292-004-0734-1. [DOI] [PubMed] [Google Scholar]
- 2.Ohhashi K, Murakami Y, Maruyama M, et al. Four cases of mucous secreting pancreatic cancer. Prog Digest Endosc. 1982;20:348–351. [Google Scholar]
- 3.Morohoshi T, Kanda M, Asanuma K, et al. Intraductal papillary neoplasms of the pancreas. A clinicopathologic study of six patients. Cancer. 1989;64:1329–1335. doi: 10.1002/1097-0142(19890915)64:6<1329::aid-cncr2820640627>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Klöppel G, Solcia E, Longnecker DS, et al. Second Edition. New York: Springer-Verlag; 1998. Histological Typing of Tumours of the Exocrine Pancreas: World Health Organization International Histological Classification of Tumours. [Google Scholar]
- 5.Kosmahl M, Pauser U, Peters K, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: A review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168–178. doi: 10.1007/s00428-004-1043-z. [DOI] [PubMed] [Google Scholar]
- 6.Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–1226. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 7.Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: Clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410–422. doi: 10.1097/00000478-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Allen PJ, D'Angelica M, Gonen M, et al. A selective approach to the resection of cystic lesions of the pancreas: Results from 539 consecutive patients. Ann Surg. 2006;244:572–582. doi: 10.1097/01.sla.0000237652.84466.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CJ, Scheiman J, Anderson MA, et al. Risk of malignancy in resected cystic tumors of the pancreas ≤3 cm in size: Is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg. 2008;12:234–242. doi: 10.1007/s11605-007-0381-y. [DOI] [PubMed] [Google Scholar]
- 10.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukushima N, Mukai K. Pancreatic neoplasms with abundant mucus production: Emphasis on intraductal papillary-mucinous tumors and mucinous cystic tumors. Adv Anat Pathol. 1999;6:65–77. doi: 10.1097/00125480-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Su GH, Hruban RH, Bansal RK, et al. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol. 1999;154:1835–1840. doi: 10.1016/S0002-9440(10)65440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maire F, Hammel P, Terris B, et al. Intraductal papillary and mucinous pancreatic tumour: A new extracolonic tumour in familial adenomatous polyposis. Gut. 2002;51:446–449. doi: 10.1136/gut.51.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poley JW, Kluijt I, Gouma DJ, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175–2181. doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 15.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: An EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–621. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 16.Longnecker DS, Adsay NV, Fernandez-del Castillo C, et al. Histopathological diagnosis of pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms: Interobserver agreement. Pancreas. 2005;31:344–349. doi: 10.1097/01.mpa.0000186245.35716.18. [DOI] [PubMed] [Google Scholar]
- 17.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 18.Azar C, Van de Stadt J, Rickaert F, et al. Intraductal papillary mucinous tumours of the pancreas. Clinical and therapeutic issues in 32 patients. Gut. 1996;39:457–464. doi: 10.1136/gut.39.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: Clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–685. doi: 10.1097/01.sla.0000124386.54496.15. discussion 685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: An updated experience. Ann Surg. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sessa F, Solcia E, Capella C, et al. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: An investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994;425:357–367. doi: 10.1007/BF00189573. [DOI] [PubMed] [Google Scholar]
- 22.Yamada M, Kozuka S, Yamao K, et al. Mucin-producing tumor of the pancreas. Cancer. 1991;68:159–168. doi: 10.1002/1097-0142(19910701)68:1<159::aid-cncr2820680129>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Adsay NV, Conlon KC, Zee SY, et al. Intraductal papillary-mucinous neoplasms of the pancreas: An analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62–77. doi: 10.1002/cncr.10203. [DOI] [PubMed] [Google Scholar]
- 24.Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: Delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839–848. doi: 10.1097/00000478-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa T, Kloppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: A consensus study. Virchows Arch. 2005;447:794–799. doi: 10.1007/s00428-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 26.Luttges J, Zamboni G, Longnecker D, et al. The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol. 2001;25:942–948. doi: 10.1097/00000478-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Nagata K, Horinouchi M, Saitou M, et al. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg. 2007;14:243–254. doi: 10.1007/s00534-006-1169-2. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura A, Horinouchi M, Goto M, et al. New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: Its relationship with potential for malignancy. J Pathol. 2002;197:201–210. doi: 10.1002/path.1109. [DOI] [PubMed] [Google Scholar]
- 29.Ban S, Naitoh Y, Ogawa F, et al. Intraductal papillary mucinous neoplasm (IPMN) of the gastric-type with focal nodular growth of the arborizing papillae: A case of high-grade transformation of the gastric-type IPMN. Virchows Arch. 2006;449:112–116. doi: 10.1007/s00428-006-0214-5. [DOI] [PubMed] [Google Scholar]
- 30.Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26–42. doi: 10.1097/00000478-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Poultsides GA, Reddy S, Cameron JL, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251:470–476. doi: 10.1097/SLA.0b013e3181cf8a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adsay NV, Adair CF, Heffess CS, et al. Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol. 1996;20:980–994. doi: 10.1097/00000478-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Ban S, Naitoh Y, Mino-Kenudson M, et al. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: Its histopathologic difference between 2 major types. Am J Surg Pathol. 2006;30:1561–1569. doi: 10.1097/01.pas.0000213305.98187.d4. [DOI] [PubMed] [Google Scholar]
- 34.Oku T, Maeda M, Wada Y, et al. Intraductal oncocytic papillary neoplasm having clinical characteristics of mucinous cystic neoplasm and a benign histology. JOP. 2007;8:206–213. [PubMed] [Google Scholar]
- 35.Kobari M, Egawa S, Shibuya K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: Differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131–1136. doi: 10.1001/archsurg.134.10.1131. [DOI] [PubMed] [Google Scholar]
- 36.Terris B, Ponsot P, Paye F, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372–1377. doi: 10.1097/00000478-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Detlefsen S, Sipos B, Feyerabend B, et al. Pancreatic fibrosis associated with age and ductal papillary hyperplasia. Virchows Arch. 2005;447:800–805. doi: 10.1007/s00428-005-0032-1. [DOI] [PubMed] [Google Scholar]
- 38.Brune K, Abe T, Canto M, et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol. 2006;30:1067–1076. [PMC free article] [PubMed] [Google Scholar]
- 39.Meckler KA, Brentnall TA, Haggitt RC, et al. Familial fibrocystic pancreatic atrophy with endocrine cell hyperplasia and pancreatic carcinoma. Am J Surg Pathol. 2001;25:1047–1053. doi: 10.1097/00000478-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Fritz S, Fernandez-del Castillo C, Mino-Kenudson M, et al. Global genomic analysis of intraductal papillary mucinous neoplasms of the pancreas reveals significant molecular differences compared to ductal adenocarcinoma. Ann Surg. 2009;249:440–447. doi: 10.1097/SLA.0b013e31819a6e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siddiqui AD, Piperdi B. KRAS mutation in colon cancer: A marker of resistance to EGFR-I therapy. Ann Surg Oncol. 2010;17:1168–1176. doi: 10.1245/s10434-009-0811-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 43.Schönleben F, Qiu W, Bruckman KC, et al. BRAF and KRAS gene mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/IPMC) of the pancreas. Cancer Lett. 2007;249:242–248. doi: 10.1016/j.canlet.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furukawa T, Fujisaki R, Yoshida Y, et al. Distinct progression pathways involving the dysfunction of DUSP6/MKP-3 in pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms of the pancreas. Mod Pathol. 2005;18:1034–1042. doi: 10.1038/modpathol.3800383. [DOI] [PubMed] [Google Scholar]
- 45.House MG, Guo M, Iacobuzio-Donahue C, et al. Molecular progression of promoter methylation in intraductal papillary mucinous neoplasms (IPMN) of the pancreas. Carcinogenesis. 2003;24:193–198. doi: 10.1093/carcin/24.2.193. [DOI] [PubMed] [Google Scholar]
- 46.Hong SM, Kelly D, Griffith M, et al. Multiple genes are hypermethylated in intraductal papillary mucinous neoplasms of the pancreas. Mod Pathol. 2008;21:1499–1507. doi: 10.1038/modpathol.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato N, Goggins M. Epigenetic alterations in intraductal papillary mucinous neoplasms of the pancreas. J Hepatobiliary Pancreat Surg. 2006;13:280–285. doi: 10.1007/s00534-005-1056-2. [DOI] [PubMed] [Google Scholar]
- 48.Sato N, Matsubayashi H, Abe T, et al. Epigenetic down-regulation of CDKN1C/p57KIP2 in pancreatic ductal neoplasms identified by gene expression profiling. Clin Cancer Res. 2005;11:4681–4688. doi: 10.1158/1078-0432.CCR-04-2471. [DOI] [PubMed] [Google Scholar]
- 49.Bauer A, Kleeff J, Bier M, et al. Identification of malignancy factors by analyzing cystic tumors of the pancreas. Pancreatology. 2009;9:34–44. doi: 10.1159/000178873. [DOI] [PubMed] [Google Scholar]
- 50.Jung CH, Jun CB, Ro SH, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Habbe N, Koorstra JB, Mendell JT, et al. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol Ther. 2009;8:340–346. doi: 10.4161/cbt.8.4.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohuchida K, Mizumoto K, Fujita H, et al. Sonic hedgehog is an early developmental marker of intraductal papillary mucinous neoplasms: Clinical implications of mRNA levels in pancreatic juice. J Pathol. 2006;210:42–48. doi: 10.1002/path.2019. [DOI] [PubMed] [Google Scholar]
- 53.Fritz S, Fernandez-del Castillo C, Iafrate AJ, et al. Novel xenograft and cell line derived from an invasive intraductal papillary mucinous neoplasm of the pancreas give new insights into molecular mechanisms. Pancreas. 2010;39:308–314. doi: 10.1097/MPA.0b013e3181bd5c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satoh K, Kanno A, Hamada S, et al. Expression of Sonic hedgehog signaling pathway correlates with the tumorigenesis of intraductal papillary mucinous neoplasm of the pancreas. Oncol Rep. 2008;19:1185–1190. [PubMed] [Google Scholar]
- 55.Izeradjene K, Combs C, Best M, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell. 2007;11:229–243. doi: 10.1016/j.ccr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 56.Siveke JT, Einwächter H, Sipos B, et al. Concomitant pancreatic activation of Kras(G12D) and Tgfa results in cystic papillary neoplasms reminiscent of human IPMN. Cancer Cell. 2007;12:266–279. doi: 10.1016/j.ccr.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Le H, Ziogas A, Rhee JM, et al. A population-based, descriptive analysis of malignant intraductal papillary mucinous neoplasms of the pancreas. Cancer Epidemiol Biomarkers Prev. 2008;17:2737–2741. doi: 10.1158/1055-9965.EPI-08-0417. [DOI] [PubMed] [Google Scholar]
- 58.Furukawa T, Takahashi T, Kobari M, et al. The mucus-hypersecreting tumor of the pancreas. Development and extension visualized by three-dimensional computerized mapping. Cancer. 1992;70:1505–1513. doi: 10.1002/1097-0142(19920915)70:6<1505::aid-cncr2820700611>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 60.Nagai K, Doi R, Kida A, et al. Intraductal papillary mucinous neoplasms of the pancreas: Clinicopathologic characteristics and long-term follow-up after resection. World J Surg. 2008;32:271–278. doi: 10.1007/s00268-007-9281-2. discussion 279–280. [DOI] [PubMed] [Google Scholar]
- 61.Niedergethmann M, Grützmann R, Hildenbrand R, et al. Outcome of invasive and noninvasive intraductal papillary-mucinous neoplasms of the pancreas (IPMN): A 10-year experience. World J Surg. 2008;32:2253–2260. doi: 10.1007/s00268-008-9692-8. [DOI] [PubMed] [Google Scholar]
- 62.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: An increasingly recognized clinicopathologic entity. Ann Surg. 2001;234:313–321. doi: 10.1097/00000658-200109000-00005. discussion 321–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D'Angelica M, Brennan MF, Suriawinata AA, et al. Intraductal papillary mucinous neoplasms of the pancreas: An analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400–408. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murakami Y, Uemura K, Hayashidani Y, et al. Predictive factors of malignant or invasive intraductal papillary-mucinous neoplasms of the pancreas. J Gastrointest Surg. 2007;11:338–344. doi: 10.1007/s11605-006-0069-8. [DOI] [PubMed] [Google Scholar]
- 65.Takahashi H, Nakamori S, Nakahira S, et al. Surgical outcomes of noninvasive and minimally invasive intraductal papillary-mucinous neoplasms of the pancreas. Ann Surg Oncol. 2006;13:955–960. doi: 10.1245/ASO.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 66.Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 67.Wada K, Kozarek RA, Traverso LW. Outcomes following resection of invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas. Am J Surg. 2005;189:632–636. doi: 10.1016/j.amjsurg.2005.01.020. discussion 637. [DOI] [PubMed] [Google Scholar]
- 68.Maire F, Hammel P, Terris B, et al. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut. 2002;51:717–722. doi: 10.1136/gut.51.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schnelldorfer T, Sarr MG, Nagorney DM, et al. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143:639–646. doi: 10.1001/archsurg.143.7.639. discussion 646. [DOI] [PubMed] [Google Scholar]
- 70.Partelli S, Fernandez-Del Castillo C, Bassi C, et al. Invasive intraductal papillary mucinous carcinomas of the pancreas: Predictors of survival and the role of lymph node ratio. Ann Surg. 2010;251:477–482. doi: 10.1097/SLA.0b013e3181cf9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodriguez JR, Salvia R, Crippa S, et al. Branch-duct intraductal papillary mucinous neoplasms: Observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–79. doi: 10.1053/j.gastro.2007.05.010. quiz 309–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jang JY, Kim SW, Lee SE, et al. Treatment guidelines for branch duct type intraductal papillary mucinous neoplasms of the pancreas: When can we operate or observe? Ann Surg Oncol. 2008;15:199–205. doi: 10.1245/s10434-007-9603-5. [DOI] [PubMed] [Google Scholar]
- 73.Talamini G, Zamboni G, Salvia R, et al. Intraductal papillary mucinous neoplasms and chronic pancreatitis. Pancreatology. 2006;6:626–634. doi: 10.1159/000097605. [DOI] [PubMed] [Google Scholar]
- 74.Grützmann R, Bunk A, Kersting S, et al. Prospective evaluation of ultrasound and colour duplex imaging for the assessment of surgical resectability of pancreatic tumours. Langenbecks Arch Surg. 2003;388:392–400. doi: 10.1007/s00423-003-0408-0. [DOI] [PubMed] [Google Scholar]
- 75.Itoh T, Hirooka Y, Itoh A, et al. Usefulness of contrast-enhanced transabdominal ultrasonography in the diagnosis of intraductal papillary mucinous tumors of the pancreas. Am J Gastroenterol. 2005;100:144–152. doi: 10.1111/j.1572-0241.2005.40726.x. [DOI] [PubMed] [Google Scholar]
- 76.Sofuni A, Iijima H, Moriyasu F, et al. Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J Gastroenterol. 2005;40:518–525. doi: 10.1007/s00535-005-1578-z. [DOI] [PubMed] [Google Scholar]
- 77.Nakagawa A, Yamaguchi T, Ohtsuka M, et al. Usefulness of multidetector computed tomography for detecting protruding lesions in intraductal papillary mucinous neoplasm of the pancreas in comparison with single-detector computed tomography and endoscopic ultrasonography. Pancreas. 2009;38:131–136. doi: 10.1097/MPA.0b013e31818b0040. [DOI] [PubMed] [Google Scholar]
- 78.Baba T, Yamaguchi T, Ishihara T, et al. Distinguishing benign from malignant intraductal papillary mucinous tumors of the pancreas by imaging techniques. Pancreas. 2004;29:212–217. doi: 10.1097/00006676-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 79.Sedlack R, Affi A, Vazquez-Sequeiros E, et al. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest Endosc. 2002;56:543–547. doi: 10.1067/mge.2002.128106. [DOI] [PubMed] [Google Scholar]
- 80.Ahmad NA, Kochman ML, Brensinger C, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc. 2003;58:59–64. doi: 10.1067/mge.2003.298. [DOI] [PubMed] [Google Scholar]
- 81.Gress F, Gottlieb K, Cummings O, et al. Endoscopic ultrasound characteristics of mucinous cystic neoplasms of the pancreas. Am J Gastroenterol. 2000;95:961–965. doi: 10.1111/j.1572-0241.2000.01976.x. [DOI] [PubMed] [Google Scholar]
- 82.Brugge WR. The use of EUS to diagnose cystic neoplasms of the pancreas. Gastrointest Endosc. 2009;69(2 suppl):S203–S209. doi: 10.1016/j.gie.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 83.Sahani DV, Kadavigere R, Blake M, et al. Intraductal papillary mucinous neoplasm of pancreas: Multi-detector row CT with 2D curved reformations–correlation with MRCP. Radiology. 2006;238:560–569. doi: 10.1148/radiol.2382041463. [DOI] [PubMed] [Google Scholar]
- 84.Yamada Y, Mori H, Matsumoto S. Intraductal papillary mucinous neoplasms of the pancreas: Correlation of helical CT and dynamic MR imaging features with pathologic findings. Abdom Imaging. 2008;33:474–481. doi: 10.1007/s00261-007-9289-y. [DOI] [PubMed] [Google Scholar]
- 85.Carbognin G, Zamboni G, Pinali L, et al. Branch duct IPMTs: Value of cross-sectional imaging in the assessment of biological behavior and follow-up. Abdom Imaging. 2006;31:320–325. doi: 10.1007/s00261-004-0127-1. [DOI] [PubMed] [Google Scholar]
- 86.Manfredi R, Graziani R, Motton M, et al. Main pancreatic duct intraductal papillary mucinous neoplasms: Accuracy of MR imaging in differentiation between benign and malignant tumors compared with histopathologic analysis. Radiology. 2009;253:106–115. doi: 10.1148/radiol.2531080604. [DOI] [PubMed] [Google Scholar]
- 87.Waters JA, Schmidt CM, Pinchot JW, et al. CT vs MRCP: Optimal classification of IPMN type and extent. J Gastrointest Surg. 2008;12:101–109. doi: 10.1007/s11605-007-0367-9. [DOI] [PubMed] [Google Scholar]
- 88.Baiocchi GL, Portolani N, Bertagna F, et al. Possible additional value of 18FDG-PET in managing pancreas intraductal papillary mucinous neoplasms: Preliminary results. J Exp Clin Cancer Res. 2008;27:10. doi: 10.1186/1756-9966-27-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maker AV, Lee LS, Raut CP, et al. Cytology from pancreatic cysts has marginal utility in surgical decision-making. Ann Surg Oncol. 2008;15:3187–3192. doi: 10.1245/s10434-008-0110-0. [DOI] [PubMed] [Google Scholar]
- 90.Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: A multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230:152–161. doi: 10.1097/00000658-199908000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maire F, Voitot H, Aubert A, et al. Intraductal papillary mucinous neoplasms of the pancreas: Performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. Am J Gastroenterol. 2008;103:2871–2877. doi: 10.1111/j.1572-0241.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 92.Pais SA, Attasaranya S, Leblanc JK, et al. Role of endoscopic ultrasound in the diagnosis of intraductal papillary mucinous neoplasms: Correlation with surgical histopathology. Clin Gastroenterol Hepatol. 2007;5:489–495. doi: 10.1016/j.cgh.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 93.Pitman MB, Michaels PJ, Deshpande V, et al. Cytological and cyst fluid analysis of small (< or =3 cm) branch duct intraductal papillary mucinous neoplasms adds value to patient management decisions. Pancreatology. 2008;8:277–284. doi: 10.1159/000134276. [DOI] [PubMed] [Google Scholar]
- 94.Michaels PJ, Brachtel EF, Bounds BC, et al. Intraductal papillary mucinous neoplasm of the pancreas: Cytologic features predict histologic grade. Cancer. 2006;108:163–173. doi: 10.1002/cncr.21838. [DOI] [PubMed] [Google Scholar]
- 95.Sugiyama M, Izumisato Y, Abe N, et al. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90:1244–1249. doi: 10.1002/bjs.4265. [DOI] [PubMed] [Google Scholar]
- 96.Salvia R, Crippa S, Falconi M, et al. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: To operate or not to operate? Gut. 2007;56:1086–1090. doi: 10.1136/gut.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weinberg BM, Spiegel BM, Tomlinson JS, et al. Asymptomatic pancreatic cystic neoplasms: Maximizing survival and quality of life using Markov-based clinical nomograms. Gastroenterology. 2010;138:531–540. doi: 10.1053/j.gastro.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schmidt CM, White PB, Waters JA, et al. Intraductal papillary mucinous neoplasms: Predictors of malignant and invasive pathology. Ann Surg. 2007;246:644–651. doi: 10.1097/SLA.0b013e318155a9e5. discussion 651–654. [DOI] [PubMed] [Google Scholar]
- 99.Tanno S, Nakano Y, Nishikawa T, et al. Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: Long-term follow-up results. Gut. 2008;57:339–343. doi: 10.1136/gut.2007.129684. [DOI] [PubMed] [Google Scholar]
- 100.Shimada K, Sakamoto Y, Esaki M, et al. Role of medial pancreatectomy in the management of intraductal papillary mucinous neoplasms and islet cell tumors of the pancreatic neck and body. Dig Surg. 2008;25:46–51. doi: 10.1159/000117823. [DOI] [PubMed] [Google Scholar]
- 101.Beger HG, Rau BM, Gansauge F, et al. Duodenum-preserving subtotal and total pancreatic head resections for inflammatory and cystic neoplastic lesions of the pancreas. J Gastrointest Surg. 2008;12:1127–1132. doi: 10.1007/s11605-008-0472-4. [DOI] [PubMed] [Google Scholar]
- 102.Bendix Holme J, Jacobsen NO, Rokkjaer M, et al. Total pancreatectomy in six patients with intraductal papillary mucinous tumour of the pancreas: The treatment of choice. HPB (Oxford) 2001;3:257–262. doi: 10.1080/136518201753335539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eguchi H, Ishikawa O, Ohigashi H, et al. Role of intraoperative cytology combined with histology in detecting continuous and skip type intraductal cancer existence for intraductal papillary mucinous carcinoma of the pancreas. Cancer. 2006;107:2567–2575. doi: 10.1002/cncr.22301. [DOI] [PubMed] [Google Scholar]
- 104.Couvelard A, Sauvanet A, Kianmanesh R, et al. Frozen sectioning of the pancreatic cut surface during resection of intraductal papillary mucinous neoplasms of the pancreas is useful and reliable: A prospective evaluation. Ann Surg. 2005;242:774–778. doi: 10.1097/01.sla.0000188459.99624.a2. discussion 778–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uehara H, Nakaizumi A, Ishikawa O, et al. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut. 2008;57:1561–1565. doi: 10.1136/gut.2007.145631. [DOI] [PubMed] [Google Scholar]
- 106.Yoon WJ, Ryu JK, Lee JK, et al. Extrapancreatic malignancies in patients with intraductal papillary mucinous neoplasm of the pancreas: Prevalence, associated factors, and comparison with patients with other pancreatic cystic neoplasms. Ann Surg Oncol. 2008;15:3193–3198. doi: 10.1245/s10434-008-0143-4. [DOI] [PubMed] [Google Scholar]
- 107.Fasanella KE, McGrath K. Cystic lesions and intraductal neoplasms of the pancreas. Best Pract Res Clin Gastroenterol. 2009;23:35–48. doi: 10.1016/j.bpg.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 108.Buscaglia JM, Giday SA, Kantsevoy SV, et al. Patient- and cyst-related factors for improved prediction of malignancy within cystic lesions of the pancreas. Pancreatology. 2009;9:631–638. doi: 10.1159/000181173. [DOI] [PubMed] [Google Scholar]
- 109.Sakorafas GH, Sarr MG, van de Velde CJ, et al. Intraductal papillary mucinous neoplasms of the pancreas: A surgical perspective. Surg Oncol. 2005;14:155–178. doi: 10.1016/j.suronc.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 110.Sho M, Nakajima Y, Kanehiro H, et al. Pattern of recurrence after resection for intraductal papillary mucinous tumors of the pancreas. World J Surg. 1998;22:874–878. doi: 10.1007/s002689900485. [DOI] [PubMed] [Google Scholar]
- 111.Bernard P, Scoazec JY, Joubert M, et al. Intraductal papillary-mucinous tumors of the pancreas: Predictive criteria of malignancy according to pathological examination of 53 cases. Arch Surg. 2002;137:1274–1278. doi: 10.1001/archsurg.137.11.1274. [DOI] [PubMed] [Google Scholar]
- 112.Bassi C, Sarr MG, Lillemoe KD, et al. Natural history of intraductal papillary mucinous neoplasms (IPMN): Current evidence and implications for management. J Gastrointest Surg. 2008;12:645–650. doi: 10.1007/s11605-007-0447-x. [DOI] [PubMed] [Google Scholar]
- 113.Raut CP, Cleary KR, Staerkel GA, et al. Intraductal papillary mucinous neoplasms of the pancreas: Effect of invasion and pancreatic margin status on recurrence and survival. Ann Surg Oncol. 2006;13:582–594. doi: 10.1245/ASO.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 114.Wang SE, Shyr YM, Chen TH, et al. Comparison of resected and non-resected intraductal papillary mucinous neoplasms of the pancreas. World J Surg. 2005;29:1650–1657. doi: 10.1007/s00268-005-0035-8. [DOI] [PubMed] [Google Scholar]