Vascular endothelial growth factor inhibitors have corticosteroid-sparing effects in patients with high-grade gliomas. Bevacizumab may have corticosteroid-sparing effects, and corticosteroid use over time while receiving bevacizumab should be systematically evaluated, and dose should be decreased as tolerated by the patient. Corticosteroid reduction may positively affect patient health-related quality of life.
Keywords: Corticosteroid, Glioblastoma, Anti-VEGF, Antiangiogenic
Abstract
Background.
Vascular endothelial growth factor inhibitors have corticosteroid-sparing effects in patients with high-grade gliomas. We assessed corticosteroid use in patients with recurrent glioblastoma treated with bevacizumab (BEV) in the BRAIN study (J Clin Oncol 2009;27:4733–4740).
Methods.
BRAIN was a phase II, multicenter, randomized, noncomparative trial of BEV alone (n = 85) or in combination with irinotecan (CPT-11) (n = 82) in adults with recurrent glioblastoma. Median corticosteroid dose for patients who used corticosteroids at baseline was summarized by treatment arm; the percentage of patients who had sustained (≥50% corticosteroid dose reduction for ≥50% of time on study drug) or complete (discontinuation of corticosteroid for ≥25% of time on study drug) reduction in corticosteroid dose overall and by objective response and progression-free survival was calculated. The incidence of corticosteroid-related adverse events was summarized.
Results.
In each treatment group, 50% of patients were using systemic corticosteroids at baseline. The majority of those experienced a reduction in dose while receiving BEV-based therapy. Thirteen (30.2%) BEV and 20 (46.5%) BEV + CPT-11 patients had a sustained reduction of corticosteroid dose; 7 (16.3%) BEV and 9 (20.9%) BEV + CPT-11 patients had a complete reduction of corticosteroid dose. The majority of patients who had an objective response or progression-free survival >6 months experienced corticosteroid dose reduction. Approximately 64% of patients who used corticosteroids while receiving BEV-based therapy experienced infection.
Conclusion.
BEV may have corticosteroid-sparing effects in patients with recurrent glioblastoma. Corticosteroid reduction may positively affect patient health-related quality of life. Given the exploratory nature of the analyses in a noncomparative study, these results should be interpreted cautiously.
Introduction
Corticosteroids are the most frequently used drugs in neuro-oncology, and dexamethasone doses up to 100 mg/day have been used to treat intracranial edema and control symptoms [1]. Chronic high-dose corticosteroids contribute to overall treatment morbidity and predispose patients to opportunistic infections and other complications, including weight gain, osteoporosis, myopathy, diabetes, and psychiatric and neurocognitive complications [1–3]. Accordingly, discontinuation of corticosteroids or reduction of dose to physiological levels is desirable, but does not typically occur with ineffective treatments.
Vascular endothelial growth factor (VEGF) inhibitors are known to have potent antiedema and, therefore, corticosteroid-sparing, effects in patients with high-grade gliomas, enabling some patients to stabilize, reduce, or discontinue their corticosteroid dose during treatment [4–7]. Corticosteroid use in patients with recurrent glioblastoma (GBM) receiving antiangiogenic treatment has not been systematically documented, and there are no formal guidelines for corticosteroid use in recurrent GBM setting.
Bevacizumab (BEV; Avastin; Genentech, Inc., South San Francisco, CA), a monoclonal antibody that inhibits VEGF, has been associated with radiological response and prolonged progression-free survival and overall survival in recurrent GBM [5, 6, 8–10], and it has received FDA approval for use in patients with GBM with progressive disease after prior therapy.
To better understand the effects of anti-VEGF treatment, specifically bevacizumab, on corticosteroid requirements, we assessed patterns of corticosteroid use in patients with recurrent GBM who were treated with bevacizumab in the BRAIN study [8]. BRAIN offers the single largest data set of patients with recurrent GBM treated with bevacizumab. Our results indicated that although 50% of patients were using corticosteroids at baseline, the majority of them experienced decreased corticosteroid use over a sustained period while receiving BEV-based therapy.
Methods: BRAIN Study
BRAIN was a phase II, multicenter, randomized, noncomparative trial that evaluated efficacy and safety of BEV alone (n = 85) or in combination with irinotecan (CPT-11; n = 82) in 167 adult (≥18 years of age) patients with histologically confirmed GBM in first or second relapse who had received prior standard radiotherapy and temozolomide. All patients provided written informed consent prior to study participation.
Co-primary endpoints of BRAIN, objective response (OR) rate and 6-month progression-free survival (PFS), were assessed by a blinded, independent radiology facility (IRF; RadPharm, Inc., Princeton, NJ), according to WHO Response Evaluation Criteria [11], with corticosteroid dose taken into account [12]. Achievement of an OR (i.e., partial or complete response) required that a patient's corticosteroid dose at the time of magnetic resonance imaging was stable or reduced relative to baseline, in accordance with modified Macdonald [12] criteria.
Longitudinal steroid dosing was recorded for all patients while they were receiving study drug. Baseline corticosteroid dose was defined as the average dose ≤4 days prior to the first study treatment, not including corticosteroids used for chemoprophylaxis or inhaled/topical corticosteroid use. All corticosteroid doses were converted to a dexamethasone equivalent dose [13]. Postbaseline corticosteroid use was defined as any use of corticosteroid from the first day of treatment to the last day of treatment.
For patients who were using corticosteroids at baseline, median corticosteroid dose over time was summarized by treatment arm. We also calculated the percentage of these patients who had sustained or complete reduction in corticosteroid dose overall and by OR and PFS status. Sustained reduction was defined as ≥50% corticosteroid dose reduction, relative to baseline, for ≥50% of the time on study drug. Complete reduction was defined as discontinuation of corticosteroid use for ≥25% of the time on study drug. Corticosteroid-related adverse events were not specifically identified; however, we summarized those events that are known to be associated with corticosteroid use [1, 3] for all patients who received at least one dose of study drug.
Results
Patient Baseline Characteristics
Between June 2006 and February 2007, 167 patients with GBM in first or second relapse were randomized to receive BEV (n = 85) or BEV + CPT-11 (n = 82) in the BRAIN study. A comprehensive list of demographics and baseline characteristics of patients who participated in BRAIN has been published [8]. The median age of patients was approximately 55 years, and 69% of patients were male. The majority of patients were at first relapse.
Corticosteroid Use in BRAIN
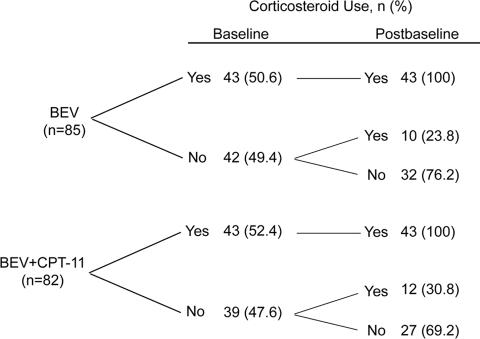
At baseline, 43 (50.6%) patients in the BEV group and 43 (52.4%) in the BEV + CPT-11 group were using systemic corticosteroids, primarily dexamethasone. Of the patients who were not using corticosteroids at baseline, 10 (23.8%) in the BEV group and 12 (30.8%) in the BEV + CPT-11 group used corticosteroids after baseline, whereas 32 (76.2%) and 27 (69.2%), respectively, did not use corticosteroids at any time while on study treatment (Fig 1).
Figure 1.
Corticosteroid use in the BRAIN study.
Abbreviations: BEV, bevacizumab; CPT-11, irinotecan.
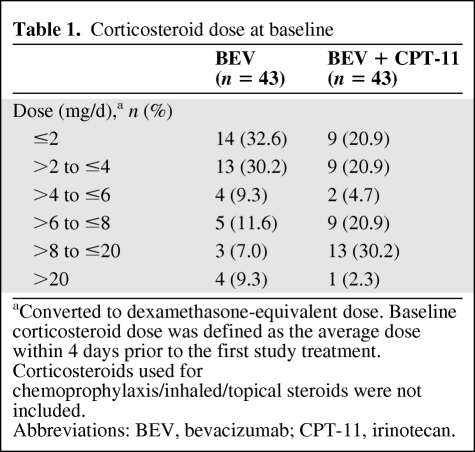
The majority of patients who used corticosteroids at baseline were taking 1–4 mg/d. More patients in the BEV + CPT-11 group (58.1%) were taking >4 mg/d (dexamethasone equivalent) compared with the BEV group (37.2%) (Table 1).
Table 1.
Corticosteroid dose at baseline
aConverted to dexamethasone-equivalent dose. Baseline corticosteroid dose was defined as the average dose within 4 days prior to the first study treatment. Corticosteroids used for chemoprophylaxis/inhaled/topical steroids were not included.
Abbreviations: BEV, bevacizumab; CPT-11, irinotecan.
Efficacy Outcomes for Patients Who Were Using Corticosteroids at Baseline
Of patients who were using corticosteroids at baseline, 14 (32.6%) each in the BEV and BEV + CPT-11 groups had an IRF-determined OR (i.e., complete or partial response) while receiving treatment in the BRAIN study; 12 (27.9%) and 14 (32.6%) patients in the BEV and BEV + CPT-11 groups, respectively, had PFS >6 months.
Corticosteroid Dose over Time
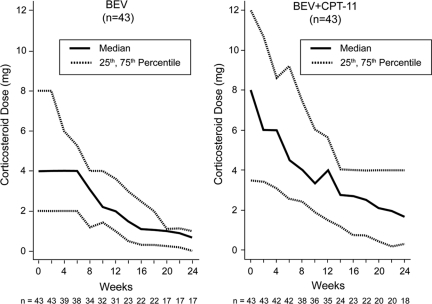
There was a consistent reduction in median corticosteroid dose over time, relative to baseline, for patients receiving BEV-based treatment on the BRAIN study (Fig. 2).
Figure 2.
Change from baseline corticosteroid dose over time. Includes all patients who were using corticosteroids at baseline. Dose reported is dexamethasone equivalent. Numbers under the x-axis label are the number of evaluable patients at each corresponding time point.
Abbreviations: BEV, bevacizumab; CPT-11, irinotecan.
Sustained Reduction
During treatment, 13 (30.2%) patients in the BEV group and 20 (46.5%) in the BEV + CPT-11 group who were using corticosteroids at baseline had a sustained reduction of corticosteroid dose (i.e., ≥50% corticosteroid dose reduction, relative to baseline, for ≥50% of the time on study drug).
Sustained Reduction by Response and PFS
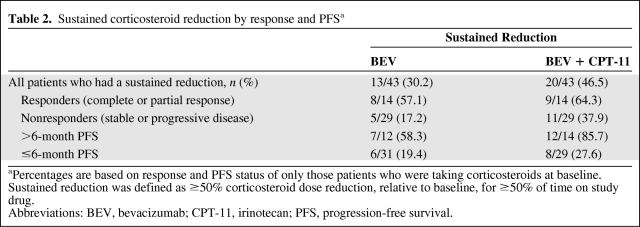
The majority of patients who had used corticosteroids at baseline and had an IRF-determined OR (i.e., responders) or PFS >6 months had sustained reduction of corticosteroid use while on the BRAIN study (Table 2).
Table 2.
Sustained corticosteroid reduction by response and PFSa
aPercentages are based on response and PFS status of only those patients who were taking corticosteroids at baseline. Sustained reduction was defined as ≥50% corticosteroid dose reduction, relative to baseline, for ≥50% of time on study drug.
Abbreviations: BEV, bevacizumab; CPT-11, irinotecan; PFS, progression-free survival.
Complete Reduction
Seven (16.3%) BEV patients and 9 (20.9%) BEV + CPT-11 patients who were using corticosteroids at baseline had a complete reduction of corticosteroid use (i.e., did not use corticosteroids for ≥25% of the time on study treatment).
Complete Reduction by Response and PFS
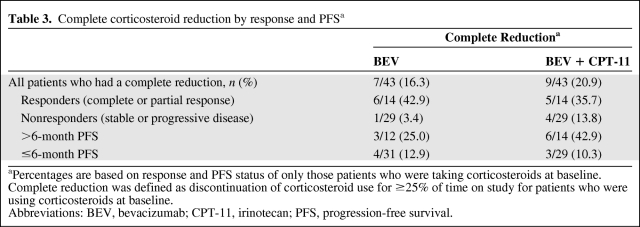
The majority of patients who had used corticosteroids at baseline and had an IRF-determined complete or partial response (i.e., responders) or PFS >6 months had complete reduction of corticosteroid use while on the BRAIN study (Table 3).
Table 3.
Complete corticosteroid reduction by response and PFSa
aPercentages are based on response and PFS status of only those patients who were taking corticosteroids at baseline. Complete reduction was defined as discontinuation of corticosteroid use for ≥25% of time on study for patients who were using corticosteroids at baseline.
Abbreviations: BEV, bevacizumab; CPT-11, irinotecan; PFS, progression-free survival.
Adverse Events
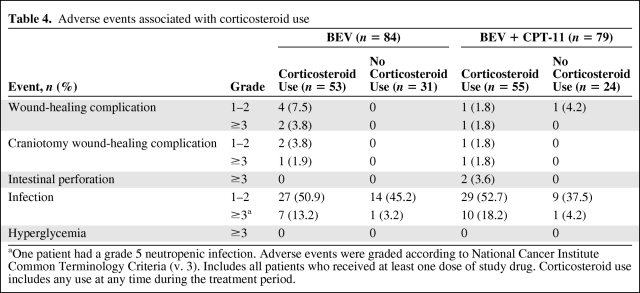
The incidence of AEs that are known to be associated with corticosteroid use were low, with the exception of infection, which occurred in approximately 64% of patients in the BEV group and 71% of patients in the BEV + CPT-11 group who used corticosteroids at any time during the treatment period (Table 4).
Table 4.
Adverse events associated with corticosteroid use
aOne patient had a grade 5 neutropenic infection. Adverse events were graded according to National Cancer Institute Common Terminology Criteria (v. 3). Includes all patients who received at least one dose of study drug. Corticosteroid use includes any use at any time during the treatment period.
Discussion
The majority of patients who participated in the BRAIN study experienced a decrease in corticosteroid dose over time while receiving BEV-based treatment. This result is consistent with previous reports of corticosteroid-sparing properties of BEV and other anti-VEGF agents. For instance, Kreisl et al. [5] reported that 58% of patients with recurrent GBM reduced their baseline corticosteroid dose by an average of 59% while receiving BEV monotherapy; Norden et al. [6] observed that 33% of their patients with recurrent malignant gliomas experienced corticosteroid dose reduction while receiving BEV-based treatment; and Vredenburgh et al. [7] reported that patients who demonstrated a response to BEV therapy tapered their corticosteroid dose. Cediranib, a tyrosine kinase inhibitor of VEGF receptors, was shown to have a normalizing effect on tumor vasculature in patients with recurrent GBM that was associated with reduction or discontinuation of corticosteroid use [4]. Corticosteroid dose reduction in BRAIN was not limited to patients who experienced an OR or remained progression free for >6 months; however, the majority of those patients did tend to have the associated benefit of corticosteroid reduction.
In the BRAIN study, the incidence of AEs that are known to be associated with corticosteroid use was, in fact, slightly higher in patients who were using corticosteroids. Although some of the evaluated AEs are known to be related to bevacizumab use (i.e., wound-healing complications, infection, and gastrointestinal perforation) [14], they are also commonly observed with corticosteroid use [1, 3]. Monitoring a patient's need for corticosteroids while being treated with BEV and making appropriate adjustments may help to minimize the incidence of corticosteroid-associated infection.
Corticosteroid use in patients with GBM receiving anti-VEGF treatment has not been systematically evaluated, and there are no formal guidelines for corticosteroid use or reassessment in the recurrent GBM setting. BRAIN is the first large-scale trial to evaluate longitudinal corticosteroid use from baseline in patients with recurrent GBM receiving bevacizumab. The lack of a control arm in the BRAIN study limits our ability to fully evaluate corticosteroid use in BEV-treated patients. However, our results suggest that BEV has corticosteroid-sparing effects and that corticosteroid use over time while receiving BEV should be systematically evaluated, and dose should be decreased as tolerated by the patient, with special attention paid to signs and symptoms of premature corticosteroid withdrawl, including neurological disability, adrenal insufficiency, headache, and lethargy [2].
Acknowledgments
Liang Fang and Dafeng Chen, Genentech, Inc., provided biostatistics support. Portions of the data were presented at the 2009 meetings of the European Society for Medical Oncology (ESMO) and the Society for NeuroOncology (SNO).
Author Contributions
Conception/Design: James J. Vredenburgh, Timothy Cloughesy, Meghna Samant, Patrick Y. Wen, W.K. Alfred Yung, Asha Das, Henry S. Friedman
Provision of study material or patients: James J. Vredenburgh, Timothy Cloughesy, Meghna Samant, Michael Prados, Patrick Y. Wen, Tom Mikkelsen, David Schiff, Lauren E. Abrey, W.K. Alfred Yung, Nina Paleologos, Martin K. Nicholas, Randy Jensen, Henry S. Friedman
Collection and/or assembly of data: James J. Vredenburgh, Timothy Cloughesy, Michael Prados, Tom Mikkelsen, David Schiff, Lauren E. Abrey, Nina Paleologos, Martin K. Nicholas, Randy Jensen, Henry S. Friedman
Data analysis and interpretation: James J. Vredenburgh, Timothy Cloughesy, Meghna Samant, Patrick Y. Wen, David Schiff, W.K. Alfred Yung, Nina Paleologos, Asha Das, Henry S. Friedman
Manuscript writing: James J. Vredenburgh, David Schiff, Nina Paleologos, Randy Jensen, Asha Das, Henry S. Friedman
Final approval of manuscript: James J. Vredenburgh, Timothy Cloughesy, Meghna Samant, Michael Prados, Patrick Y. Wen, Tom Mikkelsen, David Schiff, Lauren E. Abrey, W.K. Alfred Yung, Nina Paleologos, Martin K. Nicholas, Randy Jensen, Asha Das, Henry S. Friedman
The authors take full responsibility for the content of the paper but thank Roberta M. Kelly, Ph.D. (Genentech, Inc.), for her assistance in providing writing and editorial support.
References
- 1.Wen PY, Schiff D, Kesari S, et al. Medical management of patients with brain tumors. J Neurooncol. 2006;80:313–332. doi: 10.1007/s11060-006-9193-2. [DOI] [PubMed] [Google Scholar]
- 2.DeAngelis LM, Posner JB. Neurological complications of cancer. 2nd edition. Oxford: Oxford University Press; 2009. [Google Scholar]
- 3.Koehler PJ. Use of corticosteroids in neuro-oncology. Anticancer Drugs. 1995;6(1):19–33. doi: 10.1097/00001813-199502000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 7.Vredenburgh JJ, Desjardins A, Herndon JE, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 8.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 9.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 10.Zuniga RM, Torcuator R, Jain R, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91:329–336. doi: 10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 13.Buttgereit F, Brand MD, Burmester GR. Equivalent doses and relative drug potencies for non-genomic glucocorticoid effects: a novel glucocorticoid hierarchy. Biochem Pharmacol. 1999;58:363–368. doi: 10.1016/s0006-2952(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 14.Avastin Package insert 7/09. South San Francisco, CA: Genentech, Inc.; 2009. [Google Scholar]