This paper reports a trial performed by a National Cancer Institute–funded cooperative group evaluating the antitumor efficacy and safety of sunitinib malate in patients with previously treated pancreas adenocarcinoma.
Keywords: Sunitinib, Phase II, Refractory, Pancreas adenocarcinoma, CALGB 80603
Abstract
Background.
The Cancer and Leukemia Group B (CALGB) conducted a phase II study evaluating sunitinib in patients with progressive metastatic pancreas adenocarcinoma following prior gemcitabine-based therapy (trial CALGB 80603; ClinicalTrials.gov identifier, NCT00397787). The primary endpoint was to determine the disease control rate (DCR) as measured by the Response Evaluation Criteria in Solid Tumors (complete response, partial response [PR], and stable disease) at 6 weeks.
Patients and Methods.
Patients aged ≥18 years with an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2 and with progressive pancreas adenocarcinoma following treatment with gemcitabine were eligible. Sunitinib was dosed at 50 mg orally days 1–28, every 42 days (1 cycle). The statistical plan called for a three-stage design. A DCR ≥15% was considered worthy of further study.
Results.
In total, 77 patients were enrolled. Forty-two (54.6%) enrollees were male. The median age was 65 years. The ECOG performance status score distribution was: 0, 39%; 1, 50%; 2, 11%. The DCR was 21.6%; one patient (1.4%) had a PR and 15 patients (20.3%) had stable disease as their best response. The progression-free survival time was 1.31 months (95% confidence interval [CI] 1.25–1.38 months) and overall survival time was 3.68 months (95% CI, 3.06–4.24 months).
Conclusions.
The study met its primary endpoint; however sunitinib had minimal activity and moderate toxicity in a population of gemcitabine-refractory pancreas adenocarcinoma patients. For future studies, limiting enrollment to patients with an ECOG performance status score of 0–1 is recommended.
Introduction
Pancreas adenocarcinoma is a very challenging malignancy because of its refractoriness to available treatments. About 42,000 people were estimated to have been diagnosed with this disease in the U.S. in 2009, the substantial majority of whom would have either locally advanced or metastatic pancreas adenocarcinoma. These patients received either gemcitabine or a gemcitabine-based combination as initial therapy for locally advanced or metastatic disease [1, 2]. Most patients derive modest benefit from frontline therapy, with a median time to progression of 2–4 months. About half of all patients who receive frontline therapy are well enough and eligible for second-line therapy [3, 4]. There is not a standard second-line therapy for pancreas adenocarcinoma, nor a set of well-defined prognostic factors for second-line therapy, and there is a relative dearth of trials conducted in this disease setting [5]. Data from the randomized Charité Onkologie (CONKO)-003 trial and single-institution data have suggested that a fluoropyrimidine combined with oxaliplatin may represent a reasonable second-line option [6–8]. Also, recent observations have demonstrated that only a tiny fraction, approximately 2%, of patients with pancreas adenocarcinoma who are potentially eligible for a second-line therapy actually receive such therapy in the context of a clinical trial [9]. This latter observation may be related to the limited availability of second-line trials in this disease setting.
Sunitinib malate (SU11248, NSC #736511) is an orally bioavailable, multitargeted small molecule inhibitor of several receptor tyrosine kinases that are involved in tumor proliferation and angiogenesis, including vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2, VEGFR-3, platelet-derived growth factor receptor, and stem cell factor receptor (KIT) [10, 11]. VEGF and its receptors are over expressed in pancreas adenocarcinoma and have been associated with the development of metastases and a poor prognosis [12]. In part, the rationale for assessing sunitinib in pancreas adenocarcinoma in this study relates to the contribution of multiple signaling pathways to the pathogenesis of this disease and the potential to impact these pathways with a relatively broad-spectrum targeted therapy along with, at the time, promising data from other anti-VEGF inhibitors in this disease [13]. There is a precedent for the development of novel targeted agents in pancreatic cancer [14]. Treatment with erlotinib combined with gemcitabine has been shown to result in a longer time to tumor progression and higher median and 1-year survival rates than with gemcitabine alone in advanced pancreas cancer [15]. Pancreatic adenocarcinoma is also characterized by a profound desmoplastic and stromal reaction that has rarely been considered as a potential therapeutic target [16, 17]. Sunitinib, via its broad inhibition of receptor tyrosine kinases, offers the potential to target both the tumor directly and the stromal matrix. This paper reports a trial performed by a National Cancer Institute (NCI)-funded cooperative group evaluating the antitumor efficacy and safety of sunitinib malate in patients with previously treated pancreas adenocarcinoma.
Patients and Methods
Patients
This phase II study was conducted by the Cancer and Leukemia Group B (CALGB). Men and women aged ≥18 years with pancreas adenocarcinoma with histologic or cytologic proof of malignancy and with evidence of disease progression on a gemcitabine-containing frontline regimen were eligible. Patients had to fall into one of the following groups: (a) only one prior regimen of gemcitabine or a gemcitabine-containing combination was to have been administered; (b) one prior combined chemoradiotherapy regimen containing gemcitabine for inoperable locally advanced pancreas adenocarcinoma was permitted, as long as the patient had subsequently progressed with measurable disease outside the radiation port; (c) one prior adjuvant gemcitabine-containing regimen or combined chemoradiotherapy regimen containing gemcitabine could have been administered, if the patient subsequently experienced progression of disease within 3 months of completion of adjuvant therapy. Prior erlotinib was allowed. Measurable, metastatic disease was required (≥20 mm by conventional computerized tomography [CT] or ≥10 mm by spiral CT); locally advanced pancreatic cancer with the primary tumor as the sole site of disease was not allowed. An Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2 was required. Required initial laboratory criteria included the following: absolute neutrophil count ≥1,500/ul, platelet count ≥100,000/μl, bilirubin <1.5 mg/dl, prothrombin time and partial thromboplastin time <1.5× the upper limit of normal (ULN), serum creatinine ≤1.5 mg/dl, and aspartate aminotransferase ≤2.5× ULN if no liver metastases r ≤5× ULN if liver metastases were present. Written informed consent was required and the protocol was reviewed and approved by the local institutional review board at each participating site.
Exclusion criteria included the following. No prior therapy with any other antiangiogenic agent (e.g., bevacizumab, sorafenib, etc.) was permitted. No significant cardiac disease was permitted; specifically, the QTc interval had to be ≤500 msec. No prior myocardial infarction, cardiac arrhythmia, active angina, active congestive cardiac failure (New York Heart Association class III or class IV), or coronary artery bypass grafting or stenting in the previous 12 months prior to registration was allowed. No history of a cerebrovascular accident or transient ischemic attack within 12 months or pulmonary emboli within 6 months prior to registration was allowed. Patients with a nonhealing wound, ulcer, or bone fracture were not enrolled. No history of significant bleeding (within 6 months), abdominal fistula, gastrointestinal perforation, or intra-abdominal abscess was permitted. Patients with duodenal invasion by tumor on CT were not enrolled. Inhibitors and inducers of cytochrome P450 3A4 had to be discontinued prior to and during treatment with sunitinib. Also, drugs with proarrhythmic potential were not allowed during the study. Use of warfarin anticoagulants was not allowed. No “currently active” second malignancy, other than nonmelanoma skin cancer, was permissible. Patients were not considered to have a “currently active” malignancy if they had completed therapy and were considered by their physician to have a <30% risk for relapse. Brain metastases excluded patients from enrollment.
Study Procedures
Prior to registration, patients underwent a history and physical examination including notation of height, weight, ECOG performance status score, vital signs (blood pressure, temperature, pulse, respiration), and a pregnancy test for all females of child-bearing potential. A baseline CT or magnetic resonance imaging scan documenting all sites of metastatic disease was required within 4 weeks of study enrollment. A baseline electrocardiogram and either echocardiogram (ECHO) or multigated acquisition (MUGA) scan were performed prior to the initiation of therapy. Patients were evaluated weekly for toxicity and blood pressure monitoring during the first cycle. Tumor restaging was performed every cycle (every 6 weeks) for each of the first four cycles and then every other cycle thereafter. A follow up ECHO or MUGA scan was performed after the second cycle of therapy (every 12 weeks). Treatment was continued indefinitely until either disease progression or unacceptable toxicity occurred.
As part of the quality assurance program of the CALGB, members of the audit committee visit all participating institutions at least once every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in a sample of protocols at each institution. Such on-site review of medical records was performed for a subgroup of 11 patients (14%) of the 77 patients under this study.
Treatment and Dose Adjustments
Sunitinib malate was provided by the Division of Cancer Treatment and Diagnosis (DCTD), NCI, under a collaborative agreement between Pfizer and the DCTD. The starting dose of sunitinib was 50 mg. Sunitinib was dosed at 50 mg orally daily irrespective of timing of food, for days 1–28 followed by 14 days of rest, constituting one treatment cycle (42 days, 6 weeks). Dose level −1 of sunitinib was 37.5 mg and level −2 was 25 mg. No dose re-escalation was permitted following dose adjustment for toxicity. Patients requiring dose reduction beyond the −2 level were to discontinue protocol therapy. A treatment break of up to 4 weeks was permitted for toxicity or other disease-related complications. Protocol-specific dose modifications were prescribed for grade 3 or 4 hematologic toxicity (neutropenia, thrombocytopenia), grade 3 or 4 fatigue, hypertension, QTc prolongation, decreases in left ventricular ejection fraction, and grade 3 or 4 skin toxicity or grade 3 or 4 bleeding. For all other grade 3 toxicities, sunitinib was held until toxicity improved to grade ≤2. For other grade 4 toxicities, protocol therapy was permanently discontinued.
Statistical Plan and Analysis
The study was designed as a single-arm, nonrandomized, multicenter cooperative group (CALGB) phase II trial of single-agent sunitinib for previously treated pancreas adenocarcinoma in patients with measurable metastatic disease. The primary endpoint of the study was disease the control rate (DCR), specified as either a complete response (CR), partial response (PR), or stable disease (SD) as measured by the Response Evaluation Criteria in Solid Tumors (RECIST) 6 weeks following the initiation of protocol therapy [18, 19]. The null hypothesis that the DCR according to the RECIST was ≤5%, versus the alternative that the DCR was ≥15%, was tested using a three-stage design. If the DCR was ≥15%, then single-agent sunitinib was to be considered worthy of further study in gemcitabine-refractory pancreas adenocarcinoma. Nineteen patients were to be entered in stage 1. If a DCR of 0% was observed during the first 19 patients studied, the trial was to be closed because of a lack of efficacy. If a DCR >5% was observed among the first 19 patients studied, 20 additional patients were to be enrolled in stage 2. If a DCR ≤2% was observed among the first 39 patients studied, the trial was to close at stage 2 because of a lack of efficacy. If two or more cases of a CR, PR, or SD (DCR >5%) were observed among the first 39 patients studied, 21 additional patients were to be enrolled in stage 3. Sunitinib was to be considered worthy of further investigation at stage 3 if six cases of CR, PR, or SD (DCR of 10%) were observed among 60 eligible patients treated. The simulated power and significance level under this design (10,000 simulations) are 0.9 and 0.08, respectively. The sample size was increased to 64 to allow for replacement of patients who did not initiate protocol therapy. All patients who met the eligibility criteria and received at least one dose of protocol therapy were included in the analysis of the primary endpoint.
The secondary endpoints of the study were: response duration, progression-free survival (PFS), toxicity, and overall survival. Duration of response was determined for the subset of patients who achieved a confirmed response (CR or PR). Duration of an objective response was defined as the time from the first tumor assessment indicating response to the time of disease progression or death from any cause. PFS and overall survival times were assessed using the Kaplan–Meier method. Toxicity data were graded using the NCI Common Toxicity Criteria for Adverse Events, version 3.0. Particular attention was paid to hypertension, cardiac events, bleeding, and thrombotic events. All adverse events were reported to the CALGB. All patients who received at least one dose of sunitinib were evaluable for toxicity.
Results
Patient Characteristics
Patient registration and data collection were managed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson (E.O.'R.). Statistical analyses were performed by CALGB statisticians.
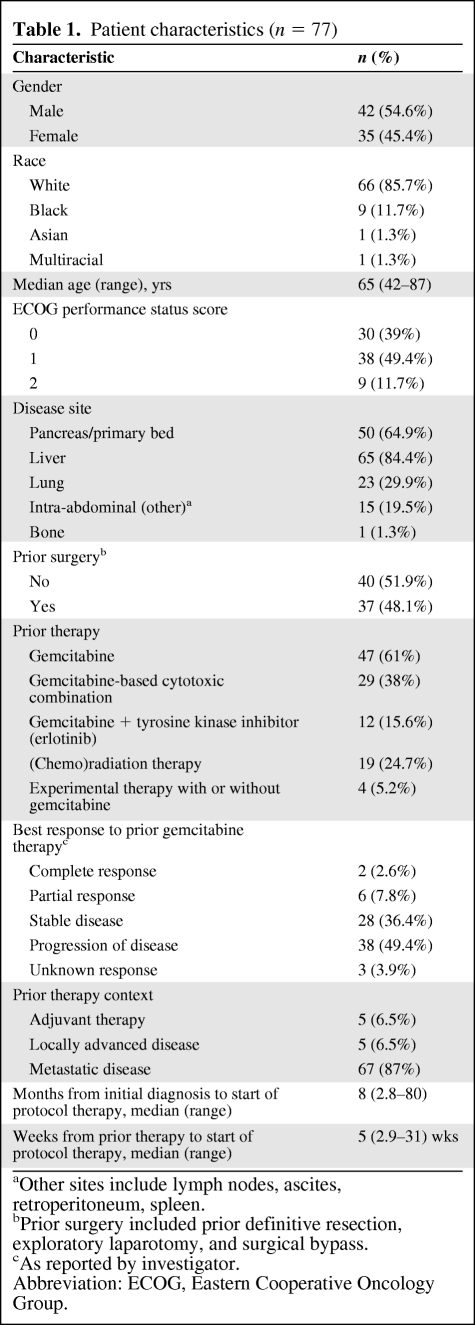
In total, 77 patients were enrolled from November 2006 through November 2007. The study met the criteria for proceeding to the third stage. Enrollment to the study was very brisk in the last several weeks and hence, in view of commitment to multiple study sites, the study recruitment exceeded the goal of 64 patients. Forty-two patients (54.5%) were male. The median age was 65 years (range, 42–87 years). The ECOG performance status score was 0 in 30 patients (38.9%), 1 in 38 patients (49.4%), and 2 in nine patients (11.7%). Eight-four percent of patients had liver metastases on entry to the study. Thirty-six percent of patients had had stable disease to prior gemcitabine and 10.4% had experienced a response to gemcitabine therapy. A detailed summary of patient characteristics is provided in Table 1.
Table 1.
Patient characteristics (n = 77)
aOther sites include lymph nodes, ascites, retroperitoneum, spleen.
bPrior surgery included prior definitive resection, exploratory laparotomy, and surgical bypass.
cAs reported by investigator.
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Primary Endpoint
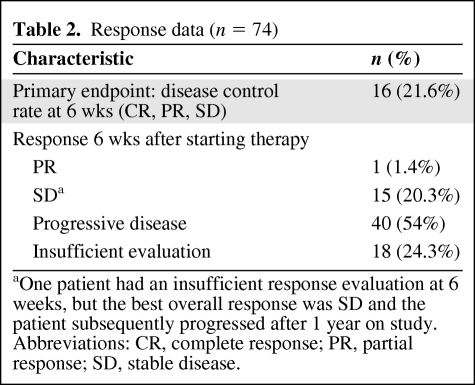
Seventy-four patients were eligible for analysis of the primary study endpoint. Three of the 77 patients enrolled never received treatment. The median follow-up time was 22.1 months. The median number of cycles delivered was one. The (CR, PR, or SD by the RECIST at 6 weeks) was 21.6%, with one patient (1.4%) with a PR and 15 patients (20.3%) with SD. Forty patients (54%) had progressive disease as their best response. Eighteen patients (24.3%) did not have a follow-up scan and were not evaluable for the primary endpoint. Those patients ended treatment prior to completing one cycle of therapy as a result of progressive disease (n = 3), death resulting from progressive disease (n = 9), and withdrawal of consent prior to receiving any treatment (n = 3). Only one of those patients was reported to have gone on to receive further treatment. For the patient with an observed PR, this was maintained for 16 weeks. For the 15 patients who had SD as their best response, the median SD duration was 11.3 weeks (range, 5.7–55 weeks). Response data are summarized in Table 2.
Table 2.
Response data (n = 74)
aOne patient had an insufficient response evaluation at 6 weeks, but the best overall response was SD and the patient subsequently progressed after 1 year on study.
Abbreviations: CR, complete response; PR, partial response; SD, stable disease.
Secondary Endpoints
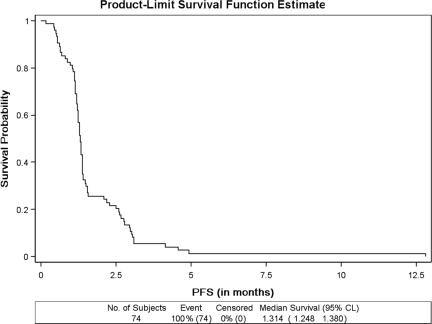
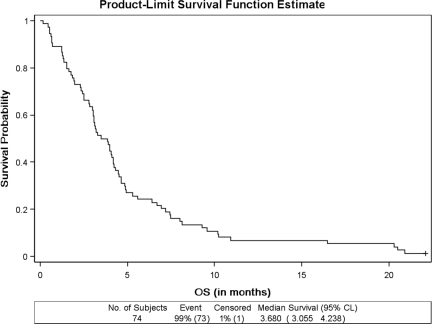
Seventy-four patients were eligible for the survival analysis. The median duration of protocol therapy was 28 days (range, 2–126 days). The median PFS interval for all 74 patients was 1.31 months (95% confidence interval [CI], 1.25–1.38 months) (Fig. 1). Seventy-three patients had died and one was alive at the time of the final study analysis. The overall median survival duration was 3.68 months (95% CI, 3.06–4.24 months) (Fig. 2).
Figure 1.
PFS in patients treated with sunitinib in the setting of prior gemcitabine treatment for metastatic pancreas adenocarcinoma.
Abbreviations: CI, confidence interval; PFS, progression-free survival.
Figure 2.
OS in patients treated with sunitinib in the setting of prior gemcitabine treatment for metastatic pancreas adenocarcinoma.
Abbreviations: CI, confidence interval; OS, overall survival.
Toxicity
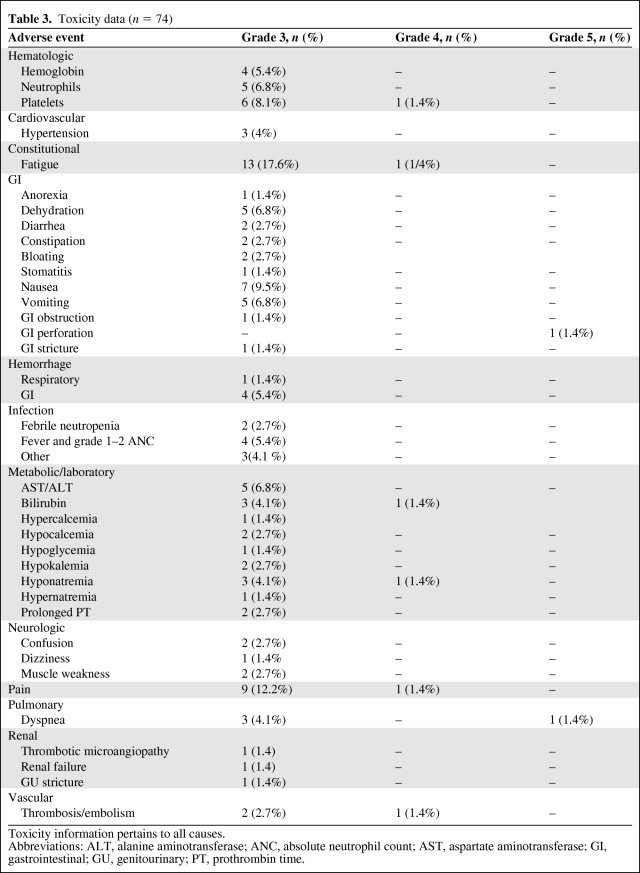
All 74 patients who received any dose of study drug were eligible for toxicity assessment. Twenty-one (27.1%) patients required a dose modification during cycle 1. The principal grade 3–5 toxicities observed were hematologic (n = 16, 21.7%, and nonhematologic (n = 32, 43.2%). Specifically, relevant grade 3 toxicities included: hypertension in three patients (4%), fatigue in 13 patients (17.6%), bleeding in five patients (6.8%), thrombotic microangiopathy/renal failure in two patients (2.7%), and thrombosis in two patients (2.7%). There were two (2.7%) grade 5 events on study, a gastrointestinal perforation and respiratory distress. Both of these grade 5 toxicities were attributed to the study drug and related to progression of underlying pancreas adenocarcinoma. The commonest reasons for study discontinuation were progression of disease (POD) during therapy (61%), adverse event (9.1%), death during treatment/POD (14.3%), or declining further treatment (9.1%). Toxicity information is summarized in Table 3.
Table 3.
Toxicity data (n = 74)
Toxicity information pertains to all causes.
Abbreviations: ALT, alanine aminotransferase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; GI, gastrointestinal; GU, genitourinary; PT, prothrombin time.
Discussion
Progressive metastatic pancreas adenocarcinoma following frontline gemcitabine-based therapy represents a very poor prognostic disease setting with median survival times measured in the several month range [20, 21]. However, this patient group represents a significant patient number in that about 40%–60% of patients who receive frontline therapy are well enough and eligible to receive further therapy [4]. New therapies are desperately needed for this patient population. This study assessed the single-agent utility of sunitinib, a broad-spectrum receptor tyrosine kinase inhibitor, in this patient population in the context of a large, cooperative group, single-arm, phase II clinical trial. Sunitinib represents an attractive drug to assess given its inhibition of multiple targets and the importance of the VEGF pathway in pancreas adenocarcinoma [12, 22] and, notwithstanding, potential activity against the desmoplastic stromal matrix, which appears to be fundamental to the development and maintenance of the pancreatic neoplastic phenotype [23]. This study technically met its primary endpoint of disease control in that 21.7% of patients had a CR, PR, or SD at 6 weeks (mostly SD). However, the very short time on study of less than one treatment cycle, reflected in the short PFS time of 1.31 months, the moderate toxicity, and the limited survival argue against any usefulness of sunitinib in this patient population. The experience and results observed in this study have been mirrored by other trials conducted with other targeted agents in a similar treatment population [20, 24–27]. One positive note was the interest in this trial evident in its very brisk recruitment, suggesting that a cooperative group can provide a good forum for conducting second-line clinical trials with novel agents in patients with previously treated pancreas adenocarcinoma.
Since the time of study conception, notwithstanding the strong preclinical rationale for antivascular therapy in pancreas adenocarcinoma [12, 22, 28], the accumulating clinical data speak against the utility of targeting VEGFR pathways as being a useful clinical approach. In the treatment of frontline advanced pancreas adenocarcinoma, there are now two fully reported randomized trials that have failed to meet a primary survival endpoint of demonstrating superiority for the addition of the antivascular agent [29–34], and two other studies evaluating the addition of axitinib to gemcitabine and the addition of aflibercept (VEGF-Trap) to gemcitabine have been preliminarily reported as not meeting a survival endpoint. The reasons for the failure of antivascular therapy remain relatively poorly understood and may relate to the hypoxic microenvironment of pancreas adenocarcinoma, along with drug delivery and distribution challenges [17, 35].
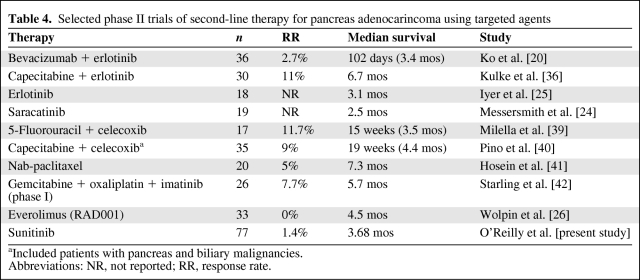
Targeted agents have been extensively assessed in both the first- and second-line setting in pancreas adenocarcinoma. Using single or combination targeted agents in a second-line setting has yielded rare responses, short PFS and overall survival times, and limited value to this strategy (Table 4). Drugs assessed include erlotinib [25], saracatinib [24], everolimus [26], bevacizumab plus erlotinib [20], and sunitinib. Somewhat more activity has been observed for combining a targeted agent with cytotoxic therapy in the refractory disease setting [36], although here the benefit may relate to the cytotoxic backbone alone because no randomized trials have evaluated the addition of a targeted agent in the second-line setting when combined with cytotoxic therapy.
Table 4.
Selected phase II trials of second-line therapy for pancreas adenocarincoma using targeted agents
aIncluded patients with pancreas and biliary malignancies.
Abbreviations: NR, not reported; RR, response rate.
Where do we go from here in the second-line treatment of pancreas adenocarcinoma? Treating patients with advanced pancreas cancer refractory to frontline therapy remains very challenging. Of fundamental importance is the patient's functional status at the time of second-line treatment initiation [5, 30]. Arguably, performance status may be one of the key predictors of outcome in the second-line setting of treatment for pancreas adenocarcinoma, along with whether or not patients responded to initial gemcitabine-based therapy. The latter characteristic was not formally assessed as part of this particular study, but was provided by the investigator. In the trial reported herein, response to prior therapy did not appear to correlate with outcome; however, other cytotoxic-based trials have stratified for response to frontline therapy [21]. In another retrospective analysis, the first-line PFS interval was identified to be the main prognostic factor for benefit from second-line therapy [3]. The current study permitted patients with an ECOG performance status score of 2. In reality, these patients probably never really had a chance to benefit from second-line therapy because their disease-related complications/comorbidities were always likely to overwhelm the potential benefit of the therapeutic agent under study. Restricting enrollment in this second-line setting to patients with an ECOG performance status score of 0–1 is recommended going forward, particularly in studies accrued from a broad base spanning academic centers through community practice settings.
Regardless, the fundamental challenge for making progress in the treatment of pancreas adenocarcinoma, irrespective of disease setting, does not relate to patient selection. It relates to the dearth of truly active drugs with meaningful impacts on pathways that are important for maintenance and progression of the malignant metastatic state and the natural history of the disease. In these authors' opinions, cytotoxic therapy remains entrenched as part of the treatment of pancreas cancer either first- or second-line. Conroy et al. [37] recently reported preliminary results from the Partenariat de Recherche Oncologie Digestive (PRODIGE) 4/ACCORD 11 trial demonstrating clear superiority for 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (the FOLFIRINOX regimen) over gemcitabine in patients with untreated metastatic pancreas cancer. This signal deserves further evaluation, and indeed, variants of it (infusional 5-fluorouracil and oxaliplatin [6, 21, 38]) are already established as a second-line treatment. For now, the role of targeted agents in previously treated pancreas cancer patients appears to be with integration with cytotoxic therapy. Olive and colleagues elegantly demonstrated that inhibition of Hedgehog signaling in a pancreas cancer mouse model refractory to gemcitabine may enhance drug delivery and effect short-term disease stabilization in the presence of IPI-926, a hedgehog signaling inhibitor [35]. If the proof of principle of this strategy holds, the implications could be significant for the treatment of pancreas adenocarcinoma.
Acknowledgments
The research for CALGB 80603 trial was supported, in part, by grants from the NCI (CA31946) to the CALGB (Richard L. Schilsky, M.D., Chairman) and to the CALGB Statistical Center (Stephen George, Ph.D., CA33601). This trial was also supported by the Cancer Treatment Evaluation Program and Pfizer. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NCI.
The following institutions participated in the study: Christiana Care Health Services, Inc. CCOP, Wilmington, DE—Stephen Grubbs, M.D., supported by CA45418; Dana-Farber Cancer Institute, Boston, MA—Harold J Burstein, M.D., Ph.D., supported by CA32291; Dartmouth Medical School–Norris Cotton Cancer Center, Lebanon, NH—Konstantin Dragnev, M.D., supported by CA04326; Duke University Medical Center, Durham, NC—Jeffrey Crawford, M.D., supported by CA47577; Georgetown University Medical Center, Washington, DC—Minetta C. Liu, M.D., supported by CA77597; Hematology-Oncology Associates of Central New York CCOP, Syracuse, NY—Jeffrey Kirshner, M.D., supported by CA45389; Illinois Oncology Research Association, Peoria, IL—John W. Kugler, M.D., supported by CA35113; Long Island Jewish Medical Center, Lake Success, NY—Kanti R. Rai, M.D., supported by CA35279; Memorial Sloan-Kettering Cancer Center, New York, NY—Clifford A. Hudis, M.D., supported by CA77651; Missouri Valley Consortium-Ccop, Omaha, NE—Gamini S. Soori, M.D.; New Hampshire Oncology-Hematology PA, Concord, NH—Douglas J. Weckstein; Northern Indiana Cancer Research Consortium CCOP, South Bend, IN—Rafat Ansari, M.D., supported by CA86726; Rhode Island Hospital, Providence, RI—William Sikov, M.D., supported by CA08025; Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC—James N. Atkins, M.D., supported by CA45808; The Ohio State University Medical Center, Columbus, OH—Clara D. Bloomfield, M.D., supported by CA77658; University of California at San Diego, San Diego, CA—Barbara A. Parker, M.D., supported by CA11789; University of Chicago, Chicago, IL—Hedy L. Kindler, M.D., supported by CA41287; University of Iowa, Iowa City, IA—Daniel A. Vaena, M.D., supported by CA47642; University of Nebraska Medical Center, Omaha, NE—Anne Kessinger, M.D., supported by CA77298; University of Vermont, Burlington, VT—Steven M. Grunberg, M.D., supported by CA77406; Washington University School of Medicine, St. Louis, MO—Nancy Bartlett, M.D., supported by CA77440.
This work was presented previously in part at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, 2008.
Author Contributions
Conception/Design: Eileen M. O'Reilly, Donna Niedzwiecki, Margaret Hall, Richard Goldberg
Provision of study material or patients: Eileen M. O'Reilly, Ghassan K. Abou-Alfa, Hedy L. Kindler, Tanios Bekaii-Saab, Timothy Pluard
Collection and/or assembly of data: Eileen M. O'Reilly, Kathe Douglas, Donna Niedzwiecki, Margaret Hall
Data analysis and interpretation: Eileen M. O'Reilly, Donna Niedzwiecki, Margaret Hall, Donna R. Hollis
Manuscript writing: Eileen M. O'Reilly
Final approval of manuscript: Eileen M. O'Reilly, Ghassan K. Abou-Alfa, Hedy L. Kindler, Richard Schilsky, Tanios Bekaii-Saab, Richard Goldberg, Timothy Pluard, Donna R. Hollis
References
- 1.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 2.Heinemann V, Boeck S, Hinke A, et al. Meta-analysis of randomized trials: Evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008;8:82. doi: 10.1186/1471-2407-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reni M, Berardi R, Mambrini A, et al. A multi-centre retrospective review of second-line therapy in advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2008;62:673–678. doi: 10.1007/s00280-007-0653-y. [DOI] [PubMed] [Google Scholar]
- 4.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Nakachi K, Furuse J, Ishii H, et al. Prognostic factors in patients with gemcitabine-refractory pancreatic cancer. Jpn J Clin Oncol. 2007;37:114–120. doi: 10.1093/jjco/hyl144. [DOI] [PubMed] [Google Scholar]
- 6.Xiong HQ, Varadhachary GR, Blais JC, et al. Phase 2 trial of oxaliplatin plus capecitabine (XELOX) as second-line therapy for patients with advanced pancreatic cancer. Cancer. 2008;113:2046–2052. doi: 10.1002/cncr.23810. [DOI] [PubMed] [Google Scholar]
- 7.Pelzer U, Kubica K, Stieler J, et al. A randomized trial in patients with gemcitabine refractory pancreatic cancer. Final results of the CONKO 003 study. J Clin Oncol. 2008;26(15 suppl):4508. [Google Scholar]
- 8.Xiong HQ, Wolff RA, Hess KR, et al. A phase II trial of oxaliplatin plus capecitabine (xelox) as second line therapy for patients with advanced pancreatic cancer. J Clin Oncol. 2006;24:207s. doi: 10.1002/cncr.23810. [DOI] [PubMed] [Google Scholar]
- 9.Schrag D, Archer L, Wang X, et al. A patterns-of-care study of post-progression treatment (Rx) among patients (pts) with advanced pancreas cancer (APC) after gemcitabine therapy on Cancer and Leukemia Group B (CALGB) study #80303. J Clin Oncol. 2007;25(18 suppl):4524. [Google Scholar]
- 10.Hartmann JT, Haap M, Kopp HG, et al. Tyrosine kinase inhibitors—a review on pharmacology, metabolism and side effects. Curr Drug Metab. 2009;10:470–481. doi: 10.2174/138920009788897975. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto KM. Su-11248 Sugen. Curr Opin Investig Drugs. 2004;5:1329–1339. [PubMed] [Google Scholar]
- 12.Seo Y, Baba H, Fukuda T, et al. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239–2245. doi: 10.1002/(sici)1097-0142(20000515)88:10<2239::aid-cncr6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Kindler HL, Friberg G, Singh DA, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033–8040. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 14.Mackenzie RP, McCollum AD. Novel agents for the treatment of adenocarcinoma of the pancreas. Expert Rev Anticancer Ther. 2009;9:1473–1485. doi: 10.1586/era.09.109. [DOI] [PubMed] [Google Scholar]
- 15.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 16.Guturu P, Shah V, Urrutia R. Interplay of tumor microenvironment cell types with parenchymal cells in pancreatic cancer development and therapeutic implications. J Gastrointest Cancer. 2009;40:1–9. doi: 10.1007/s12029-009-9071-1. [DOI] [PubMed] [Google Scholar]
- 17.Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut. 2007;56:1134–1152. doi: 10.1136/gut.2006.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lara PN, Jr, Redman MW, Kelly K, et al. Disease control rate at 8 weeks predicts clinical benefit in advanced non-small-cell lung cancer: Results from Southwest Oncology Group randomized trials. J Clin Oncol. 2008;26:463–467. doi: 10.1200/JCO.2007.13.0344. [DOI] [PubMed] [Google Scholar]
- 19.Dhani N, Tu D, Sargent DJ, et al. Alternate endpoints for screening phase II studies. Clin Cancer Res. 2009;15:1873–1882. doi: 10.1158/1078-0432.CCR-08-2034. [DOI] [PubMed] [Google Scholar]
- 20.Ko AH, Venook AP, Bergsland EK, et al. A phase II study of bevacizumab plus erlotinib for gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2010;66:1051–1057. doi: 10.1007/s00280-010-1257-5. [DOI] [PubMed] [Google Scholar]
- 21.Pelzer U, Kubica K, Stieler J, et al. A randomized trial in patients with gemcitabine refractory pancreatic cancer. Final results of the CONKO 003 study. J Clin Oncol. 2008;26(15 suppl):4508. [Google Scholar]
- 22.Hotz HG, Gill PS, Masood R, et al. Specific targeting of tumor vasculature by diphtheria toxin-vascular endothelial growth factor fusion protein reduces angiogenesis and growth of pancreatic cancer. J Gastrointest Surg. 2002;6:159–166. doi: 10.1016/s1091-255x(01)00040-3. discussion 166. [DOI] [PubMed] [Google Scholar]
- 23.Buchholz M, Gress TM. Molecular changes in pancreatic cancer. Expert Rev Anticancer Ther. 2009;9:1487–1497. doi: 10.1586/era.09.107. [DOI] [PubMed] [Google Scholar]
- 24.Messersmith WA, Nallapareddy S, Arcaroli J, et al. A phase II trial of saracatinib (AZD0530), an oral Src inhibitor, in previously treated metastatic pancreatic cancer. J Clin Oncol. 2010;28(15 suppl):e14515. [Google Scholar]
- 25.Iyer R, Khushalani NI, Tan W, et al. A phase II study of erlotinib in patients (pts) with advanced pancreatic cancer (APC) who are refractory to gemcitabine (G). Presented at the 2010 Gastrointestinal Cancers Symposium; January 22–24, 2010; Orlando, Florida. [Google Scholar]
- 26.Wolpin BM, Hezel AF, Abrams T, et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193–198. doi: 10.1200/JCO.2008.18.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nallapareddy S, Arcaroli J, Touban B, et al. A phase II trial of sarcatanib (AZD0530), an oral Src inhibitor in previously treated metastatic pancreatic cancer. Presented at the 2010 Gastrointestinal Cancers Symposium; January 22–24, 2010; Orlando, Florida. [Google Scholar]
- 28.Fujimoto K, Hosotani R, Wada M, et al. Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer. 1998;34:1439–1447. doi: 10.1016/s0959-8049(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 29.Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: Phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kindler HL, Niedzwiecki D, Hollis DR, et al. A double-blind, placebo-controlled, randomized phase III trial of gemcitabine (G) plus bevacizumab (B) versus gemcitabine plus placebo in patients (pts) with advanced pancreatic cancer (PC): A preliminary analysis of Cancer and Leukemia Group B (CALGB) [abstract] J Clin Oncol. 2007;25(18 suppl):4508. [Google Scholar]
- 31.Hruban RH, Petersen GM, Goggins M, et al. Familial pancreatic cancer. Ann Oncol. 1999;10(suppl 4):69–73. [PubMed] [Google Scholar]
- 32.Wallace JA, Locker G, Nattam S, et al. Sorafenib (S) plus gemcitabine (G) for advanced pancreatic cancer (PC): A phase II trial of the University of Chicago Phase II Consortium. J Clin Oncol. 2007;25(18 suppl):4608. [Google Scholar]
- 33.Cohen DJ, Ryan T, Moskovits T, et al. Safety and tolerability of combined gemcitabine (G) and erlotinib (E) plus sorafenib (S) in the first-line treatment of metastatic pancreatic cancer. J Clin Oncol. 2009;27(15 suppl):e15594. [Google Scholar]
- 34.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 35.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kulke MH, Blaszkowsky LS, Ryan DP, et al. Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol. 2007;25:4787–4792. doi: 10.1200/JCO.2007.11.8521. [DOI] [PubMed] [Google Scholar]
- 37.Conroy T, Desseigne F, Ychou M, et al. Randomized phase III trial comparing FOLFIRINOX (F: 5FU/leucovorin [LV], irinotecan [I], and oxaliplatin [O]) versus gemcitabine (G) as first-line treatment for metastatic pancreatic adenocarcinoma (MPA): Preplanned interim analysis results of the PRODIGE 4/ACCORD 11 trial. J Clin Oncol. 2010;28(15 suppl):4010. [Google Scholar]
- 38.Tsavaris N, Kosmas C, Skopelitis H, et al. Second-line treatment with oxaliplatin, leucovorin and 5-fluorouracil in gemcitabine-pretreated advanced pancreatic cancer: A phase II study. Invest New Drugs. 2005;23:369–375. doi: 10.1007/s10637-005-1446-y. [DOI] [PubMed] [Google Scholar]
- 39.Milella M, Gelibter A, Di Cosimo S, et al. Pilot study of celecoxib and infusional 5-fluorouracil as second-line treatment for advanced pancreatic carcinoma. Cancer. 2004;101:133–138. doi: 10.1002/cncr.20338. [DOI] [PubMed] [Google Scholar]
- 40.Pino MS, Milella M, Gelibter A, et al. Capecitabine and celecoxib as second-line treatment of advanced pancreatic and biliary tract cancers. Oncology. 2009;76:254–261. doi: 10.1159/000205388. [DOI] [PubMed] [Google Scholar]
- 41.Hosein PJ, Lopes Gd, Jr., Gomez CM, et al. A phase II trial of nab-paclitaxel (NP) in patients with advanced pancreatic cancer (PC) who have progressed on gemcitabine (G)-based therapy. J Clin Oncol. 2010;28(15 suppl):4120. [Google Scholar]
- 42.Starling N, Hawkes EA, Chau I, et al. A dose-escalation study of gemcitabine (Gem) plus oxaliplatin (Ox) in combination with imatinib in patients (pts) with gemcitabine-refractory advanced pancreatic adenocarcinoma (PC) J Clin Oncol. 2010;28(15 suppl):4155. doi: 10.1093/annonc/mdr317. [DOI] [PubMed] [Google Scholar]