The authors report the results of a phase III clinical trial comparing five different treatment regimens in patients with cancer cachexia. Combination treatment with medroxyprogesterone or megestrol acetate, oral supplementation with eicosapentaenoic acid, L-carnitine, and thalidomide was most effective in these patients.
Keywords: Cancer cachexia, Randomized phase III trial, Combined approach, Lean body mass, Fatigue, Resting energy expenditure
Abstract
Purpose.
A phase III, randomized study was carried out to establish the most effective and safest treatment to improve the primary endpoints of cancer cachexia—lean body mass (LBM), resting energy expenditure (REE), and fatigue—and relevant secondary endpoints: appetite, quality of life, grip strength, Glasgow Prognostic Score (GPS) and proinflammatory cytokines.
Patients and Methods.
Three hundred thirty-two assessable patients with cancer-related anorexia/cachexia syndrome were randomly assigned to one of five treatment arms: arm 1, medroxyprogesterone (500 mg/day) or megestrol acetate (320 mg/day); arm 2, oral supplementation with eicosapentaenoic acid; arm 3, L-carnitine (4 g/day); arm 4, thalidomide (200 mg/day); and arm 5, a combination of the above. Treatment duration was 4 months.
Results.
Analysis of variance showed a significant difference between treatment arms. A post hoc analysis showed the superiority of arm 5 over the others for all primary endpoints. An analysis of changes from baseline showed that LBM (by dual-energy X-ray absorptiometry and by L3 computed tomography) significantly increased in arm 5. REE decreased significantly and fatigue improved significantly in arm 5. Appetite increased significantly in arm 5; interleukin (IL)-6 decreased significantly in arm 5 and arm 4; GPS and Eastern Cooperative Oncology Group performance status (ECOG PS) score decreased significantly in arm 5, arm 4, and arm 3. Toxicity was quite negligible, and was comparable between arms.
Conclusion.
The most effective treatment in terms of all three primary efficacy endpoints and the secondary endpoints appetite, IL-6, GPS, and ECOG PS score was the combination regimen that included all selected agents.
Introduction
Cachexia is a multifactorial syndrome characterized by tissue wasting; body weight loss, mostly loss of lean body mass (LBM); increased resting energy expenditure (REE); metabolic alterations (the latter two comprising hypermetabolic syndrome); fatigue; and a reduced performance status [1], very often accompanied by anorexia leading to reduced food intake. Cachexia accompanies the end stage of several chronic diseases, in particular, cancer, and therefore the term “cancer-related anorexia/cachexia syndrome” (CACS) is used [2–5]. The prevalence of CACS increases from 50% to >80% before death, and in >20% of cancer patients it is the cause of death [6].
The proinflammatory cytokines interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α play central roles in the pathophysiology of CACS [7–12]. There is evidence that chronic, low-grade, tumor-induced activation of the host immune system, which shares several characteristics with the “acute-phase response,” is involved in CACS [13].
Consequently, the management of CACS is a complex challenge that should address the different causes underlying this clinical event with an integrated or multimodal treatment approach. Optimal management of CACS patients would be to cure the cancer, but, unfortunately, this remains an infrequent achievement in adults with advanced solid tumors. A second option is to increase nutritional intake, but a large number of randomized, controlled trials of nutritional intervention failed to show a significant benefit in terms of weight change or quality of life (QoL). These results have led to attempts to manipulate the process of cachexia with a variety of pharmacological agents, with the main purpose of providing symptomatic improvement. To date, however, despite several years of coordinated efforts in basic and clinical research, practice guidelines for the prevention and treatment of CACS are lacking [14].
On the basis of this rationale, we carried out an open, early phase II study according to the Simon two-stage design to test the efficacy and safety of integrated oral treatment based on pharmaconutritional support, antioxidants, and drugs in advanced cancer patients with CACS. Twenty-two of 39 evaluable patients responded to the treatment, achieving a significant improvement in the key endpoint variables LBM, fatigue, appetite, QoL (measured by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 [EORTC QLQ-C30]), IL-6, and TNF-α. The observed body weight increase (1.9 kg) was almost completely sustained by a parallel increase in LBM (1.7 kg), independently correlated with an IL-6 decrease, thus strengthening the role of proinflammatory cytokines. Treatment was safe without any toxic effects [15, 16]. These promising results warranted a phase III study.
Aim of the Study
In April 2005, we started a phase III, randomized study with the aim of establishing the most effective and safest treatment able to improve the identified “key” variables (primary endpoints) of CACS—an increase in LBM, a decrease of REE, an improvement in fatigue—and some relevant secondary endpoints, such as an improvement in appetite, an improvement in QoL (as measured by the EORTC QLQ-C30 and EuroQoL [EQ]-5D), an increase in grip strength, a decrease in Glasgow Prognostic Score (GPS), and a decrease in proinflammatory cytokines.
Patients and Methods
Study Design
The study was a phase III, randomized trial. The protocol was approved by the reference ethics committee. Written informed consent was obtained from all patients. Procedures were in accordance with Good Clinical Practices and the Helsinki Declaration.
Eligibility Criteria
Patients (aged ≥18 years) with a histologically confirmed advanced stage tumor at any site, loss of >5% of ideal or preillness body weight in the previous 3 months with or without abnormal values of proinflammatory cytokines predictive of the onset of clinical cachexia, and a life expectancy ≥4 months, were eligible. Patients could be receiving concomitant antineoplastic chemotherapy or hormone therapy with palliative intent or supportive care.
Exclusion Criteria
Women of child-bearing age and patients with a mechanical obstruction to feeding, medical treatments inducing significant changes in patient metabolism or body weight, and a history of thromboembolism were excluded.
Intervention
All patients included in the study were given, as basic treatment, polyphenols (300 mg/days) obtained by dietary sources or supplemented with tablets (Nova-Q®; Pharma Gam, Cagliari, Italy), lipoic acid (300 mg/day, present in Nova-Q tablets), carbocysteine (Fluifort®; Dompé, Milan, Italy) (2.7 g/day), vitamin E (400 mg/day), vitamin A (30,000 IU/day), and vitamin C (500 mg/day), all orally. Patients were then randomized to one of five arms: arm 1, a progestational agent, that is, medroxyprogesterone acetate (MPA) (500 mg/day) or megestrol acetate (MA) (320 mg/day), which we considered equivalent and which were prescribed according to specific circumstances; arm 2, an oral eicosapentaenoic acid (EPA)-enriched (2.2 g/day) nutritional supplement, in prescribed dosages of two cartons/day for both ProSure (Abbott Laboratories, North Chicago, IL) and Resource® Support (Novartis Medical Nutrition, Saronno, Italy) or 3 cartons/day for Forticare® (Nutricia, Lainate, Italy); arm 3, L-carnitine (Carnitene®; SigmaTau, Rome, Italy) (4 g/day); arm 4, thalidomide (Celgene, Milan, Italy) (200 mg/day); arm 5, MPA or MA plus EPA-enriched nutritional supplement plus L-carnitine plus thalidomide.
The planned treatment duration was 4 months. A placebo arm was not included because it was not considered ethical partly because of the results of our phase II study [15] and mainly because an approved drug for this specific indication is currently available, that is, MA/MPA. We could have considered the MA/MPA arm as a control arm; however, we did not because results so far on the efficacy of MA/MPA do not allow the consideration of it as a standard treatment. Indeed, our choice not to consider MA/MPA as a control arm was confirmed post hoc by the results of the present study.
The doses were derived from the results of our previous studies [16–18].
Efficacy Endpoints
Primary Endpoints
The primary efficacy endpoints were: an increase in LBM, a decrease in REE, and a decrease in fatigue. LBM was assessed by conventional bioelectrical impedance analysis (BIA) (Bioelectric Impedance Analyser 101, Akern Spa, Firenze, Italy) [15] in all patients; by dual-energy X-ray absorptiometry (DEXA) in 144 patients, using a Hologic Delphi W scanner (Hologic Inc., Bedford, MA); and by regional computed tomography (CT) at L3, currently considered the highest precision method, able to provide detail on fat-free mass and specific muscles not provided by DEXA or BIA [19], in 25 patients. REE was assessed by indirect calorimetry (Medgem; SensorMedics Italia Srl, Milan, Italy). Fatigue was assessed by the Multidimensional Fatigue Symptom Inventory–Short Form [20, 21].
Secondary Endpoints
Secondary endpoints were: appetite, by visual analog scale (VAS); grip strength, by dynamometer (Jamar Hydraulic Hand Dynamometer; Sammons Preston, Bolingbrook, IL); QoL, by the EORTC QLQ-C30, EQ-5Dindex, and EQ-5DVAS; serum levels of IL-6 and TNF-α, by enzyme-linked immunosorbent assays (Immunotech, Marseille, France); GPS, currently considered a significant predictive index for survival in advanced stage cancer patients [22, 23]; blood levels of reactive oxygen species (FORT test, Callegari SpA, Italy) and the antioxidant enzyme glutathione peroxidase (Randox, Crumlin, UK), by photometer; total daily physical activity and the associated energy expenditure, carried out with an appropriate electronic device (SenseWear PRO2 Armband, SensorMedics Italia, Milan, Italy) able to assess total energy expenditure (TEE), that is, the sum of REE plus the energy spent in physical activity (active energy expenditure [AEE]), which is able to identify the specific type of physical activity (e.g., walking, running, lying down) in such a way as to attribute to it a “functional quality” [24]; and performance status (PS), according to the Eastern Cooperative Oncology Group (ECOG) PS scale [25].
As for clinical outcome variables such as objective clinical response, progression-free survival (PFS), and overall survival (OS), considering that patients did not begin anticancer treatment at the same time that they received the anti-CACS intervention, it did not appear feasible to measure these clinical variables in the present study. Notwithstanding, we roughly assessed the differences between arms to ascertain whether different cancer treatments could have influenced per se these clinical variables.
All methods were reported in detail in our previous papers [15, 16]. The endpoints were evaluated before treatment and at 4, 8, and 16 weeks after treatment start.
Safety Endpoints
Adverse events were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0) [26].
Statistical Analyses
Differences between groups at baseline were analyzed by the χ2 test for categoric variables and by Student's t-test (or Wilcoxon rank sum test when appropriate) for continuous variables.
The original intention was to compare arms in terms of changes in the primary endpoints from before to after treatment (16 weeks versus baseline) by conducting a one-way analysis of variance (ANOVA) for multiple comparisons using Bonferroni's correction. Moreover, changes across time points (4, 8, and 16 weeks) for the primary endpoints were also assessed using ANOVA for repeated measures.
The benefit obtained for primary and secondary endpoints in each arm (difference between baseline and after-treatment values) was assessed using a paired Student's t-test or Wilcoxon signed-rank test when appropriate.
The analysis was performed on an intent-to treat basis. An interim analysis was planned every 100 randomized patients to test the efficacy (primary efficacy endpoints) and the toxicity of the different arms according to the following “early stopping rules”: the arm(s) in which the efficacy values were significantly lower (p < .05), by a t-test for changes, than in the other arms would be stopped. Likewise, the arm(s) in which grade 3 or 4 toxicity values were significantly higher (p < .05), by a t-test for changes, than in the other arms would be stopped.
A very low level for the significance of p-values (p ≤ .001) was chosen considering that there are 10 possible pairs of between-arm comparisons and three endpoints, implying 30 possible candidate analyses; p-values are reported including Bonferroni's corrections for multiple comparisons. All analyses were carried out with two-sided tests using a 5% type I error rate. SPSS version 15.0 (SPSS Inc., Chicago, IL) was used.
Sample Size Calculation
Hypothesizing a difference between arms of 20% and considering an α type error of 0.05 and a β type error of 0.20, 95 patients needed to be enrolled in each arm.
Results
Patients
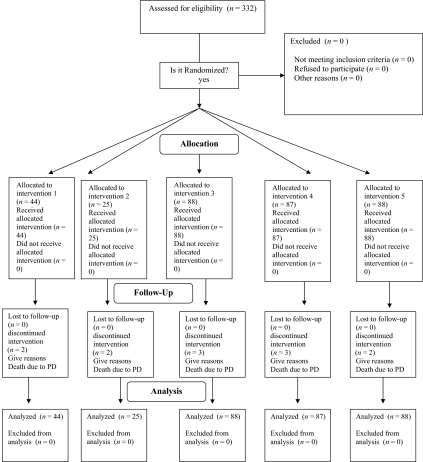
In total, 332 patients were recruited between April 2005 and December 2008, and all were deemed assessable (Fig. 1). More than 90% of the patients were recruited from the Department of Medical Oncology, University of Cagliari. The five arms consisted of patient groups comparable at baseline on the basis of the most common stratification factors (Table 1). Twelve patients withdrew as a result of early death caused by progressive disease. The percentage of dropouts was similar between arms. The original sample size should have included 475 patients, but this was reduced because of the early stopping of arm 1 and arm 2. Arm 3, arm 4, and arm 5 accrued the planned number of patients.
Figure 1.
Consort diagram.
Abbreviation: PD, progressive disease.
Table 1.
Baseline patient characteristics
aχ2 test.
Abbreviations: BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance status; CRP, C-reactive protein.
Primary Efficacy Endpoints
According to our original intention, that is, a comparison between arms, ANOVA showed a significant difference. A post hoc analysis showed the superiority of arm 5 over the other arms for all primary endpoints, as reported in Table 2.
Table 2.
Comparison of primary efficacy endpoints among arms 3, 4, and 5 by ANOVA
The table reports the mean change ± SD of the primary endpoints before and after treatment (16 weeks versus baseline).
Post hoc analysis showed: LBM (DEXA), arm 5 versus arm 3 and 4, p < .001; REE, arm 5 versus arm 3, p = 0.004, arm 5 versus arm 4, p = .056; fatigue, arm 5 versus arm 3, p = .004; arm 5 versus arm 4, p = .07.
aOne-way ANOVA using Bonferroni's correction for multiple comparisons.
Abbreviations: ANOVA, analysis of variance; BIA, bioimpedance analysis; CI, confidence interval; DEXA, dual energy x-ray absorptiometry; LBM, lean body mass; REE, resting energy expenditure; SD, standard deviation.
An analysis of changes from baseline showed that LBM, as assessed by DEXA, significantly increased (p = .015) in arm 5 whereas LBM as assessed by BIA did not change significantly. The L3 CT analysis showed an improvement in the estimated LBM (kg) (p = .001) and a trend toward an increase in muscle mass surface area (mm2) in arm 5.
The improvement of LBM by DEXA is of great significance since this technique is considered, apart from L3 TC which is not yet available in clinical practice, the most reliable and precise method currently available to assess LBM. Indeed, DEXA measures directly the weight of LBM whilst in contrast BIA indirectly estimates fat-free mass. Indeed, BIA evaluation is currently considered an obsolete technique.
REE, which was elevated at enrollment in 85% of patients, decreased significantly (p = .044) in arm 5. Fatigue improved significantly (p = .047) in arm 5. Results are reported in Table 3. Moreover, ANOVA for repeated measures showed a trend across the time points for the primary endpoints in arm 3, arm 4, and arm 5.
Table 3.
Primary and secondary endpoints before and after treatment
aStudent's t-test for paired data.
bCalculated using the equation as described in Mantovani et al. [16].
Abbreviations: BIA, bioimpedance analysis; DEXA, dual energy x-ray absorptiometry; ECOG PS, Eastern Cooperative Oncology Group performance status; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30; EQ-5D, EuroQoL 5D; FORT, free oxygen radicals test; GPS, Glasgow prognostic score; GPx, glutathione peroxidase; IL, Interleukin; L3 CT, computed tomography at 3rd lumbar vertebra; LBM, lean body mass; MFSI-SF, Multidimensional Fatigue Symptom Inventory–Short Form; REE, resting energy expenditure; ROS, reactive oxygen species; TNF, tumor necrosis factor.
Secondary Efficacy Endpoints
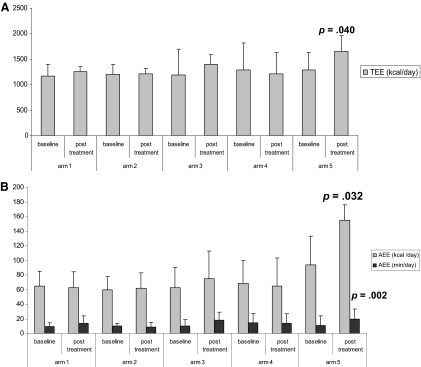
Results are reported in Table 3. Appetite increased significantly (p = .0003) in arm 5; IL-6 decreased significantly in arm 5 and arm 4; GPS and ECOG-PS score decreased significantly in arm 5, arm 4, and arm 3. A trend toward an increase in grip strength in arm 4 (p = .08), a trend toward an improvement in EQ-5Dindex in arm 5 (p = .09), and a trend toward a decrease in TNF-α in arm 5 were observed. TEE and AEE (kcal/day and min/day) increased significantly in arm 5 (p < .05) (Fig. 2A, 2B).
Figure 2.
Assessment of total daily physical activity and the associated energy expenditure. Total energy expenditure (TEE) (A) as well as active energy expenditure (AEE) (B) increased significantly in arm 5. Bars in (A) show TEE calculated as kcal/24-hour consumption. Bars in (B) show AEE expressed as the number of kcal/24 hours consumed beyond the limit of 3.0 metabolic equivalents (METs) and the number of minutes of activity >3.0 METs. 1 MET equals oxygen consumption of 3.5 ml O2/kg per minute or 1 kcal/kg per hour, both equal to resting energy expenditure.
The objective clinical response at the end of treatment was roughly not different among the different arms. This suggests that the different antineoplastic treatments administered did not have different impacts on the clinical outcome, nor was there interaction among intervention arms. Likewise, the median PFS and median OS times were roughly not different among arms (see Patients and Methods).
Interim Analyses
At the first interim analysis on 125 randomized patients, arm 2 was significantly inferior for the primary endpoints LBM (p < .05 versus arm 4 and arm 5), REE (p < .001 versus arm 1, arm 3, and arm 5), and fatigue (p = .002 versus arm 1 and p < .001 versus arm 3, arm 4, and arm 5), on the basis of t-test for changes. Therefore, arm 2 was withdrawn from the study [18] in accordance with the “early stopping rules” set out in Statistical Analyses.
A second interim analysis on 204 patients showed that arm 1 was inferior to the others in terms of the primary efficacy endpoints LBM (p = .02 versus arm 5), REE (p = .03 versus arm 5), and fatigue (p = .02 versus arm 4 and p = .002 versus arm 5), and therefore it was withdrawn from the study [27] in accordance with the “early stopping rules” set out in Statistical Analyses.
Toxicity
Toxicity was quite negligible and was comparable among treatment arms. Only two patients with grade 3 or 4 diarrhea were reported in arm 3 and arm 5. Overall, patient compliance was very good (Table 4).
Table 4.
Toxicity assessed as the worst toxicity per patient
aχ2 test.
Abbreviations: NS, not significant.
Discussion
The aim of our trial was to search for a potentially effective treatment for CACS, which must be considered critical among the as yet unavailable oncologic treatments with high impact. Among the selected efficacy endpoints, we highlighted LBM, REE, and fatigue as primary endpoints, considered the “core” symptoms of CACS, and among the secondary endpoints, we considered appetite, proinflammatory cytokines, and a scoring system based on systemic inflammation (i.e., GPS), the prognostic value of which is independent of tumor stage and conventional scoring systems [28] and superior to PS [29, 30] and to other markers of systemic inflammatory response.
Thus far, attempts at CACS therapy with a variety of single interventions have had limited success. The predominant features of CACS, that is, progressive loss of muscle mass and function, have been shown to be only minimally affected by the nutritional or pharmacologic tools currently available. Conversely, a combination of dietary, nutritional, and pharmacologic approaches to normalize the metabolic environment may have the potential to reverse CACS and improve the associated symptoms that affect QoL [31, 32].
The different single agents used in the present study were selected on the following basis. The antioxidant agents were shown to be effective in our previous studies [33–38]—polyphenols, in particular, quercetin, were included for their high antioxidant activity [39]. Synthetic progestagens, MA and MPA, are currently the only approved drugs for CACS in Europe; their mechanism of action may be partly related to glucocorticoid activity and the ability to downregulate the synthesis of proinflammatory cytokines [40] and to increase food intake by neuropeptide Y release [41]. To date, >15 randomized, controlled studies in weight-losing cancer patients have demonstrated that MPA and MA significantly improve appetite, food intake, body weight, and sometimes nausea and emesis, whereas in most trials, no definite improvement in global QoL was observed [42–46]. The weight gain observed with progestagens consists mainly of water and fat mass [47], having virtually no influence on LBM and functional activity [48]. A Cochrane Database systematic review [49] concluded that MA improves appetite and weight gain in cancer patients, whereas no overall conclusion about QoL could be drawn. In keeping with the above studies, the present trial does not confirm the efficacy of MPA (500 mg/day) or MA (320 mg/day) alone.
The ω-3 EPA and docosahexaenoic acid have been shown to inhibit the production of proinflammatory cytokines and thereby to act positively on cancer cachexia both in experimental tumor models and in humans [50]. A double-blind, randomized study [51] in 200 pancreatic cancer patients demonstrated a significantly positive correlation between the consumption of an EPA supplementation of at least 1.5 cartons per day and an increase in weight and LBM. In the present study, EPA-enriched nutritional supplementation alone demonstrated no benefit in terms of the CACS primary endpoints. This is in keeping with Jatoi et al. [52], who showed that EPA supplementation did not result in an improvement in weight, survival, or QoL, compared with MA, and is also in keeping with the results of a large multicenter study [53] comparing two different dosages of EPA with placebo in cachectic cancer patients. Indeed, a recent meta-analysis [54] concluded that “there were insufficient data to establish whether oral EPA was better than placebo.”
Carnitine, which may be deficient in cancer patients, is an essential cofactor for the mitochondrial production of acetyl-coenzyme A through the β-oxidation pathway and plays a pivotal role in cell energy metabolism: coenzyme A is required for β-oxidation, amino acid metabolism, and pyruvate dehydrogenase synthesis, and thus for triggering the tricarboxylic acid cycle [55, 56]. Carnitine thus may be considered a very intriguing drug. Indeed, we recently showed that L-carnitine administration (6 g/day for 30 days) was effective in improving fatigue and increasing LBM and appetite in 12 patients with advanced cancer [57]. However, this early promise notwithstanding, in the present study L-carnitine did not impact any of the primary endpoints and only had an impact on the secondary efficacy endpoints GPS and ECOG PS score.
Thalidomide has multiple immunomodulatory and anti-inflammatory properties, mainly by downregulating TNF-α and IL-6 production. Thus, thalidomide has been used for treatment of cachexia associated with cancer and AIDS. Whereas Bruera et al. [58] and Gordon et al. [59] showed that thalidomide was only able to attenuate weight loss and LBM in cachectic patients, Khan et al. [1] demonstrated an LBM gain after a short thalidomide (200 mg/day) treatment in 10 cachectic patients with advanced esophageal cancer. Thalidomide, at a dose of 300 mg/day for 3 months, was used by us [60] in 18 advanced stage cancer patients. Body weight did not change, whereas appetite improved and IL-6 and TNF-α decreased significantly. In the present study, thalidomide was shown to be effective in terms of the secondary efficacy endpoints IL-6, GPS, and ECOG PS score. No significant adverse events were observed. Only two patients reported grade 1 somnolence; no sensorial neuropathy was seen.
In the present study, the most effective treatment for all three primary efficacy endpoints and for the secondary endpoints appetite, IL-6, TNF-α, GPS, and ECOG PS score was the combination regimen. This is perfectly in keeping with the assumption that CACS is a multifactorial process and therefore an effective approach should be multitargeted.
In selecting the agents, we had originally planned to include the most selective cyclo-oxygenase 2 inhibitor, celecoxib, on the basis of the results observed in our phase II study [16] and in other authors' studies [61–63]; however, we eventually decided to not include this drug because of cardiotoxicity safety concerns that emerged in 2005 and that led to the withdrawal of rofecoxib from the market and restriction on the use of celecoxib.
Similarly, we did not include other potentially promising agents, such as infliximab, which was later shown to be ineffective in a clinical trial in cancer cachexia [64], as well as ghrelin and ghrelin mimetics [65, 66].
We obviously did not include agents or treatments that were developed for their anticachectic potential later on, such as insulin treatment [67], oxandrolone [68], and olanzapine [69].
The present study is, to our knowledge, the first randomized study with such a high number of patients enrolled and an ample range of treatments carried out in CACS patients. The results require some considerations.
The selected primary endpoints were very well chosen. Indeed, the combination arm was demonstrated to successfully target them as well as some important secondary endpoints.
The efficacy of the combined treatment in terms of the inflammatory response symptoms (cytokines, GPS) and primary efficacy endpoints adds further evidence to the assumption that the core symptoms of cachexia are systemic inflammation driven.
We do not have an indisputable explanation as to why the different single agents, ineffective or mildly effective alone, became effective when combined. Thus, an additive or even synergistic effect may be hypothesized.
The combined treatment consists mainly of diet, low-cost pharmacologic nutritional support, and low-cost drugs, having a favorable cost-benefit profile while achieving optimal patient compliance.
The promising results of our study suggest wide clinical application of the combined treatment; however, we are aware that our results may not be easily translated into current practice because the treatment may appear, at first, to be not simple to administer or obtain adequate compliance in cachectic cancer patients who often have a huge drug burden. To overcome these issues, proper patient communication and motivation are paramount.
The results of the present study, showing the efficacy of a combined treatment approach, seem to confirm the basic assumption that the treatment of cancer cachexia, a multifactorial syndrome, is more likely to yield success with a multitargeted approach.
As for future trends, on which experimental research has been focused recently, it can be suggested that drugs or treatments currently tested in animal models and in phase I and phase II clinical studies may be shortly translated into clinical phase III trials, namely, drugs downregulating the production and/or release of proinflammatory cytokines, particularly, IL-6, ghrelin, ghrelin mimetics or antagonists, and steroid androgen-receptor modulators such as ostarine.
Acknowledgments
The following centers contributed to patient enrollment and recruitment.
2nd Medical Oncology Unit, “Ospedale Oncologico Regionale Businco,” Cagliari, (Dott. Carlo Floris).
Department of Medical Oncology, “Azienda Ospedaliero Universitaria,” Ferrara, Dr. Giorgio Lelli.
Day Hospital of Medical Oncology, “Ospedale Sirai,” Carbonia, Dr. Daniela Massa.
2nd Medical Oncology Unit, “Azienda Ospedaliero Universitaria,“ Cagliari, Prof. Bruno Massidda.
Department of Medical Oncology, University of Palermo, Professor Nicola Gebbia.
Professor Quirico Mela, M.D., and Dr. Lorenza Montaldo, M.D. (Department of Internal Medicine, University of Cagliari, Cagliari, Italy) contributed to the performance of the DEXA analysis. Professor Giorgio Mallarini, M.D., Dr. Marco Mura, M.D., and Dr., Stefano Marini (Department of Radiology, University of Cagliari, Cagliari, Italy) contributed to the performance of the L3 CT imaging.
The authors thank Dr. Antonio Vigano' and Dr. Enriqueta Lucàr F. (Nutrition and Performance Laboratory, McGill University, Montreal, Canada) for sharing their expertise on L3 CT imaging.
Author Contributions
Conception/Design: Giovanni Mantovani, Clelia Madeddu
Financial support: Giovanni Mantovani
Provision of study material or patients: Antonio Macciò, Clelia Madeddu, Elena Massa, Mariele Dessì, Filomena Panzone
Collection and/or assembly of data: Clelia Madeddu, Roberto Serpe, Elena Massa, Mariele Dessì, Filomena Panzone
Data analysis and interpretation: Giovanni Mantovani, Clelia Madeddu, Roberto Serpe, Mariele Dessì, Filomena Panzone, Paolo Contu
Manuscript writing: Giovanni Mantovani, Clelia Madeddu
Final approval of manuscript: Giovanni Mantovani, Antonio Macciò, Clelia Madeddu, Roberto Serpe, Elena Massa, Mariele Dessì, Filomena Panzone, Paolo Contu
References
- 1.Khan ZH, Simpson EJ, Cole AT, et al. Oesophageal cancer and cachexia: The effect of short-term treatment with thalidomide on weight loss and lean body mass. Aliment Pharmacol Ther. 2003;17:677–682. doi: 10.1046/j.1365-2036.2003.01457.x. [DOI] [PubMed] [Google Scholar]
- 2.Heber D, Byerley LO, Chi J, et al. Pathophysiology of malnutrition in the adult cancer patient. Cancer. 1986;58(8 suppl):1867–1873. doi: 10.1002/1097-0142(19861015)58:8+<1867::aid-cncr2820581413>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Bruera E. Clinical management of anorexia and cachexia in patients with advanced cancer. Oncology. 1992;49(suppl 2):35–42. doi: 10.1159/000227126. [DOI] [PubMed] [Google Scholar]
- 4.Brennan MF. Uncomplicated starvation versus cancer cachexia. Cancer Res. 1977;37:2359–2364. [PubMed] [Google Scholar]
- 5.Nelson K, Walsh D. Management of the anorexia/cachexia syndrome. Cancer Bull. 1991;43:403–406. [Google Scholar]
- 6.Bruera E. ABC of palliative care. Anorexia, cachexia, and nutrition. BMJ. 1997;315:1219–1222. doi: 10.1136/bmj.315.7117.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moldawer LL, Gelin J, Scherstén T, et al. Circulating interleukin 1 and tumor necrosis factor during inflammation. Am J Physiol. 1987;253:R922–R928. doi: 10.1152/ajpregu.1987.253.6.R922. [DOI] [PubMed] [Google Scholar]
- 8.Strassmann G, Fong M, Kenney JS, et al. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest. 1992;89:1681–1684. doi: 10.1172/JCI115767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busbridge J, Dascombe MJ, Hoopkins S. Acute central effects of interleukin-6 on body temperature, thermogenesis and food intake in the rat. Proc Nutr Soc. 1989;38:48A. [Google Scholar]
- 10.Gelin J, Moldawer LL, Lönnroth C, et al. Role of endogenous tumor necrosis factor alpha and interleukin 1 for experimental tumor growth and the development of cancer cachexia. Cancer Res. 1991;51:415–421. [PubMed] [Google Scholar]
- 11.McLaughlin CL, Rogan GJ, Tou J, et al. Food intake and body temperature responses of rats to recombinant human interleukin-1 beta and a tripeptide interleukin-1 beta antagonist. Physiol Behav. 1992;52:1155–1160. doi: 10.1016/0031-9384(92)90475-h. [DOI] [PubMed] [Google Scholar]
- 12.Perboni S, Inui A. Anorexia in cancer: Role of feeding-regulatory peptides. Philos Trans R Soc Lond B Biol Sci. 2006;361:1281–1289. doi: 10.1098/rstb.2006.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantovani G, Macciò A, Massa E, et al. Managing cancer-related anorexia/cachexia. Drugs. 2001;61:499–514. doi: 10.2165/00003495-200161040-00004. [DOI] [PubMed] [Google Scholar]
- 14.Boddaert MS, Gerritsen WR, Pinedo HM. On our way to targeted therapy for cachexia in cancer? Curr Opin Oncol. 2006;18:335–340. doi: 10.1097/01.cco.0000228738.85626.ac. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani G, Madeddu C, Macciò A, et al. Cancer-related anorexia/cachexia syndrome and oxidative stress: An innovative approach beyond current treatment. Cancer Epidemiol Biomarkers Prev. 2004;13:1651–1659. [PubMed] [Google Scholar]
- 16.Mantovani G, Macciò A, Madeddu C, et al. A phase II study with antioxidants, both in the diet and supplemented, pharmaconutritional support, progestagen, and anti-cyclooxygenase-2 showing efficacy and safety in patients with cancer-related anorexia/cachexia and oxidative stress. Cancer Epidemiol Biomarkers Prev. 2006;15:1030–1034. doi: 10.1158/1055-9965.EPI-05-0538. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani G, Macciò A, Bianchi A, et al. Megestrol acetate in neoplastic anorexia/cachexia: Clinical evaluation and comparison with cytokine levels in patients with head and neck carcinoma treated with neoadjuvant chemotherapy. Int J Clin Lab Res. 1995;25:135–141. doi: 10.1007/BF02592554. [DOI] [PubMed] [Google Scholar]
- 18.Mantovani G, Macciò A, Madeddu C, et al. Randomized phase III clinical trial of five different arms of treatment for patients with cancer cachexia: Interim results. Nutrition. 2008;24:305–313. doi: 10.1016/j.nut.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 20.Stein KD, Martin SC, Hann DM, et al. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 21.Prue G, Rankin J, Cramp F, et al. Fatigue in gynaecological cancer patients: A pilot study. Support Care Cancer. 2006;14:78–83. doi: 10.1007/s00520-005-0830-7. [DOI] [PubMed] [Google Scholar]
- 22.Forrest LM, McMillan DC, McArdle CS, et al. A prospective longitudinal study of performance status, an inflammation-based score (GPS) and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2005;92:1834–1836. doi: 10.1038/sj.bjc.6602591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc. 2008;67:257–262. doi: 10.1017/S0029665108007131. [DOI] [PubMed] [Google Scholar]
- 24.Dahele M, Skipworth RJ, Wall L, et al. Objective physical activity and self-reported quality of life in patients receiving palliative chemotherapy. J Pain Symptom Manage. 2007;33:676–685. doi: 10.1016/j.jpainsymman.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 26.National Cancer Institute. Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, version 3.0. August 9, 2006. [accessed February 8, 2010]. Available at http://ctep.cancer.gov.
- 27.Mantovani G, Madeddu C, Serpe R, et al. Randomized phase III clinical trial of 5 different arms of treatment for patients with cancer-related anorexia/cachexia syndrome (CACS) [abstract 1676]. Presented at the 100th Annual Meeting of the American Association for Cancer Research; April 18–22, 2009; Denver, CO. [Google Scholar]
- 28.Ramsey S, Lamb GW, Aitchison M, et al. Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer. 2007;109:205–212. doi: 10.1002/cncr.22400. [DOI] [PubMed] [Google Scholar]
- 29.Forrest LM, McMillan DC, McArdle CS, et al. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer. 2004;90:1704–1706. doi: 10.1038/sj.bjc.6601789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crumley AB, McMillan DC, McKernan M, et al. Evaluation of an inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancer. Br J Cancer. 2006;94:637–641. doi: 10.1038/sj.bjc.6602998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NIH guide. Cachexia: Research into biobehavioral management and quality of life. June 11, 2001. [accessed February 8, 2010]. Available at http://grants.nih.gov/grants/guide/pa-files/pa-01-109.html.
- 32.Bossola M, Pacelli F, Tortorelli A, et al. Cancer cachexia: It's time for more clinical trials. Ann Surg Oncol. 2007;14:276–285. doi: 10.1245/s10434-006-9179-5. [DOI] [PubMed] [Google Scholar]
- 33.Mantovani G, Macciò A, Madeddu C, et al. The impact of different antioxidant agents alone or in combination on reactive oxygen species, antioxidant enzymes and cytokines in a series of advanced cancer patients at different sites: Correlation with disease progression. Free Radic Res. 2003;37:213–223. doi: 10.1080/10715760303849. [DOI] [PubMed] [Google Scholar]
- 34.Mantovani G, Macciò A, Madeddu C, et al. Antioxidant agents are effective in inducing lymphocyte progression through cell cycle in advanced cancer patients: Assessment of the most important laboratory indexes of cachexia and oxidative stress. J Mol Med. 2003;81:664–673. doi: 10.1007/s00109-003-0476-1. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani G, Macciò A, Madeddu C, et al. Reactive oxygen species, antioxidant mechanisms, and serum cytokine levels in cancer patients: Impact of an antioxidant treatment. J Environ Pathol Toxicol Oncol. 2003;22:17–28. doi: 10.1615/jenvpathtoxoncol.v22.i1.20. [DOI] [PubMed] [Google Scholar]
- 36.Mantovani G, Madeddu C, Gramignano G, et al. Subcutaneous interleukin-2 in combination with medroxyprogesterone acetate and antioxidants in advanced cancer responders to previous chemotherapy: Phase II study evaluating clinical, quality of life, and laboratory parameters. J Exp Ther Oncol. 2003;3:205–219. doi: 10.1046/j.1359-4117.2003.01096.x. [DOI] [PubMed] [Google Scholar]
- 37.Mantovani G, Macciò A, Madeddu C, et al. Phase II study of subcutaneously administered interleukin-2 in combination with medroxyprogesterone acetate and antioxidant agents as maintenance treatment in advanced cancer responders to previous chemotherapy. Oncol Rep. 2002;9:887–896. [PubMed] [Google Scholar]
- 38.Mantovani G, Macciò A, Madeddu C, et al. Selenium is effective in inducing lymphocyte progression through cell cycle in cancer patients: Potential mechanisms for its activity. J Exp Ther Oncol. 2004;4:69–78. [PubMed] [Google Scholar]
- 39.Higdon JV, Frei B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani G, Macciò A, Esu S, et al. Medroxyprogesterone acetate reduces the in vitro production of cytokines and serotonin involved in anorexia/cachexia and emesis by peripheral blood mononuclear cells of cancer patients. Eur J Cancer. 1997;33:602–607. doi: 10.1016/s0959-8049(96)00486-8. [DOI] [PubMed] [Google Scholar]
- 41.McCarthy HD, Crowder RE, Dryden S, et al. Megestrol acetate stimulates food and water intake in the rat: Effects on regional hypothalamic neuropeptide Y concentrations. Eur J Pharmacol. 1994;265:99–102. doi: 10.1016/0014-2999(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 42.Beller E, Tattersall M, Lumley T, et al. Improved quality of life with megestrol acetate in patients with endocrine-insensitive advanced cancer: A randomised placebo-controlled trial. Australasian Megestrol Acetate Cooperative Study Group. Ann Oncol. 1997;8:277–283. doi: 10.1023/a:1008291825695. [DOI] [PubMed] [Google Scholar]
- 43.Bruera E, Macmillan K, Kuehn N, et al. A controlled trial of megestrol acetate on appetite, caloric intake, nutritional status, and other symptoms in patients with advanced cancer. Cancer. 1990;66:1279–1282. doi: 10.1002/1097-0142(19900915)66:6<1279::aid-cncr2820660630>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 44.Loprinzi CL, Ellison NM, Schaid DJ, et al. Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia. J Natl Cancer Inst. 1990;82:1127–1132. doi: 10.1093/jnci/82.13.1127. [DOI] [PubMed] [Google Scholar]
- 45.Tchekmedyian NS, Hickman M, Siau J, et al. Megestrol acetate in cancer anorexia and weight loss. Cancer. 1992;69:1268–1274. doi: 10.1002/cncr.2820690532. [DOI] [PubMed] [Google Scholar]
- 46.Loprinzi CL, Michalak JC, Schaid DJ, et al. Phase III evaluation of four doses of megestrol acetate as therapy for patients with cancer anorexia and/or cachexia. J Clin Oncol. 1993;11:762–767. doi: 10.1200/JCO.1993.11.4.762. [DOI] [PubMed] [Google Scholar]
- 47.Simons JP, Schols AM, Hoefnagels JM, et al. Effects of medroxyprogesterone acetate on food intake, body composition, and resting energy expenditure in patients with advanced, nonhormone-sensitive cancer: A randomized, placebo-controlled trial. Cancer. 1998;82:553–560. [PubMed] [Google Scholar]
- 48.Inui A. Cancer anorexia-cachexia syndrome: Current issues in research and management. CA Cancer J Clin. 2002;52:72–91. doi: 10.3322/canjclin.52.2.72. [DOI] [PubMed] [Google Scholar]
- 49.Berenstein EG, Ortiz Z. Megestrol acetate for the treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2005;(2):CD004310. doi: 10.1002/14651858.CD004310.pub2. [DOI] [PubMed] [Google Scholar]
- 50.Barber MD, Ross JA, Voss AC, et al. The effect of an oral nutritional supplement enriched with fish oil on weight-loss in patients with pancreatic cancer. Br J Cancer. 1999;81:80–86. doi: 10.1038/sj.bjc.6690654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fearon KC, Von Meyenfeldt MF, Moses AG, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: A randomised double blind trial. Gut. 2003;52:1479–1486. doi: 10.1136/gut.52.10.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jatoi A, Rowland K, Loprinzi CL, et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer-associated wasting: A North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J Clin Oncol. 2004;22:2469–2476. doi: 10.1200/JCO.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 53.Fearon KC, Barber MD, Moses AG, et al. Double-blind, placebo-controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J Clin Oncol. 2006;24:3401–3407. doi: 10.1200/JCO.2005.04.5724. [DOI] [PubMed] [Google Scholar]
- 54.Dewey A, Baughan C, Dean T, et al. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst Rev. 2007;(1):CD004597. doi: 10.1002/14651858.CD004597.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pettegrew JW, Levine J, McClure RJ. Acetyl-L-carnitine physical-chemical, metabolic, and therapeutic properties: Relevance for its mode of action in Alzheimer's disease and geriatric depression. Mol Psychiatry. 2000;5:616–632. doi: 10.1038/sj.mp.4000805. [DOI] [PubMed] [Google Scholar]
- 56.Kelly GS. L-carnitine: Therapeutic applications of a conditionally-essential amino acid. Altern Med Rev. 1998;3:345–360. [PubMed] [Google Scholar]
- 57.Gramignano G, Lusso MR, Madeddu C, et al. Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition. 2006;22:136–145. doi: 10.1016/j.nut.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Bruera E, Neumann CM, Pituskin E, et al. Thalidomide in patients with cachexia due to terminal cancer: Preliminary report. Ann Oncol. 1999;10:857–859. doi: 10.1023/a:1008329821941. [DOI] [PubMed] [Google Scholar]
- 59.Gordon JN, Trebble TM, Ellis RD, et al. Thalidomide in the treatment of cancer cachexia: A randomised placebo controlled trial. Gut. 2005;54:540–545. doi: 10.1136/gut.2004.047563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Massa E, Madeddu C, Gramignano G, et al. Clinical trial with thalidomide in patients with tumors at different sites in progression of disease after previous treatments. Effect on clinical response and cachexia. Suppor Palliat Cancer Care. 2004;1:21–28. [Google Scholar]
- 61.Lai V, George J, Richey L, et al. Results of a pilot study of the effects of celecoxib on cancer cachexia in patients with cancer of the head, neck, and gastrointestinal tract. Head Neck. 2008;30:67–74. doi: 10.1002/hed.20662. [DOI] [PubMed] [Google Scholar]
- 62.Cerchietti LC, Navigante AH, Peluffo GD, et al. Effects of celecoxib, medroxyprogesterone, and dietary intervention on systemic syndromes in patients with advanced lung adenocarcinoma: A pilot study. J Pain Symptom Manage. 2004;27:85–95. doi: 10.1016/j.jpainsymman.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 63.Cerchietti LC, Navigante AH, Castro MA. Effects of eicosapentaenoic and docosahexaenoic n-3 fatty acids from fish oil and preferential Cox-2 inhibition on systemic syndromes in patients with advanced lung cancer. Nutr Cancer. 2007;59:14–20. doi: 10.1080/01635580701365068. [DOI] [PubMed] [Google Scholar]
- 64.Wiedenmann B, Malfertheiner P, Friess H, et al. A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J Support Oncol. 2008;6:18–25. [PubMed] [Google Scholar]
- 65.Strasser F, Lutz TA, Maeder MT, et al. Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: A randomised, placebo-controlled, double-blind, double-crossover study. Br J Cancer. 2008;98:300–308. doi: 10.1038/sj.bjc.6604148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neary NM, Small CJ, Wren AM, et al. Ghrelin increases energy intake in cancer patients with impaired appetite: Acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:2832–2836. doi: 10.1210/jc.2003-031768. [DOI] [PubMed] [Google Scholar]
- 67.Lundholm K, Körner U, Gunnebo L, et al. Insulin treatment in cancer cachexia: Effects on survival, metabolism, and physical functioning. Clin Cancer Res. 2007;13:2699–2706. doi: 10.1158/1078-0432.CCR-06-2720. [DOI] [PubMed] [Google Scholar]
- 68.Lesser GJ, Case D, Ottery F, et al. A phase III randomized study comparing the effects of oxandrolone (Ox) and megestrol acetate (Meg) on lean body mass (LBM), weight (wt) and quality of life (QOL) in patients with solid tumors and weight loss receiving chemotherapy [abstract 9513] J Clin Oncol. 2008;26(15 suppl):505s. [Google Scholar]
- 69.Braiteh F, Dalal S, Khuwaja A, et al. Phase I pilot study of the safety and tolerability of olanzapine (OZA) for the treatment of cachexia in patients with advanced cancer [abstract 20529] J Clin Oncol. 2008;26(15 suppl):727s. [Google Scholar]