The article reviews the currently available treatment options for patients with limited-stage small cell lung cancer.
Keywords: Combined modality therapy, Lung cancer, Review article, Radiation therapy
Abstract
In the U.S., the prevalence of small cell lung cancer (SCLC) is declining, probably reflecting the decreasing prevalence of tobacco use. However, a significant number of patients will receive a diagnosis of SCLC, and approximately 40% of patients with SCLC will have limited-stage (LS) disease, which is potentially curable with the combination of chemotherapy and radiation therapy. The standard therapy for LS-SCLC is concurrent chemoradiotherapy, and the 5-year survival rate observed in clinical trials is approximately 25%. The standard chemotherapy remains cisplatin and etoposide, but carboplatin is frequently used in patients who cannot tolerate or have a contraindication to cisplatin. Substantial improvements in survival have been made through improvements in radiation therapy. Concurrent chemoradiotherapy is the preferred therapy for patients who are appropriate candidates. The optimal timing of concurrent chemoradiotherapy is during the first or second cycle, based on data from meta-analyses. The optimal radiation schedule and dose remain topics of debate, but 1.5 Gy twice daily to a total of 45 Gy and 1.8–2.0 Gy daily to a total dose of 60–70 Gy are commonly used treatments. For patients who obtain a near complete or complete response, prophylactic cranial radiation reduces the incidence of brain metastases and improves overall survival. The ongoing Radiation Therapy Oncology Group and Cancer and Leukemia Group B and the European and Canadian phase III trials will investigate different radiation treatment paradigms for patients with LS-SCLC, and completion of these trials is critical.
Introduction
Lung cancer is the leading cause of cancer-related mortality in the U.S., and it was estimated that, in the U.S. in 2009, more patients would die from lung cancer than from colorectal, breast, and prostate cancer combined [1]. It is estimated that approximately 13% of the 219,000 patients who receive a diagnosis of lung cancer will have small cell histology (approximately 28,500 patients) [1, 2]. Small cell lung cancer (SCLC) is characterized by rapid growth, a high prevalence of mediastinal lymph node and distant metastases at the time of diagnosis, and high sensitivity to chemotherapy and radiotherapy. SCLC is frequently divided into limited-stage (LS)-SCLC and extensive-stage (ES)-SCLC, and it is estimated that 40% of patients will have LS-SCLC at the time of diagnosis [2]. The prevalence of SCLC is declining within the U.S., probably related to a decrease in smoking prevalence over last several decades. In an analysis of the Surveillance, Epidemiology, and End Results (SEER) database, the percentage of patients with a diagnosis of SCLC among patients with lung cancer decreased from 17% in 1986 to 13% in 2002 [2]. The percentage of patients with SCLC who are women has increased, probably reflecting a later peak in tobacco use among women, and the percentage of patients with LS-SCLC aged ≥70 years is increasing, and was 45% in 2000 [3]. The increasing prevalence of elderly patients will undoubtedly create some unique treatment challenges. The 5-year overall survival rate for LS-SCLC reported in the most recent U.S. cooperative group trial was 25% [4], but the 5-year survival rate observed in a recent review of the SEER database was 10% [2].
Staging
The most commonly used staging system is the two-stage Veterans Administration Lung Study Group (VALG) staging system for patients with inoperable lung cancer [5]. LS-SCLC is defined as disease that can be encompassed within a reasonable radiation field, and ES-SCLC is defined as disease that is greater than a reasonable radiation field. This simple staging system provides valuable prognostic information, and remains the best predictor of response to therapy and survival [6–8]. The most common interpretation of this system defines LS-SCLC as disease confined to one hemithorax, defined as the ipsilateral and contralateral mediastinal or the ipsilateral supraclavicular lymph nodes. Patients with malignant effusions are defined as having LS-SCLC according to the VALG definition, but are frequently considered to have ES-SCLC by many cooperative groups. The Radiation Therapy Oncology Group (RTOG) and Eastern Cooperative Oncology Group exclude patients with malignant pleural effusions, as well as contralateral hilar or supraclavicular lymphadenopathy, from LS-SCLC trials. The tumor–node–metastasis (TNM) system exists for SCLC; however, this system has not been routinely adopted for clinical practice. The TNM system was recently updated as part of the International Association for Study of Lung Cancer staging project [9]. The TNM staging system was able to differentiate patients' SCLC prognosis according to stage, with the exception that there was no significant difference between stage IA and stage IB patients.
There has been increasing interest in the use of fluorodeoxyglucose (FDG) positron emission tomography (PET) in the staging of patients with SCLC. Several small studies have revealed a benefit of PET scan staging in detecting distant metastatic disease or additional nodal metastases; however, the number of patients included in those trials was relatively small [10–13]. Thus, data on the use of FDG-PET staging are limited at this time, and the current guidelines from the American College of Chest Physicians state that the routine use of PET scanning outside a clinical trial cannot be recommended [14]. The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in SCLC state that a PET scan is optional but can be used as part of the initial evaluation in addition to other recommended studies, and a bone scan is optional if a PET scan is obtained [15].
Chemoradiotherapy
In LS-SCLC, treatment with chemotherapy alone results in poor intrathoracic disease control, with intrathoracic failures occurring in 75%–90% of patients [16]. The addition of thoracic radiotherapy (TRT) to chemotherapy leads to a significantly lower rate of intrathoracic failure, to 30%–60%; however, longer overall survival has not been consistently observed. In order to address this issue, two meta-analyses were performed [17, 18]. Pignon et al. [18] performed a meta-analysis based on individual data from 13 randomized trials that included 2,103 evaluable patients with LS-SCLC. The relative risk for death observed among patients receiving chemotherapy and TRT, in comparison with chemotherapy alone, was 0.86 (95% confidence interval [CI], 0.78–0.94; p = .001). An absolute different in survival of 5.4% ± 1.4% at 3 years was observed. Warde and Payne performed a literature-based meta-analysis of 11 randomized trials that included 1,911 patients, and that meta-analysis revealed longer overall survival with the combination of TRT and chemotherapy than with chemotherapy alone (odds ratio for 2-year survival rate, 1.53; 95% CI, 1.30–1.76; p < .001) [17]. The absolute difference in the 2-year survival rate was 5.4% (95% CI, 1.1%–9.7%). These meta-analyses established the combination of chemotherapy and TRT as standard of care in LS-SCLC.
Timing of Radiation
Although the timing of radiation therapy continues to be debated, the 2009 NCCN guidelines in SCLC state that there is category 1 evidence to support concurrent chemoradiotherapy over sequential therapy for fit patients [15]. The optimal timing of concurrent radiation therapy during the course of chemotherapy is generally agreed to be during the first or second cycle of chemotherapy [19–21]. This is supported by some phase III data and three meta-analyses based on literature. Interpretation of the phase III trials investigating the timing of TRT is difficult because the definitions of early and late TRT, the types of chemotherapy used, and the radiotherapy schedules employed have varied among trials [22–30]. Of the trials performed, three revealed longer survival for early versus late TRT [23, 29, 30]. Chemotherapy compliance appears to be essential in order to observe the survival benefit with early versus late TRT [22].
A meta-analysis of phase III studies combining chest irradiation and platinum-based chemotherapy concluded that the most important predictor of 5-year survival is the time from the start of any treatment until the end of radiotherapy (SER), with shorter SERs (<30 days) being associated with the highest 5-year survival rates (>20%) [31]. A subsequent meta-analysis was performed evaluating early versus late radiotherapy, with early therapy defined as within 30 days of beginning chemotherapy [32]. When platinum-based chemotherapy was used, the 2- and 5-year survival rates favored early TRT. This difference was significant only if the overall treatment time of radiation was <30 days. Compliance was important, suggesting that patient selection is important [19]. In a meta-analysis by Fried et al. [20], late TRT was defined as beginning 9 weeks after the initiation of chemotherapy or after completion of the third cycle of chemotherapy. That meta-analysis showed a statistically significant benefit of early TRT over late TRT in terms of 2-year overall survival but not for 3-year overall survival. On subset analysis of studies that used hyperfractionated TRT, treatment with early versus late TRT revealed a survival benefit, but no overall survival benefit was observed for early versus late TRT when once-daily TRT was employed. A survival benefit for early versus late TRT was seen in studies using platinum-based therapy; no significant difference in overall survival was observed for early versus late TRT in studies using nonplatinum-based chemotherapy. Despite the supportive data for early concurrent radiation therapy, early concurrent radiation therapy and dose intense therapy are not appropriate for all patients because of the greater toxicity. Delayed radiation therapy is preferable for patients who cannot tolerate concurrent treatment because of a poor performance status, weight loss, or comorbid conditions predisposing them to poor tolerance, or for patients who have large tumor volumes for which adequate coverage with radiation therapy would result in an unacceptable dose to normal tissue.
Radiation Dose and Fractionation Schedule
Radiation schedule and total dose for SCLC have been topics of continuous debate. Several radiation therapy doses and fractionation schedules are supported in the literature. The 2009 NCCN guidelines on SCLC note that, when radiotherapy is given for LS-SCLC, it should be delivered as either 1.5 Gy twice daily to a total of 45 Gy or 1.8–2.0 Gy daily to 60–70 Gy, starting with the first or second cycle of chemotherapy. A 10-year-old study demonstrated the benefits of using twice-daily dosing for 3 weeks over once-daily dosing for 5 weeks to deliver a total of 45 Gy [4]. Patients in both arms of that study received the same total dose of radiation. The study clearly demonstrated that altering the course of radiation therapy influences survival. However, the twice-daily regimen is not well accepted in clinical practice [33]. This is likely a result of the practical challenges of administering twice-daily therapy, and the associated greater acute toxicity, in particular, a higher rate of esophagitis; grade 3 or 4 esophagitis was observed in 32% and 15% of the twice-daily and daily TRT arms, respectively. One of the major criticisms of that study is that, because the biological effectiveness of 45 Gy delivered over 5 weeks is less than that of 45 Gy delivered over 3 weeks, the study does not confirm that twice-daily treatment is better than once-daily treatment delivered to comparable biologic doses. A second trial by the North Central Cancer Treatment Group investigated twice-daily TRT with a treatment break (48 Gy in 32 fractions with an initial 2.5-week break after 24 Gy) in comparison with 50.4 Gy in 28 fractions [34]. TRT began on the fourth cycle of chemotherapy in both treatment arms, and the times to completion of TRT were similar in both treatment arms. No difference in terms of overall survival was observed between the two treatment arms. Although the effective dose of the twice-daily fractionation was diminished by the 2.5-week treatment break, the results of that trial may have discouraged the use of twice-daily TRT.
A number of cooperative groups have investigated novel radiotherapy schedules in phase I or II trials. The phase I Cancer and Leukemia Group B (CALGB) 8837 study evaluated maximally tolerated twice-daily and daily doses of radiation with concurrent chemotherapy in LS-SCLC. The maximally tolerated doses (MTDs) were 45 Gy in 30 fractions over 3 weeks when given twice daily and 70 Gy in 35 fractions over 7 weeks when given daily [35]. The CALGB 39808 trial subsequently confirmed that it is feasible to safely deliver 70 Gy daily concurrently with carboplatin and etoposide following an induction regimen of paclitaxel and topotecan. That regimen was associated with a 92% response rate and median overall survival time of 22.4 months [36]. The RTOG 97-12 trial evaluated the MTD of TRT given to patients concurrently taking cisplatin and etoposide (EP). Radiation was given as 1.8 Gy/fraction daily to the clinical target volume for the first two cycles and then twice daily to the gross tumor volume for 3, 5, 7, 9, or 11 days (i.e., total dose of 50.4–64.8 Gy). The MTD was 61.2 Gy, with esophagitis being the dose-limiting toxicity [37]. A subsequent phase II study, RTOG 0239, evaluated a 61.2-Gy concomitant boost regimen with EP. Radiation was administered at 1.8 Gy/fraction, 5 days per week for 16 fractions, then twice daily for 5 days for a total dose of 61.2 Gy in 34 fractions in 5 weeks. The 2-year overall survival rate observed in that trial was 37%, and the rate of locoregional control at 2 years was 80% [38].
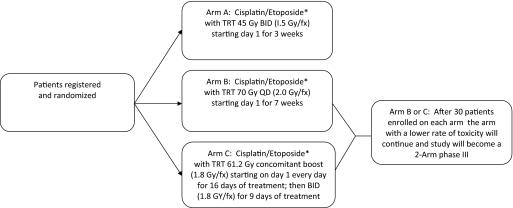
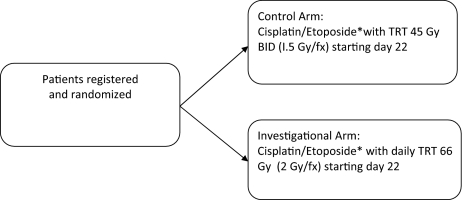
An ongoing phase III study conducted by the CALGB and RTOG is exploring the optimal dose of radiation in LS-SCLC (Fig. 1). That trial is comparing overall survival and toxicity among patients receiving EP plus 45 Gy in 30 treatments given twice daily, 5 days a week for 3 weeks (standard-dose radiation), 70 Gy in 35 treatments given daily, 5 days a week for 7 weeks, and 61.2 Gy in 34 treatments given daily, 5 days/week for 16 days, and then twice daily, 5 days a week for 9 days (National Library of Medicine [NLM] identifier, NCT00632853) [39]. The 45-Gy twice-daily dose is considered the control arm, and the other two treatment arms are considered the investigational arms. After the initial 30 patients have been enrolled in each treatment arm, an interim analysis will be performed, and the investigational treatment arm with the higher rate of treatment-related toxicity will be discontinued and the trial will be a two-arm phase III trial. Another phase III trial, Concurrent Once-daily Versus twice daily RadioTherapy (CONVERT), is running both in Europe and Canada, comparing two concomitant chemoradiation therapy regimens plus EP (Fig. 2) and conformal radiotherapy of 45 Gy in 30 fractions of 1.5 Gy given twice daily (control arm) versus 70 Gy in 35 daily fractions of 2 Gy (NLM identifier, NCT00433563) [40].
Figure 1.
CALGB 30610/RTOG 0538: Phase III trial of TRT regimens in patients with limited-stage small cell lung cancer receiving cisplatin and etoposide.
*All patients will receive cisplatin, 80 mg/m2 on day 1 and etoposide, 100 mg/m2 on days 1, 2, and 3, every 21 days for four cycles. Prophylactic cranial radiation should be offered to all patients with a complete response or near complete response.
Abbreviations: BID, twice daily; CALGB, Cancer and Leukemia Group B; fx, fraction; QD, daily; RTOG, Radiation Therapy Oncology Group; TRT, thoracic radiation therapy.
Figure 2.
CONVERT: Phase III trial of TRT in patients with limited-stage small cell lung cancer receiving cisplatin and etoposide.
*All patients receive cisplatin, 25 mg/m2 on days 1–3 or 75 mg/m2 on day 1, and etoposide, 100 mg/m2 on days 1–3 for four to six cycles.
Abbreviations: BID, twice daily; CONVERT, Concurrent Once-daily Versus twice daily RadioTherapy; fx, fraction; TRT, thoracic radiation therapy.
Radiation Therapy Fields
Historically, radiation therapy portals were large, encompassing the entire mediastinum. This was necessary to ensure adequate coverage of gross disease prior to the routine use of computed tomography (CT)-based radiation therapy planning. In addition to the routine use of CT planning, image-guided radiation therapy and techniques to control or compensate for tumor motion improve tumor coverage. This reduced field size, in most cases, translates into lower rates of toxicity. Radiation therapy fields in an intergroup study include the ipsilateral hilar nodes in all cases and the precarinal, bilateral paratracheal, and subcarinal lymph nodes in patients with N2 or N3 disease. There are no field reductions in the hyperfractionated arm. The fields are reduced to treat gross disease only after 44 Gy in the 70-Gy arm. Boost fields include gross disease only in the concomitant boost arm. The current intergroup trial allows for a resimulation for redefinition of gross disease prior to beginning the radiation therapy boost with the intent of decreasing treatment volumes.
Prophylactic Cranial Irradiation
The incidence of central nervous system (CNS) metastases in SCLC patients is >50% [41]. The use of prophylactic cranial irradiation (PCI) has consistently been shown to be effective in decreasing the incidence of CNS failures [42–45], and it is recommended therapy for patients with LS-SCLC who have had a complete response to primary therapy. Historically, the routine use of PCI was met with skepticism because of retrospective reports of a high level of toxicity and a lack of a survival advantage. Poor functional outcomes in patients treated with PCI have been attributed to treatment with a high dose per fraction and a high total dose of radiation [46] and concurrent chemotherapy [47]. Detailed data regarding the toxicity of PCI have been limited. Retrospective and prospective studies have shown deficits in baseline assessments after systemic therapy and before the administration of PCI [42, 43, 48], emphasizing the need for prospective evaluation. Studies that have prospectively assessed the cognitive impact of PCI have not shown significant impairment attributed to PCI [42, 48, 49].
Meta-analysis and SEER data review have shown a survival advantage with the use of PCI in patients with LS-SCLC. Aupérin et al. [50] published a meta-analysis of individual data from patients treated in seven prospective randomized studies. This was the first study to demonstrate a survival advantage with PCI. The relative risk for death in the treatment group, as compared with the control group, was 0.84 (95% CI, 0.73–0.97; p = .01), which corresponds to a 5.4% higher rate of survival at 3 years (15.3% in the control group versus 20.7% in the treatment group). Patel et al. [51] reported a SEER data review of 7,995 patients with LS-SCLC. Of them, 670 were identified as having PCI. Greater overall and cause-specific survival were observed in patients treated with PCI. The 2- and 5-year survival rates were 23% and 11% without PCI and 42% and 19% with PCI, respectively.
PCI does not completely eliminate the risk for CNS failure. Data have shown that lower doses of radiation may be less effective in preventing CNS failures [42, 50]. Le Péchoux et al. [52] published the results of a large international study evaluating radiation dose for PCI in SCLC. Between September 1999 and December 2005, 720 patients with LS-SCLC in complete remission after chemotherapy and TRT from 157 centers in 22 countries were randomly assigned to standard-dose PCI to 25 Gy in 10 daily fractions or higher-dose PCI to 36 Gy delivered in 10 fractions of 2 Gy once daily or 16 fractions of 1.5 Gy twice daily. No significant difference in the total incidence of brain metastases was observed after higher-dose PCI. There was a significantly higher rate of cancer-related mortality in the higher-dose arm as a result of the unexplained finding of more deaths from extracranial disease progression. Based on the results of that study, 25 Gy delivered at 2.5 Gy per fraction per day remains the standard of care for PCI in LS-SCLC patients. The commonly used radiation schedule of 30 Gy at 2 Gy per fraction was not investigated in that trial, and it remains a commonly used and reasonable alternative.
Chemotherapy and Targeted Agents in LS-SCLC
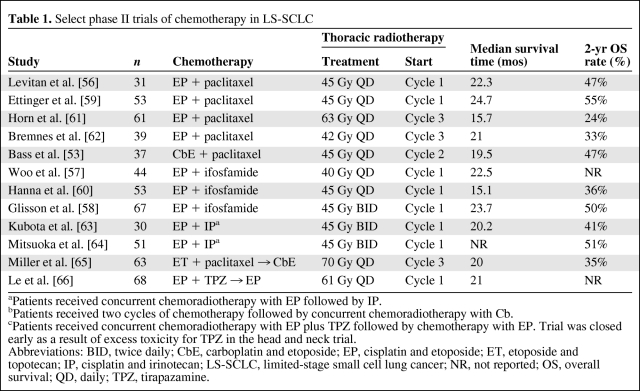
SCLC is a very chemotherapy-sensitive disease, and in ES-SCLC patients, EP, cisplatin and irinotecan (IP), and cyclophosphamide, doxorubicin, and vincristine are considered standard therapies; in LS-SCLC, EP is the preferred combination because it can easily be integrated with concurrent TRT. Several phase II trials have investigated the role of carboplatin and etoposide with concurrent TRT [53, 54], and a retrospective review has revealed outcomes similar to those with cisplatin-based therapy [55]. However, phase III data comparing the efficacy of carboplatin with that of cisplatin in LS-SCLC are lacking and phase III data from carboplatin-based chemoradiotherapy are limited. Given the curative intent of chemoradiotherapy, cisplatin is considered the standard therapy unless the patient cannot tolerate or has a contraindication to cisplatin-based therapy. The percentage of patients with LS-SCLC who are aged ≥70 years is increasing, and there is a higher prevalence of comorbidities such as renal insufficiency in this patient population. These changing demographics may reduce the proportion of patients who are eligible for cisplatin-based therapy. Several phase I and phase II trials have investigated different chemotherapy platforms in LS-SCLC patients, and those trials have involved the addition of a third agent, most frequently paclitaxel or ifosfamide (Table 1) [53, 56–66]. The other strategy that has been investigated is the replacement of etoposide or treatment with a different chemotherapy combination before or after completion of concurrent chemoradiotherapy with EP [65, 66]. None of these chemotherapy strategies has revealed results promising enough to pursue a phase III trial.
Table 1.
Select phase II trials of chemotherapy in LS-SCLC
aPatients received concurrent chemoradiotherapy with EP followed by IP.
bPatients received two cycles of chemotherapy followed by concurrent chemoradiotherapy with Cb.
cPatients received concurrent chemoradiotherapy with EP plus TPZ followed by chemotherapy with EP. Trial was closed early as a result of excess toxicity for TPZ in the head and neck trial.
Abbreviations: BID, twice daily; CbE, carboplatin and etoposide; EP, cisplatin and etoposide; ET, etoposide and topotecan; IP, cisplatin and irinotecan; LS-SCLC, limited-stage small cell lung cancer; NR, not reported; OS, overall survival; QD, daily; TPZ, tirapazamine.
The one exception has been the use of concurrent chemoradiotherapy with EP followed by consolidation therapy with IP [63, 64], which is being investigated in the Japanese Clinical Oncology Group Trial 0202. All patients will receive initial treatment with EP concurrent with TRT (twice daily to 45 Gy) and then patients will receive either three additional cycles of EP or IP. Treatment with IP versus EP in ES-SCLC patients resulted in longer survival in one phase III study performed in Japan [67]; however, two phase III trials performed in North America did not reveal a survival benefit for IP in comparison with EP in ES-SCLC patients [68, 69]. Differences in the patient populations and the pharmacogenomics of the patient populations related to the metabolism of irinotecan may have contributed to the different results observed between the two trials. The results of this trial are awaited, but if the new combination does provide a survival benefit, there will be a question as to whether the results apply to the North American patient population.
Targeted therapies have been investigated in LS-SCLC, and the results to date have been disappointing as well. Vandetanib is a tyrosine kinase inhibitor of vascular endothelial growth factor receptor-2 and epidermal growth factor receptor, and it was investigated in comparison with placebo in patients with LS-SCLC and ES-SCLC who experienced a response after initial chemotherapy [70]. The progression-free and overall survival times were not longer in the overall study population, but in a subgroup analysis, patients with LS-SCLC who received vandetanib (n = 23), versus placebo (n = 23), experienced a longer overall survival time (hazard ratio, 0.45; p = .07). A phase II trial investigated the combination of carboplatin, irinotecan, and bevacizumab in combination with TRT, and that trial was stopped early after two patients developed tracheoesophageal (TE) fistula and a third patient died from an aerodigestive hemorrhage [71]. Of note, the two patients developed TE fistula 2 and 5 months after completion of TRT. These safety data have resulted in concerns about the safety of bevacizumab concurrently or after the completion of chemoradiotherapy in LS-SCLC patients, and it does not appear that antiangiogenic agents will have a significant role in LS-SCLC treatment. Other targeted agents that have been investigated include matrix metalloproteinase inhibitors, tamoxifen, and thalidomide, in trials for patients with LS-SCLC or in which LS-SCLC constituted a substantial proportion of the patients enrolled, and none of those agents led to a significant improvement in overall survival [72–74].
Conclusions
LS-SCLC is a potentially curable disease with chemoradiotherapy, and advances in radiation therapy have significantly improved overall survival. The initiation of TRT concurrent with chemotherapy, during the first two cycles, and a start to end of radiotherapy <30 days appear to improve the efficacy of chemoradiotherapy. The data from individual trials investigating the timing of TRT are contradictory, but three meta-analyses [20, 21, 32], based on published data, suggest that TRT should be started early, and an accelerated regimen should be considered in combination with platinum-based therapy. A meta-analysis based on individual data may provide additional information about the optimal time to initiation of TRT and a better estimate of the magnitude of benefit. EP remains the standard chemotherapy. PCI reduces the incidence of brain metastases and improves overall survival. There are two ongoing phase III trials: an RTOG and CALGB trial for patients with LS-SCLC will investigate three different TRT paradigms and the CONVERT trial is comparing two radiation therapy paradigms. The continued enrollment and completion of these trials are critical for further advances in the treatment of this disease.
Author Contributions
Conception/Design: Elizabeth Gore, Thomas Stinchcombe
Provision of study material or patients: Elizabeth Gore, Thomas Stinchcombe
Collection and/or assembly of data: Elizabeth Gore, Thomas Stinchcombe
Data analysis and interpretation: Elizabeth Gore, Thomas Stinchcombe
Manuscript writing: Elizabeth Gore, Thomas Stinchcombe
Final approval of manuscript: Elizabeth Gore, Thomas Stinchcombe
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the Surveillance, Epidemiologic, and End Results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 3.Gaspar LE, Gay EG, Crawford J, et al. Limited-stage small-cell lung cancer (stages I-III): Observations from the National Cancer Data Base. Clin Lung Cancer. 2005;6:355–360. doi: 10.3816/CLC.2005.n.015. [DOI] [PubMed] [Google Scholar]
- 4.Turrisi AT, 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 5.Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3. 1973;4:31–42. [PubMed] [Google Scholar]
- 6.Shepherd FA, Ginsberg RJ, Haddad R, et al. Importance of clinical staging in limited small-cell lung cancer: A valuable system to separate prognostic subgroups. The University of Toronto Lung Oncology Group. J Clin Oncol. 1993;11:1592–1597. doi: 10.1200/JCO.1993.11.8.1592. [DOI] [PubMed] [Google Scholar]
- 7.Albain KS, Crowley JJ, LeBlanc M, et al. Determinants of improved outcome in small-cell lung cancer: An analysis of the 2,580-patient Southwest Oncology Group data base. J Clin Oncol. 1990;8:1563–1574. doi: 10.1200/JCO.1990.8.9.1563. [DOI] [PubMed] [Google Scholar]
- 8.Osterlind K, Hansen HH, Hansen M, et al. Mortality and morbidity in long-term surviving patients treated with chemotherapy with or without irradiation for small-cell lung cancer. J Clin Oncol. 1986;4:1044–1052. doi: 10.1200/JCO.1986.4.7.1044. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: Proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol. 2007;2:1067–1077. doi: 10.1097/JTO.0b013e31815bdc0d. [DOI] [PubMed] [Google Scholar]
- 10.Brink I, Schumacher T, Mix M, et al. Impact of [18F]FDG-PET on the primary staging of small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2004;31:1614–1620. doi: 10.1007/s00259-004-1606-x. [DOI] [PubMed] [Google Scholar]
- 11.Chin R, Jr, McCain TW, Miller AA, et al. Whole body FDG-PET for the evaluation and staging of small cell lung cancer: A preliminary study. Lung Cancer. 2002;37:1–6. doi: 10.1016/s0169-5002(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 12.Kut V, Spies W, Spies S, et al. Staging and monitoring of small cell lung cancer using [18F]fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET) Am J Clin Oncol. 2007;30:45–50. doi: 10.1097/01.coc.0000239095.09662.19. [DOI] [PubMed] [Google Scholar]
- 13.Bradley JD, Dehdashti F, Mintun MA, et al. Positron emission tomography in limited-stage small-cell lung cancer: A prospective study. J Clin Oncol. 2004;22:3248–3254. doi: 10.1200/JCO.2004.11.089. [DOI] [PubMed] [Google Scholar]
- 14.Simon GR, Turrisi A American College of Chest Physicians. Management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 suppl):324S–339S. doi: 10.1378/chest.07-1385. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer v. 2.2009. [accessed July 29, 2009]. Available at http://www.nccn.org/professionals/physician.
- 16.Faivre-Finn C, Lorigan P, West C, et al. Thoracic radiation therapy for limited-stage small-cell lung cancer: Unanswered questions. Clin Lung Cancer. 2005;7:23–29. doi: 10.3816/CLC.2005.n.018. [DOI] [PubMed] [Google Scholar]
- 17.Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992;10:890–895. doi: 10.1200/JCO.1992.10.6.890. [DOI] [PubMed] [Google Scholar]
- 18.Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327:1618–1624. doi: 10.1056/NEJM199212033272302. [DOI] [PubMed] [Google Scholar]
- 19.Pijls-Johannesma MC, De Ruysscher D, Lambin P, et al. Early versus late chest radiotherapy for limited stage small cell lung cancer. Cochrane Database Syst Rev. 2005;(1):CD004700. doi: 10.1002/14651858.CD004700.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried DB, Morris DE, Poole C, et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol. 2004;22:4837–4845. doi: 10.1200/JCO.2004.01.178. [DOI] [PubMed] [Google Scholar]
- 21.De Ruysscher D, Pijls-Johannesma M, Vansteenkiste J, et al. Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann Oncol. 2006;17:543–552. doi: 10.1093/annonc/mdj094. [DOI] [PubMed] [Google Scholar]
- 22.Spiro SG, James LE, Rudd RM, et al. Early compared with late radiotherapy in combined modality treatment for limited disease small-cell lung cancer: A London Lung Cancer Group multicenter randomized clinical trial and meta-analysis. J Clin Oncol. 2006;24:3823–3830. doi: 10.1200/JCO.2005.05.3181. [DOI] [PubMed] [Google Scholar]
- 23.Jeremic B, Shibamoto Y, Acimovic L, et al. Initial versus delayed accelerated hyperfractionated radiation therapy and concurrent chemotherapy in limited small-cell lung cancer: A randomized study. J Clin Oncol. 1997;15:893–900. doi: 10.1200/JCO.1997.15.3.893. [DOI] [PubMed] [Google Scholar]
- 24.Skarlos DV, Samantas E, Briassoulis E, et al. Randomized comparison of early versus late hyperfractionated thoracic irradiation concurrently with chemotherapy in limited disease small-cell lung cancer: A randomized phase II study of the Hellenic Cooperative Oncology Group (HeCOG) Ann Oncol. 2001;12:1231–1238. doi: 10.1023/a:1012295131640. [DOI] [PubMed] [Google Scholar]
- 25.Work E, Nielsen OS, Bentzen SM, et al. Randomized study of initial versus late chest irradiation combined with chemotherapy in limited-stage small-cell lung cancer. Aarhus Lung Cancer Group. J Clin Oncol. 1997;15:3030–3037. doi: 10.1200/JCO.1997.15.9.3030. [DOI] [PubMed] [Google Scholar]
- 26.Gregor A, Drings P, Burghouts J, et al. Randomized trial of alternating versus sequential radiotherapy/chemotherapy in limited-disease patients with small-cell lung cancer: A European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group study. J Clin Oncol. 1997;15:2840–2849. doi: 10.1200/JCO.1997.15.8.2840. [DOI] [PubMed] [Google Scholar]
- 27.Perry MC, Eaton WL, Propert KJ, et al. Chemotherapy with or without radiation therapy in limited small-cell carcinoma of the lung. N Engl J Med. 1987;316:912–918. doi: 10.1056/NEJM198704093161504. [DOI] [PubMed] [Google Scholar]
- 28.Perry MC, Herndon JE, 3rd, Eaton WL, et al. Thoracic radiation therapy added to chemotherapy for small-cell lung cancer: An update of Cancer and Leukemia Group B Study 8083. J Clin Oncol. 1998;16:2466–2467. doi: 10.1200/JCO.1998.16.7.2466. [DOI] [PubMed] [Google Scholar]
- 29.Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1993;11:336–344. doi: 10.1200/JCO.1993.11.2.336. [DOI] [PubMed] [Google Scholar]
- 30.Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: Results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20:3054–3060. doi: 10.1200/JCO.2002.12.071. [DOI] [PubMed] [Google Scholar]
- 31.De Ruysscher D, Pijls-Johannesma M, Bentzen SM, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol. 2006;24:1057–1063. doi: 10.1200/JCO.2005.02.9793. [DOI] [PubMed] [Google Scholar]
- 32.Pijls-Johannesma M, De Ruysscher D, Vansteenkiste J, et al. Timing of chest radiotherapy in patients with limited stage small cell lung cancer: A systematic review and meta-analysis of randomised controlled trials. Cancer Treat Rev. 2007;33:461–473. doi: 10.1016/j.ctrv.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Movsas B, Moughan J, Komaki R, et al. Radiotherapy patterns of care study in lung carcinoma. J Clin Oncol. 2003;21:4553–4559. doi: 10.1200/JCO.2003.04.018. [DOI] [PubMed] [Google Scholar]
- 34.Schild SE, Bonner JA, Shanahan TG, et al. Long-term results of a phase III trial comparing once-daily radiotherapy with twice-daily radiotherapy in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;59:943–951. doi: 10.1016/j.ijrobp.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 35.Choi NC, Herndon JE, 2nd, Rosenman J, et al. Phase I study to determine the maximum-tolerated dose of radiation in standard daily and hyperfractionated-accelerated twice-daily radiation schedules with concurrent chemotherapy for limited-stage small-cell lung cancer. J Clin Oncol. 1998;16:3528–3536. doi: 10.1200/JCO.1998.16.11.3528. [DOI] [PubMed] [Google Scholar]
- 36.Bogart JA, Herndon JE, 2nd, Lyss AP, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: Analysis of Cancer and Leukemia Group B Study 39808. Int J Radiat Oncol Biol Phys. 2004;59:460–468. doi: 10.1016/j.ijrobp.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 37.Komaki R, Swann RS, Ettinger DS, et al. Phase I study of thoracic radiation dose escalation with concurrent chemotherapy for patients with limited small-cell lung cancer: Report of Radiation Therapy Oncology Group (RTOG) Protocol 97-12. Int J Radiat Oncol Biol Phys. 2005;62:342–350. doi: 10.1016/j.ijrobp.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 38.Komaki R, Paulus R, Ettinger DS, et al. A phase II study of accelerated high-dose thoracic radiation therapy (AHTRT) with concurrent chemotherapy for limited small cell lung cancer: RTOG 0239 [abstract 7527] J Clin Oncol. 2009;27(15 suppl):388. [Google Scholar]
- 39.ClinicalTrials.gov. Three different radiation therapy regimens in treating patients with limited-stage small cell lung cancer receiving cisplatin and etoposide. [accessed September 9, 2009]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00632853.
- 40.ClinicalTrials.gov. Cisplatin, etoposide, and two different schedules of radiation therapy in treating patients with limited-stage small cell lung cancer. [accessed January 7, 2009]. Available at http://clinicaltrials.gov/ct2/show/NCT00433563.
- 41.Bunn PA, Jr, Nugent JL, Matthews MJ. Central nervous system metastases in small cell bronchogenic carcinoma. Semin Oncol. 1978;5:314–322. [PubMed] [Google Scholar]
- 42.Gregor A, Cull A, Stephens RJ, et al. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: Results of a multicentre randomised trial. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) and the European Organization for Research and Treatment of Cancer (EORTC) Eur J Cancer. 1997;33:1752–1758. doi: 10.1016/s0959-8049(97)00135-4. [DOI] [PubMed] [Google Scholar]
- 43.Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87:183–190. doi: 10.1093/jnci/87.3.183. [DOI] [PubMed] [Google Scholar]
- 44.Laplanche A, Monnet I, Santos-Miranda JA, et al. Controlled clinical trial of prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Lung Cancer. 1998;21:193–201. doi: 10.1016/s0169-5002(98)00056-7. [DOI] [PubMed] [Google Scholar]
- 45.Aroney RS, Aisner J, Wesley MN, et al. Value of prophylactic cranial irradiation given at complete remission in small cell lung carcinoma. Cancer Treat Rep. 1983;67:675–682. [PubMed] [Google Scholar]
- 46.Crossen PE, Morrison MJ, Colls BM. Increased frequency of the S allele of the L-myc oncogene in non-Hodgkin's lymphoma. Br J Cancer. 1994;69:759–761. doi: 10.1038/bjc.1994.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahles TA, Silberfarb PM, Herndon J, 2nd, et al. Psychologic and neuropsychologic functioning of patients with limited small-cell lung cancer treated with chemotherapy and radiation therapy with or without warfarin: A study by the Cancer and Leukemia Group B. J Clin Oncol. 1998;16:1954–1960. doi: 10.1200/JCO.1998.16.5.1954. [DOI] [PubMed] [Google Scholar]
- 48.Komaki R, Meyers CA, Shin DM, et al. Evaluation of cognitive function in patients with limited small cell lung cancer prior to and shortly following prophylactic cranial irradiation. Int J Radiat Oncol Biol Phys. 1995;33:179–182. doi: 10.1016/0360-3016(95)00026-U. [DOI] [PubMed] [Google Scholar]
- 49.Arriagada R, Le Chevalier T, Rivière A, et al. Patterns of failure after prophylactic cranial irradiation in small-cell lung cancer: Analysis of 505 randomized patients. Ann Oncol. 2002;13:748–754. doi: 10.1093/annonc/mdf123. [DOI] [PubMed] [Google Scholar]
- 50.Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 51.Patel S, Macdonald OK, Suntharalingam M. Evaluation of the use of prophylactic cranial irradiation in small cell lung cancer. Cancer. 2009;115:842–850. doi: 10.1002/cncr.24105. [DOI] [PubMed] [Google Scholar]
- 52.Le Péchoux C, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): A randomised clinical trial. Lancet Oncol. 2009;10:467–474. doi: 10.1016/S1470-2045(09)70101-9. [DOI] [PubMed] [Google Scholar]
- 53.Baas P, Belderbos JS, Senan S, et al. Concurrent chemotherapy (carboplatin, paclitaxel, etoposide) and involved-field radiotherapy in limited stage small cell lung cancer: A Dutch multicenter phase II study. Br J Cancer. 2006;94:625–630. doi: 10.1038/sj.bjc.6602979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sunpaweravong P, Magree L, Rabinovitch R, et al. A phase I/II study of docetaxel, etoposide, and carboplatin before concurrent chemoradiotherapy with cisplatin and etoposide in limited-stage small cell lung cancer. Invest New Drugs. 2006;24:213–221. doi: 10.1007/s10637-005-3669-3. [DOI] [PubMed] [Google Scholar]
- 55.Spigel DR, Hainsworth JD, Burris HA, et al. Long-term follow-up of limited stage small cell lung cancer patients treated with carboplatin-based chemotherapy and radiotherapy by the Minnie Pearl Cancer Research Network (MPCRN) [abstract 7222] J Clin Oncol. 2004;22:671. [Google Scholar]
- 56.Levitan N, Dowlati A, Shina D, et al. Multi-institutional phase I/II trial of paclitaxel, cisplatin, and etoposide with concurrent radiation for limited-stage small-cell lung carcinoma. J Clin Oncol. 2000;18:1102–1109. doi: 10.1200/JCO.2000.18.5.1102. [DOI] [PubMed] [Google Scholar]
- 57.Woo IS, Park YS, Kwon SH, et al. A phase II study of VP-16-fosfamide-cisplatin combination chemotherapy plus early concurrent thoracic irradiation for previously untreated limited small cell lung cancer. Jpn J Clin Oncol. 2000;30:542–546. doi: 10.1093/jjco/hyd135. [DOI] [PubMed] [Google Scholar]
- 58.Glisson B, Scott C, Komaki R, et al. Cisplatin, ifosfamide, oral etoposide, and concurrent accelerated hyperfractionated thoracic radiation for patients with limited small-cell lung carcinoma: Results of Radiation Therapy Oncology Group Trial 93-12. J Clin Oncol. 2000;18:2990–2995. doi: 10.1200/JCO.2000.18.16.2990. [DOI] [PubMed] [Google Scholar]
- 59.Ettinger DS, Berkey BA, Abrams RA, et al. Study of paclitaxel, etoposide, and cisplatin chemotherapy combined with twice-daily thoracic radiotherapy for patients with limited-stage small-cell lung cancer: A Radiation Therapy Oncology Group 9609 phase II study. J Clin Oncol. 2005;23:4991–4998. doi: 10.1200/JCO.2005.00.414. [DOI] [PubMed] [Google Scholar]
- 60.Hanna N, Ansari R, Fisher W, et al. Etoposide, ifosfamide and cisplatin (VIP) plus concurrent radiation therapy for previously untreated limited small cell lung cancer (SCLC): A Hoosier Oncology Group (HOG) phase II study. Lung Cancer. 2002;35:293–297. doi: 10.1016/s0169-5002(01)00429-9. [DOI] [PubMed] [Google Scholar]
- 61.Horn L, Bernardo P, Sandler A, et al. A phase II study of paclitaxel + etoposide + cisplatin + concurrent radiation therapy for previously untreated limited stage small cell lung cancer (E2596): A trial of the Eastern Cooperative Oncology Group. J Thorac Oncol. 2009;4:527–533. doi: 10.1097/JTO.0b013e31819c7daf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bremnes RM, Sundstrom S, Vilsvik J, et al. Multicenter phase II trial of paclitaxel, cisplatin, and etoposide with concurrent radiation for limited-stage small-cell lung cancer. J Clin Oncol. 2001;19:3532–3538. doi: 10.1200/JCO.2001.19.15.3532. [DOI] [PubMed] [Google Scholar]
- 63.Kubota K, Nishiwaki Y, Sugiura T, et al. Pilot study of concurrent etoposide and cisplatin plus accelerated hyperfractionated thoracic radiotherapy followed by irinotecan and cisplatin for limited-stage small cell lung cancer: Japan Clinical Oncology Group 9903. Clin Cancer Res. 2005;11:5534–5538. doi: 10.1158/1078-0432.CCR-04-1771. [DOI] [PubMed] [Google Scholar]
- 64.Mitsuoka S, Kudoh S, Takada Y, et al. Phase II study of cisplatin, etoposide and concurrent thoracic radiotherapy (TRT) followed by irinotecan and cisplatin in patients with limited stage small-cell lung cancer (SCLC); updated results of WJTOG9902 [abstract 7044] J Clin Oncol. 2004;22(14 suppl):627. [Google Scholar]
- 65.Miller AA, Wang XF, Bogart JA, et al. Phase II trial of paclitaxel-topotecan-etoposide followed by consolidation chemoradiotherapy for limited-stage small cell lung cancer: CALGB 30002. J Thorac Oncol. 2007;2:645–651. doi: 10.1097/JTO.0b013e318074bbf5. [DOI] [PubMed] [Google Scholar]
- 66.Le QT, Moon J, Redman M, et al. Phase II study of tirapazamine, cisplatin, and etoposide and concurrent thoracic radiotherapy for limited-stage small-cell lung cancer: SWOG 0222. J Clin Oncol. 2009;27:3014–3019. doi: 10.1200/JCO.2008.21.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 68.Hanna N, Bunn PA, Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–2043. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- 69.Lara PN, Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: Clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27:2530–2535. doi: 10.1200/JCO.2008.20.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arnold AM, Seymour L, Smylie M, et al. Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20. J Clin Oncol. 2007;25:4278–4284. doi: 10.1200/JCO.2007.12.3083. [DOI] [PubMed] [Google Scholar]
- 71.Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol. 2010;28:43–48. doi: 10.1200/JCO.2009.24.7353. [DOI] [PubMed] [Google Scholar]
- 72.Rigas JR, Denham CA, Rinaldi DA, et al. Randomized placebo-controlled trials of the matrix metalloproteinase inhibitor (MMPI), BAY12-9566 as adjuvant therapy for patients with small cell and non-small cell lung cancer [abstract 2525] Proc Am Soc Clin Oncol. 2003;22:628. [Google Scholar]
- 73.McClay EF, Bogart J, Herndon JE, 2nd, et al. A phase III trial evaluating the combination of cisplatin, etoposide, and radiation therapy with or without tamoxifen in patients with limited-stage small cell lung cancer: Cancer and Leukemia Group B Study (9235) Am J Clin Oncol. 2005;28:81–90. doi: 10.1097/01.coc.0000139940.52625.d0. [DOI] [PubMed] [Google Scholar]
- 74.Lee SM, Woll PJ, Rudd R, et al. Anti-angiogenic therapy using thalidomide combined with chemotherapy in small cell lung cancer: A randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst. 2009;101:1049–1057. doi: 10.1093/jnci/djp200. [DOI] [PubMed] [Google Scholar]