Reported are results from a subgroup analysis of postmenopausal women with hormone receptor–positive human epidermal growth factor receptor 2–positive metastatic breast cancer from a phase III trial of letrozole plus placebo versus letrozole plus lapatinib. The combination was well tolerated and more efficacious than letrozole alone.
Keywords: Breast neoplasms, Lapatinib, Letrozole, Aromatase inhibitors, Targeted therapy, erbB-2, HER-2
Abstract
Objective.
To evaluate the efficacy and tolerability of letrozole plus lapatinib versus letrozole plus placebo in women with hormone receptor (HR)+ human epidermal growth factor receptor (HER)-2+ tumors receiving first-line therapy for metastatic breast cancer (MBC).
Patients and Methods.
Postmenopausal women (n = 1,286) with HR+ MBC were randomized to daily oral treatment with letrozole (2.5 mg) plus lapatinib (1,500 mg) versus letrozole (2.5 mg) plus placebo. Of the 1,286 patients enrolled in the phase III study, 219 had HER-2+ tumors. The primary endpoint was progression-free survival (PFS) in HER-2+ patients.
Results.
Results in the HR+ HER-2+ population (n = 219) are presented. The addition of lapatinib to letrozole resulted in a significantly lower risk for disease progression than with letrozole alone (hazard ratio, 0.71; 95% confidence interval, 0.53–0.96). The PFS time was 8.2 months, versus 3.0 months. The objective response rate (ORR) (28% versus 15%) and clinical benefit rate (CBR) (48% versus 29%) were also significantly greater in lapatinib-treated women. The most common adverse events in the lapatinib group were diarrhea (68%) and rash (46%), primarily grade 1 and 2.
Conclusions.
The addition of lapatinib to letrozole is well tolerated and leads to a significantly greater PFS time, ORR, and CBR than with letrozole alone in women with MBC who coexpress HR and HER-2.
Introduction
Estrogen deprivation with agents such as aromatase inhibitors and tamoxifen is standard treatment for postmenopausal estrogen receptor (ER)+ breast cancer. However, resistance invariably develops, leading to disease relapse. Endocrine therapy–induced upregulation of signaling pathways of the epidermal growth factor receptor (EGFR) family of receptors—ErbB-1 (EGFR) and ErbB-2 (human epidermal growth factor receptor [HER]-2)—and enhanced ER-mediated transcription, which lead to a more aggressive phenotype, are key adaptive changes associated with estrogen resistance [1, 2].
Just as estrogen deprivation is the cornerstone of treatment for ER+ postmenopausal breast cancer, anti-HER-2 therapy is the treatment of choice for HER-2+ breast cancer. Dramatic clinical success is achieved when the HER-2 signaling pathway is blocked in women with breast cancer that overexpresses HER-2 [3–6].
Approximately 50% of HER-2+ breast cancers are hormone receptor (HR)+, [4, 5, 7, 8] and HER-2 positivity is considered a marker of estrogen resistance [9, 10]. Therefore, dual treatment for tumors that overexpress both receptors is a logical approach. A randomized trial was conducted in women with HR+ and HER-2+ metastatic breast cancer (MBC) to determine the impact of combined treatment with the anti-HER-2 monoclonal antibody trastuzumab and the aromatase inhibitor anastrozole versus anastrozole alone. The combination was associated with a median progression-free survival (PFS) duration of 4.8 months, versus 2.4 months for anastrozole alone [7]. Other clinical research also supports the role of combination treatment with HER-2 inhibitors and endocrine blockade in HR+ breast cancer [11, 12].
Synergy has been demonstrated with the combination of tamoxifen and lapatinib in models of endocrine resistance [13, 14]. A phase I study demonstrated no pharmacokinetic interactions between lapatinib and the aromatase inhibitor letrozole [15]. To further examine the role of estrogen deprivation plus anti-HER-2 therapy, we compared first-line therapy using letrozole plus placebo with letrozole plus lapatinib in a phase III study conducted in postmenopausal women with HR+ MBC [16]. Full results of this phase III study have been published [16]. Results in the subgroup of postmenopausal women with HR+ HER-2+ MBC are presented here.
Materials and Methods
Eligibility Criteria
Postmenopausal women with ER+ or progesterone receptor–positive (i.e., HR+), histologically confirmed, advanced breast cancer or MBC (stage IIIb/c or stage IV) were enrolled between December 9, 2003 and December 29, 2006. Results are presented here for enrolled women whose primary or metastatic tumor was also HER-2+. A HER-2+ tumor was defined as fluorescence in situ hybridization (FISH)+ (ratio >2), 3+ staining intensity by immunohistochemistry (IHC), or 2+ staining intensity by IHC and FISH+, as described previously for methods performed in a commercial laboratory [17].
Prior therapy for advanced or metastatic disease was prohibited, but prior neoadjuvant/adjuvant chemotherapy, antiestrogens, and radiotherapy were allowed. Adjuvant aromatase inhibitors and trastuzumab were permitted if discontinued at least 1 year prior to study entry. A good performance status (Eastern Cooperative Oncology Group [ECOG] performance status score of 0–1) and normal organ function were required, with cardiac ejection fraction within the institutional range of normal. Extensive symptomatic visceral disease and current or past central nervous system metastases were causes for exclusion. Enrollment required archived tumor tissue for use in biomarker analyses.
Ethics approval was obtained from appropriate local ethics committees and the study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. All patients signed informed consent documents before enrollment in the study. This study was funded by GlaxoSmithKline.
Trial Design
This was a randomized, double-blind, controlled, parallel-group, multicenter, phase III study with stratification by interval since completion of prior adjuvant antiestrogen therapy (at least 6 months or no prior therapy versus <6 months) and location of metastatic sites (soft tissue or visceral versus bone-only disease). Measurable disease was not required at study entry. Eligible patients were randomized to once-daily oral treatment with letrozole (2.5 mg) plus lapatinib (1,500 mg) or to the same dose of letrozole plus a matching lapatinib placebo. Lapatinib dosage adjustments were made in accordance with the U.S. Food and Drug Administration–approved lapatinib prescribing information [18]. No dosage adjustments were allowed for letrozole. Patients were continued on the assigned treatment until disease progression or withdrawal from study and were not permitted to cross over to the alternate treatment in the event of disease progression. Permanent withdrawal from study treatment was required for unacceptable toxicity or grade 3 or 4 interstitial pneumonitis, hepatoxicity, or cardiac dysfunction.
Assessments
The primary endpoint was investigator-assessed PFS in the HER-2+ population determined according to the Response Evaluation Criteria in Solid Tumors (RECIST) [19]. The PFS time was defined as the time from randomization until the earliest date of disease progression or death resulting from any cause. The overall response rate (ORR), clinical benefit rate (CBR), overall survival (OS) time, and safety were secondary endpoints. The CBR was defined as a confirmed complete response, partial response, or stable disease for at least 6 months.
Initially, toxicity was assessed every 4 weeks according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0), and cardiac function was assessed every 8 weeks. Beginning week 108, toxicities and cardiac function were assessed every 12 weeks. Efficacy was assessed every 12 weeks and at the time of study treatment withdrawal, after which patients were followed only for survival.
The primary endpoint of PFS in the HER-2+ population was powered at 80% to detect a hazard ratio (HR) of 0.645 with an α of 0.05 with 173 events. PFS and OS were summarized using the Kaplan–Meier method, with the stratified log-rank test used for comparisons between treatment arms. Cox regression analysis was used to assess the prognostic significance of PFS for the known prognostic baseline characteristics after retaining treatment and stratification factors: age, ECOG performance status score (0 or ≥1), number of metastatic sites, site of disease (bone only or visceral and soft tissue), interval since prior chemotherapy, interval since prior adjuvant antiestrogen therapy, disease-free interval, and serum HER-2 (extracellular domain [ECD]) at baseline (<15 ng/ml versus ≥15 ng/ml). The Kaplan–Meier method with stratified log rank was used to retrospectively analyze investigator-assessed PFS in subpopulations within the HER-2+ cohort to compare treatment arms within each subpopulation: patients without bone as the only site of metastasis, age, presence or absence of liver metastasis, number of metastatic sites, ECOG performance status, and prior hormonal therapy.
Results
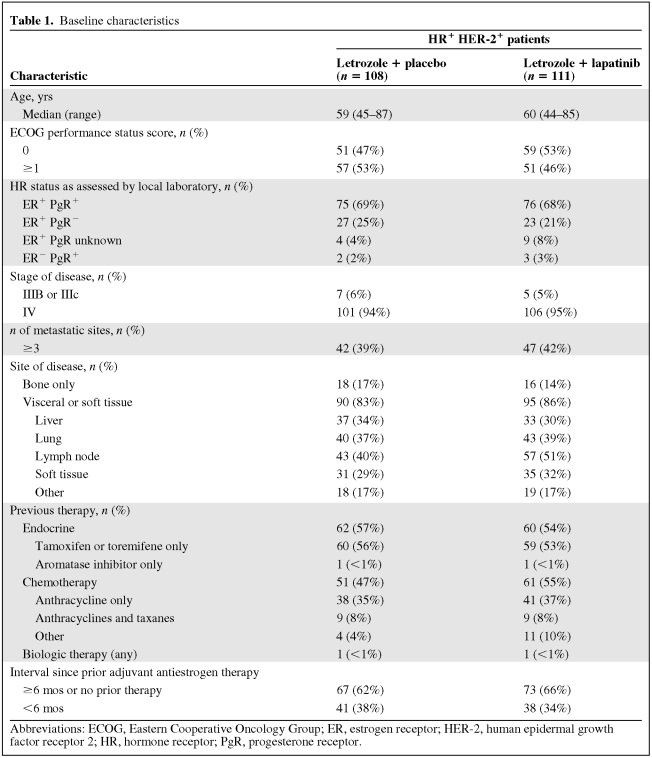
In total, 1,286 HR+ patients were enrolled, of whom 219 had HR+ HER-2+ MBC (Fig. 1). Of these 219 patients, 111 were randomized to the letrozole plus lapatinib arm and 108 were in the letrozole plus placebo arm. Baseline disease and patient characteristics were well balanced between arms. Most patients had stage IV disease and visceral or soft tissue metastases. Approximately half of the patients in each arm received prior antiestrogen therapy and/or prior chemotherapy. Approximately one third of the patients received adjuvant antiestrogen therapy within 6 months of study entry (Table 1).
Figure 1.
Study design, populations, randomization, and assessment of events.
Abbreviations: HER-2, human epidermal growth factor receptor 2; ITT, intent to treat; MBC, metastatic breast cancer; PD, progressive disease; PFS, progression-free survival; po, orally.
Table 1.
Baseline characteristics
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; HR, hormone receptor; PgR, progesterone receptor.
Efficacy
Patients were followed for a median of 1.9 years. The median PFS times in HR+ HER-2+ patients were 3.0 months in the letrozole plus placebo group and 8.2 months in the letrozole plus lapatinib group. The HR for the risk for progression was 0.71 favoring the lapatinib group (95% confidence interval [CI], 0.53–0.96; p = .019) (Fig. 2). When adjusted for baseline prognostic factors, the stepwise Cox regression analysis for PFS confirmed the benefit of letrozole plus lapatinib over letrozole alone (HR, 0.65; 95% CI, 0.47–0.89; p = .008). Younger age, a performance status score of 0, and baseline HER-2 ECD <15 ng/ml measured by quantitative enzyme-linked immunosorbent assay were identified as significant predictors of PFS.
Figure 2.
Progression-free survival in the human epidermal growth factor receptor (HER)-2+ population.
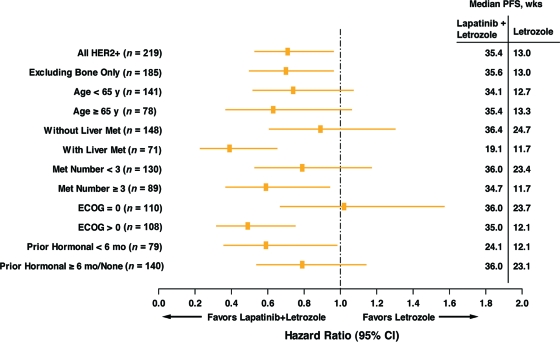
A retrospective analysis within known prognostic factor subpopulations showed consistently longer PFS time with letrozole plus lapatinib than with letrozole alone in the following groups: patients without bone as the only site of metastasis, patients with and without liver metastases, patients with fewer than three or three or more metastatic sites, patients with an ECOG performance status score of 0 or >0, and patients having received prior hormonal therapy for <6 months or for ≥6 months/none (Fig. 3). Patients with bone as the only site of metastasis were not included because of the small subpopulation size.
Figure 3.
Forest plot of hazard ratio for investigator-evaluated PFS by subgroups of baseline covariates.
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HER-2, human epidermal growth factor receptor 2; Met, metastasis; PFS, progression-free survival.
The ORR was also significantly higher in lapatinib-treated patients (28%, versus 15%; odds ratio [OR], 0.4; 95% CI, 0.2–0.9; p = .021), as was the CBR (48%, versus 29%; OR, 0.4; 95% CI, 0.2–0.8; p = .003) (Table 2). With a 47% death rate and 41% of patients still being followed for survival, the median OS times were 32.3 months in the letrozole plus placebo group and 33.3 months in the letrozole plus lapatinib group.
Table 2.
Response rate
Patients with both measurable and non-measurable disease are included in this table.
Abbreviations: CBR, clinical benefit rate (confirmed CR or PR or SD for at least 6 months); CI, confidence interval; CR, complete response; OR, odds ratio; ORR, overall response rate (confirmed CR or PR); PR, partial response; SD, stable disease.
Safety
In total, 219 HR+ HER-2+ patients were included in the safety analysis. Two subjects randomized to the letrozole plus placebo arm actually received letrozole plus lapatinib, thus the safety population reports on 106 and 113 patients, respectively. Adverse events were reported in 77% of patients in the letrozole plus placebo group and in 96% of patients in the letrozole plus lapatinib group. In both groups, adverse events were primarily grade 1 and 2. The most common adverse events in the letrozole plus lapatinib group were diarrhea (68%), rash (46%), nausea (27%), fatigue (22%), and arthralgia (18%), and in each case, with the exception of arthralgia, the incidence was greater than in the letrozole plus placebo group (Table 3). Although grade 3 and 4 events were rare (no individual grade 4 event was reported in more than one patient in either group), they were more common in patients receiving lapatinib. The most prominent grade 3 event was diarrhea, reported in 7% of patients treated with letrozole plus lapatinib. No action (dose interruption or reduction) was required in most cases of diarrhea (93%). In a small number of cases, diarrhea was managed by dose reduction (2%) or temporary interruption (4%). No patient required drug withdrawal as a result of diarrhea. There was one investigator-assessed treatment-related death in the letrozole plus lapatinib arm and none in the letrozole plus placebo arm.
Table 3.
Adverse events
Shown are events reported in ≥10% of patients in any group; discrepancies between values in the total column and the addition of the incidence rates reported for grades 1, 2, 3, and 4 are a result of mathematical rounding.
Alanine aminotransferase was increased in 6% of patients in the placebo group and in 11% of patients in the lapatinib group. Grade 1 or 2 hyperbilirubinemia was reported in 4% of lapatinib-treated patients. Details of adverse events are provided in Table 3.
A relative reduction in left ventricular ejection fraction ≥20% and below the institutional normal limit was reported in one patient receiving letrozole plus placebo and in three patients receiving letrozole plus lapatinib. None of the HER-2+ patients experienced a symptomatic cardiac event.
Discussion
Identification of mechanisms of resistance often serves as the basis for the development of more effective therapies. An association between tumor HER-2 positivity and lack of response to endocrine therapy has been observed [9, 10]. The role of growth factors in estrogen resistance was established in human breast cancer cells, whereby inhibition of crosstalk between ER and HER-2 restored the estrogen responsiveness of ER+ breast cancer cells [2]. Moreover, preclinical data suggest that EGFR/HER-2 targeted therapy combined with endocrine deprivation delays the development of resistance [20, 21].
In this study, we applied our understanding of the mechanism of resistance to endocrine-deprivation therapy to the development of a combination regimen that addresses the roles of excess hormones, hormone resistance, and HER-2 overexpression in postmenopausal women with MBC.
Lapatinib is an oral receptor tyrosine kinase inhibitor that targets HER-2 and has not been associated with significant symptomatic cardiotoxicity [22]. We combined lapatinib with the aromatase inhibitor letrozole, which has been shown to have favorable clinical efficacy, compared with tamoxifen [23].
The addition of lapatinib to letrozole as first-line therapy in postmenopausal women with HR+ HER-2+ MBC led to a significantly lower risk for disease progression and longer PFS time, 8.2 months versus 3.0 months, as well as a higher ORR and CBR. These results are consistent with the findings in a similar population in which anastrozole alone was compared with anastrozole plus trastuzumab [7]. Likewise, benefit for the combination of lapatinib and letrozole compared with letrozole alone was seen in all known prognostic factor subpopulations, including patients who were resistant to prior endocrine therapy (i.e., relapsed on or within 6 months of adjuvant tamoxifen), and those with liver metastases or more than three sites of metastatic disease. This suggests, therefore, that dual treatment for suitable patients with tumors that overexpress both HR and HER-2 is a logical approach.
Conclusions
Women with HR+ HER-2+ MBC achieved a statistically significant 29% lower risk for disease progression when treated with letrozole plus lapatinib than with letrozole alone. The combination targeted therapy was well tolerated, with primarily grade 1 and 2 toxicities. These data support the use of letrozole plus lapatinib for first-line therapy of patients with HR+ HER-2+ MBC. This trial further confirms that sustained HER-2 inhibition provides benefit in patients with HER-2+ MBC. Moreover, the addition of an oral lapatinib therapy provides a convenient option for women who receive oral endocrine therapy for an extended time.
Acknowledgments
We would like to acknowledge the women who participated in this study, the investigators and their staff, members of the independent data monitoring committee, Tim Kelly from GlaxoSmithKline, Novartis Oncology, and The Phillips Group for technical assistance in preparation of the manuscript.
This research was sponsored and funded by GlaxoSmithKline.
Portions of these data have been published in Johnston et al. [16].
Author Contributions
Conception/Design: Stephen Johnston, Lisa O'Rourke, Julie Maltzman, Allison Florance
Provision of study material or patients: Stephen Johnston, Lisa O'Rourke, Julie Maltzman
Collection and/or assembly of data: Lisa O'Rourke, Julie Maltzman, Allison Florance
Data analysis and interpretation: Stephen Johnston, Lisa O'Rourke, Julie Maltzman, Allison Florance
Manuscript writing: Stephen Johnston, Lee S. Schwartzberg, Sandra X. Franco, Lisa O'Rourke, Julie Maltzman, Allison Florance
Final approval of manuscript: Stephen Johnston, Lee S. Schwartzberg, Sandra X. Franco, Lisa O'Rourke, Julie Maltzman, Allison Florance
The authors take full responsibility for the content of the paper but thank Mary Duafala, R.Ph., and Claire Gilmore, Pharm.D., from Phillips Group Oncology Communications for their assistance in organizing the published literature, preparing an initial draft of the manuscript, and collating the comments of authors and other named contributors.
References
- 1.Arpino G, Gutierrez C, Weiss H, et al. Treatment of human epidermal growth factor receptor 2–overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst. 2007;99:694–705. doi: 10.1093/jnci/djk151. [DOI] [PubMed] [Google Scholar]
- 2.Sabnis GJ, Jelovac D, Long B, et al. The role of growth factor receptor pathways in human breast cancer cells adapted to long-term estrogen deprivation. Cancer Res. 2005;65:3903–3910. doi: 10.1158/0008-5472.CAN-04-4092. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 4.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 5.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 6.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: Results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 8.Lal P, Tan LK, Chen B. Correlation of HER-2 status with estrogen and progesterone receptors and histologic features in 3,655 invasive breast carcinomas. Am J Clin Pathol. 2005;123:541–546. doi: 10.1309/YMJ3-A83T-B39M-RUT9. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen BB, Regan MM, Lykkesfeldt AE, et al. Adjuvant letrozole versus tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: Supplementary results from the BIG 1–98 randomised trial. Lancet Oncol. 2008;9:23–28. doi: 10.1016/S1470-2045(07)70386-8. [DOI] [PubMed] [Google Scholar]
- 10.De Placido S, De Laurentiis M, Carlomagno C, et al. Twenty-year results of the Naples GUN randomized trial: Predictive factors of adjuvant tamoxifen efficacy in early breast cancer. Clin Cancer Res. 2003;9:1039–1046. [PubMed] [Google Scholar]
- 11.Osborne K, Neven P, Dirix K, et al. Randomized phase II study of gefitinib (IRESSA) or placebo in combination with tamoxifen in patients with hormone receptor positive metastatic breast cancer. Breast Cancer Res Treat. 2007;106(suppl 1):S107. [Google Scholar]
- 12.Cristofanilli M, Valero V, Mangalik A, et al. A phase II multicenter, double-blind, randomized trial to compare anastrozole plus gefitinib with anastrozole plus placebo in postmenopausal women with hormone receptor-positive (HR+) metastatic breast cancer (MBC) [abstract 1012] J Clin Oncol. 2008;26(15 suppl):44s. [Google Scholar]
- 13.Chu I, Blackwell K, Chen S, et al. The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res. 2005;65:18–25. [PubMed] [Google Scholar]
- 14.Leary AF, Martin LA, Lykkesfeldt AE, et al. Enhancing endocrine responsiveness using the dual EGFR/HER2 tyrosine kinase inhibitor lapatinib in models of endocrine resistance [abstract 303] Breast Cancer Res Treat. 2006;100(suppl 1):S29. [Google Scholar]
- 15.Chu QS, Cianfrocca ME, Goldstein LJ, et al. A phase I and pharmacokinetic study of lapatinib in combination with letrozole in patients with advanced breast cancer. Clin Cancer Res. 2008;14:4484–4490. doi: 10.1158/1078-0432.CCR-07-4417. [DOI] [PubMed] [Google Scholar]
- 16.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 17.Press MF, Finn RS, Cameron D, et al. HER-2 gene amplification, HER-2 and epidermal growth factor receptor mRNA and protein expression, and lapatinib efficacy in women with metastatic breast cancer. Clin Cancer Res. 2008;14:7861–7870. doi: 10.1158/1078-0432.CCR-08-1056. [DOI] [PubMed] [Google Scholar]
- 18.Tykerb (lapatinib) tablets [prescribing information] Research Triangle Park, NC: GlaxoSmithKline; 2008. Jul, [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Gee JM, Harper ME, Hutchinson IR, et al. The antiepidermal growth factor receptor agent gefitinib (ZD1839/Iressa) improves antihormone response and prevents development of resistance in breast cancer in vitro. Endocrinol. 2003;144:5105–5117. doi: 10.1210/en.2003-0705. [DOI] [PubMed] [Google Scholar]
- 21.Massarweh S, Osborne CK, Creighton CJ, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68:826–833. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 22.Perez EA, Koehler M, Byrne J, et al. Cardiac safety of lapatinib: Pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc. 2008;83:679–686. doi: 10.4065/83.6.679. [DOI] [PubMed] [Google Scholar]
- 23.The Breast International Group (BIG) 1–98 Collaborative Group. Thr̈limann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]