This article reviews the published literature on the use of chemoradiation as a modality in various stages of pancreatic adenocarcinoma and highlights areas that future trials in this field should target for a way forward in this malignancy.
Keywords: Pancreatic cancer, Chemoradiation, Chemoradiotherapy, IMRT
Abstract
Adenocarcinoma of the exocrine pancreas has an annual incidence of 7,400 cases in the U.K. In comparison with other common cancers of solid organs, namely, breast, colorectal, and prostate cancer, pancreatic cancer has a high morbidity and mortality. Radical resection is possible in only 15%–20% of patients, and only 3%–4% of all patients presenting with this condition achieve long-term control and cure. Various strategies in the form of neoadjuvant and adjuvant treatment have been employed over the years to improve outcome, with limited success. Systemic chemotherapy remains the gold standard in the metastatic setting in good performance status patients, and adjuvant chemotherapy after resection of localized and locally advanced cancer has been found to improve outcome. The role of radiotherapy, however, remains controversial and is an area that merits further investigation in well-conducted multicenter trials at various stages of the disease in combination with systemic agents and exploiting recent advances in the delivery of radiotherapy. In this article, we review the published literature on the use of chemoradiation as a modality in various stages of pancreatic adenocarcinoma and highlight areas that future trials in this field should target for a way forward in this malignancy.
Introduction
Pancreatic cancer is the tenth most common cancer in the western world and has become the fourth leading cause of cancer-related death. In the U.S., for 2009, an expected incidence of 42,470 new cases was accompanied by 35,240 pancreatic cancer–related deaths for a mortality rate of 82.9% [1]. In the U.K., an annual incidence of 7,400 cases was accompanied by a mortality rate of 98% [2]. These figures underline the paucity of effective treatments available. Apart from the obvious need for new breakthroughs, it is noteworthy that there remains a significant amount of uncertainty and controversy over the optimal use of even the conventional modalities at our disposal, despite years of research.
Here, we comprehensively review the published literature on the role of chemoradiation (CRT) as a strategy at several stages of the disease, highlighting questions, research into which may optimize outcomes.
Neoadjuvant (Preoperative) CRT
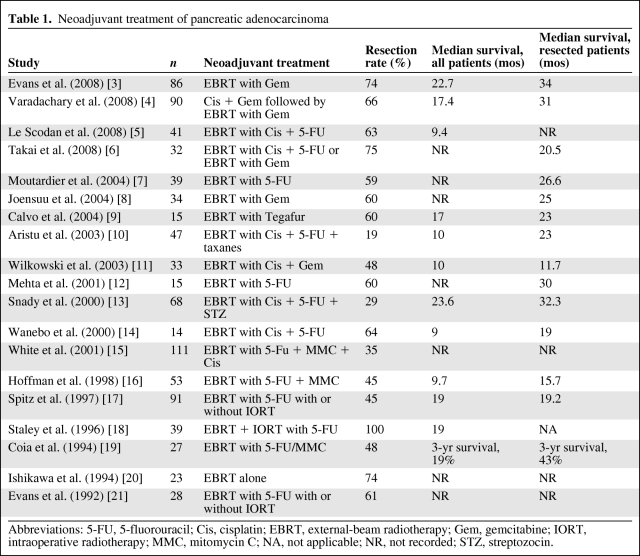
Preoperative treatment of resectable or borderline resectable cancer has several attractive benefits (Table 1) [3–21]. First, any partial response to treatment reduces the tumor volume, potentially increasing the likelihood of an R0 resection. Second, the resected tumor can serve as its own biological “marker” of treatment response. Third, the undisturbed tumor microenvironment permitting better oxygenation of tumor tissue may enhance treatment effects. Finally, multimodality therapy is likely to be better tolerated prior to, rather than after, a radical pancreaticoduodenectomy [22]. Patients who develop unresectable or metastatic disease during the induction treatment phase are also spared the morbidity of such a radical procedure. The main drawbacks are the comparatively low response rate to multimodality treatments in advanced pancreatic cancer (APC) and the potentially higher complication rate, which could result in the delay of potentially curative surgery.
Table 1.
Neoadjuvant treatment of pancreatic adenocarcinoma
Abbreviations: 5-FU, 5-fluorouracil; Cis, cisplatin; EBRT, external-beam radiotherapy; Gem, gemcitabine; IORT, intraoperative radiotherapy; MMC, mitomycin C; NA, not applicable; NR, not recorded; STZ, streptozocin.
CRT in Resectable Pancreatic Cancer
In a recent phase II study [3], preoperative radiotherapy was given to 86 patients at a dose of 30 Gy in 10 fractions over 2 weeks with 7 weekly gemcitabine doses at 400 mg/m2. Seventy-three patients (85%) went to surgery and 13 patients were found to have either progressive disease or a deterioration in performance status. At surgery, nine patients were found to have metastatic disease and 64 patients (74%) underwent radical surgery. The median survival times and 5-year overall survival rates in the whole population, resected patients, and unresectable patients were 22.7 months and 27%, 34 months and 36%, and 7 months and 0%, respectively. The same group conducted a further study [4] in which similar gemcitabine-based CRT was preceded by induction chemotherapy with four cycles of cisplatin and gemcitabine as 2-weekly schedules in a cohort of 90 patients. Although 88% of patients completed the whole course of treatment and 66% underwent the planned R0 resection, the median survival time was not improved upon (Table 1).
CRT in “Borderline Resectable” Pancreatic Cancer
The definition of “borderline resectable” is an evolving entity not founded on evidence-based criteria that have been shown to select similar patients in a validated prospective sense. Existing data, therefore, have to be viewed with caution especially because they span almost three decades, during which surgical and staging techniques have progressed substantially. Tumors that are encasing the superior mesenteric artery (SMA), celiac artery (CA), aorta, or inferior vena cava are considered unresectable. In addition, tumors with encasement of the superior mesenteric vein (SMV) or portal vein (PV) >180° over an extended segment are also considered unresectable. Tumors in which the PV and SMV are patent and there is a clear fat plane between the tumor and SMA and CA are deemed primarily resectable [23]. Borderline resectable patients are, therefore, those that fall in between these two groups. They often have abutment or encasement of the PV, SMV, or SMA over ≤180° or short-segment (≤1.5 cm) encasement of the SMV or PV, which is amenable to partial resection of the vein and reconstruction [24, 25]. These patients are, however, more likely to have R1 or R2 resections, and hence a neoadjuvant strategy could be employed to increase the prospect of an R0 resection.
Radiotherapy
One of the earlier studies in this group of patients employed external-beam radiotherapy (EBRT) alone [26]. Seventeen patients were treated with radiation at doses of 40–46 Gy over 4–5 weeks. The response rate was 29% and six patients (35%) became resectable. Only two patients (12%) had an R0 resection, and they survived for 5 years. To circumvent the dose limitations of EBRT imposed by the need to limit the dose to normal organs, strategies have been developed to deliver a higher dose to the tumor, such as intraoperative radiation therapy (IORT) and brachytherapy. Roldan et al. [27] employed a combination of EBRT and IORT versus EBRT alone in unresectable cancers. Although the local control rate at 2 years was significantly better for the combination arm (66% versus 20%; p < .0005), this did not translate into a survival benefit. A similar lack of survival benefit but higher toxicity was reported by the Memorial Sloan-Kettering Cancer Center group with 103Pd brachytherapy in unresectable patients [28].
Combining Chemotherapy with Radiotherapy
Fluoropyrimidines.
Because escalation of the radiation dose in locally APC did not translate into longer survival, focus shifted to employing multiagent chemotherapy with conventional radiation, especially because a small randomized trial (RCT) from the Gastrointestinal Tumor Study Group (GITSG) [29] had demonstrated the superiority of 5-fluorouracil (5-FU)–based CRT over radiotherapy alone in locally advanced unresectable disease (discussed in detail below). Hoffman et al. [30] performed pilot studies with 50.4 Gy of radiation with a combination of 5-FU (1,000 mg/m2 per day continuous infusion on days 2–5 and days 29–32) along with mitomycin-C (10 mg/m2). In 34 patients treated with this regimen, 25 went for surgery. Eleven had a pancreaticoduodenectomy and 10 had an R0 resection, with a 45-month median survival duration. Based on these promising results, a phase II study was set up by the Eastern Cooperative Oncology Group, which included 53 patients. The resection rates were similar to those in the pilot phase, but the median survival time was shorter [16].
Gemcitabine.
The clinical primacy of gemcitabine [31] in APC has led to preclinical studies with human pancreatic and colon cancer cell lines that have shown its potency as a powerful radiosensitizer [32, 33]. Phase I trials of gemcitabine with 50.4 Gy of radiation given in 1.8-Gy fractions established dose-limiting hematologic and gastrointestinal toxicities at a dose of 700 mg/m2 weekly. Responses were observed at doses >500 mg/m2, but late duodenal strictures were noted [34] at doses >400 mg/m2. Crane et al. [35] analyzed a retrospective series of 53 patients with unresectable pancreatic cancer treated with weekly gemcitabine doses of 250–300 mg/m2 for 7 weeks with concurrent radiation of 30–33 Gy in 10 fractions versus 61 patients treated with concurrent infusional 5-FU and radiation. The radiotherapy volumes were large and included at-risk uninvolved lymph node stations at the porta, celiac axis, and superior mesenteric vessels. The toxicity rate in the gemcitabine arm was significantly higher (23% versus 2%). Resectability was achieved in 9% of patients in the gemcitabine group, as opposed to 2% of patients in the 5-FU arm. There was, however, no significant difference in the median survival times (11 months versus 9 months). The safe weekly dose of gemcitabine, therefore, needs to remain <400 mg/m2 when used with conventional radiation, which is a suboptimal dose for systemic disease control. Subsequent studies with a smaller radiotherapy volume to include the primary tumor and involved nodes only have successfully used full doses of gemcitabine, at 1,000 mg/m2 weekly, with acceptable toxicity. Encouragingly, locoregional nodal failure outside the radiation volume was rare [36]. Gemcitabine combinations with other chemotherapy agents like cisplatin [11] and paclitaxel [37] given concurrently with radiation have resulted in R0 resections in up to 30% of patients with acceptable toxicity and no difference in the postsurgical complication rate.
A recurrent theme of neoadjuvant CRT studies is that 10%–30% of patients experience disease progression during preoperative treatment, which in turn has led to the suggestion that a period of induction chemotherapy could potentially superselect patients suitable to undergo CRT. A retrospective analysis of 323 patients with locally APC at the MD Anderson Cancer Center showed a longer median overall survival time (11 months versus 8.5 months; p < .001) in patients receiving a median of 2.5 months of gemcitabine-based upfront combination chemotherapy than in patients receiving CRT alone [38]. Fogelman et al. [39], at Columbia University, used three cycles of gemcitabine, docetaxel, and capecitabine over a 9-week period followed by CRT in a series of 14 patients with locally APC. Only one patient progressed in the induction phase and eight patients (57%) became resectable, and all had R0 resections.
Adjuvant CRT in Pancreatic Adenocarcinoma
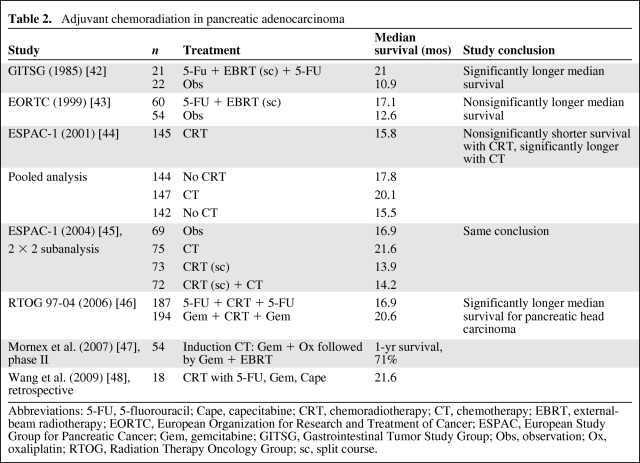
The relatively high rate of both locoregional and distant recurrence following surgery for pancreatic cancer makes a strong case for effective adjuvant therapy [40, 41]. RCTs of CRT are limited, and the available data are boosted by some phase II and single-institution studies. They are summarized in Table 2 [42–48].
Table 2.
Adjuvant chemoradiation in pancreatic adenocarcinoma
Abbreviations: 5-FU, 5-fluorouracil; Cape, capecitabine; CRT, chemoradiotherapy; CT, chemotherapy; EBRT, external-beam radiotherapy; EORTC, European Organization for Research and Treatment of Cancer; ESPAC, European Study Group for Pancreatic Cancer; Gem, gemcitabine; GITSG, Gastrointestinal Tumor Study Group; Obs, observation; Ox, oxaliplatin; RTOG, Radiation Therapy Oncology Group; sc, split course.
The first prospective, multicenter trial of CRT versus observation alone was performed by the GITSG [42]. Resected pancreatic cancer patients with R0 margins were assigned to receive either split-course radiotherapy over 6 weeks with a 2-week gap in between, with concurrent 5-FU on week 1 and week 5 followed by maintenance 5-FU for 2 years or until progression or no active treatment. In 1974–1982, only 49 patients were randomized. At an interim analysis, patients in the CRT arm had a significantly longer median survival time (21 months versus 11 months). A further 32 patients were added to the treatment cohort in a nonrandomized fashion following the interim analysis, and the final analysis showed a median survival time of 18 months with 2- and 5-year survival rates of 46% and 17%, respectively [49]. Following that trial, adjuvant CRT became the standard of care in the U.S. The study, however, has been criticized in other quarters for its poor accrual, low statistical power, suboptimal radiotherapy schedule, lack of radiotherapy quality assurance, and noncompliance with maintenance chemotherapy in 75% of patients.
The European Organization for Research and Treatment of Cancer (EORTC) conducted a similar study of CRT versus observation in Europe between 1987 and 1995 [43]. The radiation schedule was similar but the 5-FU was delivered as an infusion and there was no maintenance chemotherapy. It included pancreatic and periampullary cancers and both R0 and R1 resections, but did not prestratify for primary site or resection margin status. The trial did not show any significant benefit in terms overall survival in the whole population or in patients with pancreatic head cancer. The statistical analysis of that trial has been criticized, and it could have given a significant result if the design was more appropriate [50].
The European Study Group for Pancreatic Cancer (ESPAC) conducted the largest phase III RCT in this setting between 1994 and 2000. Five hundred forty-one patients were randomized to: (a) chemotherapy versus observation, (b) CRT versus observation, and (c) a 2 × 2 factorial design of observation versus chemotherapy versus CRT versus CRT plus maintenance chemotherapy. The radiotherapy schedule was similar to that used in the EORTC study and the chemotherapy agent was bolus 5-FU. Both R0 and R1 patients were included. At an early intent-to-treat analysis at 10 months, there was a statistically significant survival benefit for patients receiving chemotherapy (median survival time, 19.7 months versus 14 months; p = .0005) but no benefit for patients treated with CRT (median survival time, 15.5 months versus 16.1 months; p = .24) [44]. The mature results of ESPAC-1 [45] with analysis restricted to the 2 × 2 arm of the study showed a significant 5-year survival benefit for chemotherapy versus no chemotherapy (21% versus 8%; p = .009), but no benefit for CRT versus no CRT (10% versus 20%; p = .05). The conclusion from that trial was that adjuvant chemotherapy significantly improved survival, whereas CRT had a detrimental effect on survival because it delayed systemic chemotherapy. The results generated substantial controversy and the trial was criticized because of a suboptimal radiotherapy schedule, lack of central radiotherapy quality assurance, wide variation in the radiotherapy doses employed, in violation of the protocol, and the allowance of background therapy with chemotherapy or CRT prior to randomization, which could all potentially influence the final analysis.
A subsequent meta-analysis of adjuvant therapy by the Pancreatic Cancer Meta-analysis Group (PCMG) in 2005 looked at individual patient data from five randomized studies of chemotherapy and CRT along with previously unpublished results of the ESPAC-1 study [51]. They concluded that: (a) chemotherapy alone reduced the risk for death by 25% (hazard ratio [HR], 0.75; confidence interval [CI], 0.64–0.90; stratified p = .001), (b) CRT had no significant impact (HR, 1.09; CI, 0.89–1.32; stratified p = .43), and (c) subgroup analyses showed CRT as more effective than chemotherapy in patients with R1 resections. However, that meta-analysis was heavily influenced by ESPAC-1 data.
A further PCMG meta-analysis looking at the influence of resection margin status and treatment on survival suggested that resection margin involvement was not a significant factor for survival (HR, 1.10; CI, 0.94–1.29; p = .24) [52]. The 2- and 5-year survival rates were 33% and 16% for R0 and 29% and 15% for R1 patients, respectively. CRT in R1 patients resulted in a 28% lower risk for death (HR, 0.72; CI, 0.47–1.10) and there was a 19% higher risk for death in R0 patients (HR, 1.19; CI, 0.95–1.49). Chemotherapy, on the other hand, resulted in a 4% higher risk for death in R1 patients (HR, 1.04; CI, 0.78–1.40) and a 35% lower risk for death in R0 patients (HR, 0.65; CI, 0.53–0.80).
The latest trial of CRT (Radiation Therapy Oncology Group trial 97–04) was conducted between 1998 and 2002 [46]. The analysis was conducted on 442 of 538 patients randomized between 3 weeks before and 3 months after CRT with 5-FU and 3 weeks before and 3 months after CRT with gemcitabine. The CRT part in both arms delivered 50.4 Gy in 28 fractions, with concurrent 5-FU as a 250-mg/m2 per day continuous infusion. Only 5% of the patients had an unacceptable deviation from protocol. Patients were stratified for surgical margin, tumor diameter, and nodal status. At the final analysis, 381 patients with pancreatic head tumors only had a significant benefit from gemcitabine in terms of the median survival time and 3-year survival rate (20.6 months versus 16.9 months and 32% versus 21%, respectively). There was no significant difference when tumors of the body and tail were included as well.
The ESPAC group recently reported data from ESPAC-3 in their latest abstract, showing equivalence of gemcitabine and 5-FU plus leucovorin as adjuvant therapy, with a better safety profile in favor of gemcitabine [53]. ESPAC-4 has now been launched comparing gemcitabine with gemcitabine plus capecitabine in the adjuvant setting because the assessment of the ESPAC group is that CRT offers no benefit in this setting. Nevertheless, the issue of the optimal treatment of patients with positive resection margins is still far from clear.
In light of the above findings, it is difficult to formulate a “one-size-fits-all” strategy in the adjuvant setting for pancreatic cancer. Good quality trials are still needed, targeting surgical subgroups, especially because more aggressive surgery of “borderline” cases will lead to a greater number of R1 resections.
The advent of biologicals is interesting, but it is as yet difficult to see where they fit in the combination radiotherapy and adjuvant settings, given disappointing results in APC patients to date.
CRT in Locally Advanced Nonresectable Pancreatic Adenocarcinoma
Locally advanced nonresectable pancreatic adenocarcinoma (LANPC) as an entity presents a significant dilemma to multidisciplinary teams involved in the management of pancreatic cancer. The chance for a cure is low with radiation alone. Combination with chemotherapy is logical, but it can be associated with significant toxicity.
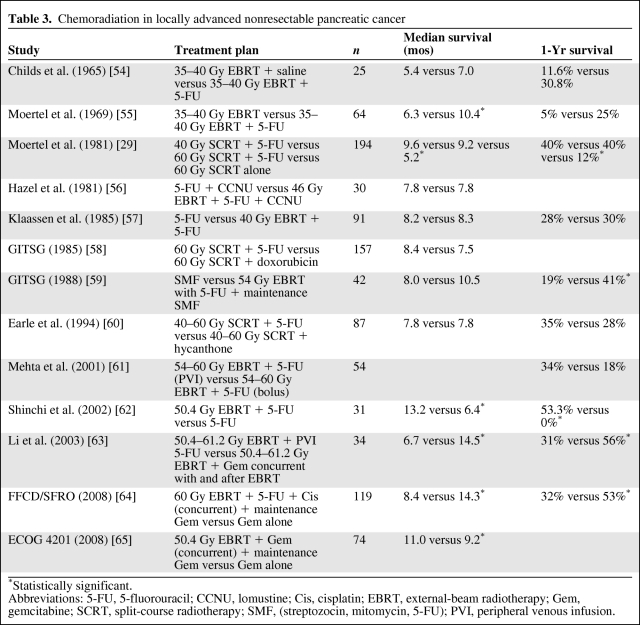
The GITSG demonstrated the superiority of 5-FU–based CRT over radiotherapy alone in locally advanced unresectable disease [29]. The median survival time was 5.7 months in the 60-Gy radiotherapy alone arm, compared with 10 months in the arms receiving bolus 5-FU with 40 Gy and 60 Gy of radiation. That trial also raised the possibility that, with chemotherapy, a higher dose of radiation is perhaps not necessary because the 1-year survival rates in the 60-Gy arm and 40-Gy arm with concurrent 5-FU were similar. This is a significant finding in terms of keeping the total radiation dose to a minimum and thereby reducing the rate of serious adverse events. Since the publication of that trial in 1981, several single-institution and cooperative group studies have employed CRT in LANPC patients with similar median survival figures (Table 3) [29, 54–65]. The rates of grade 3 and 4 toxicities were consistently higher in the CRT arms of these trials, and when compared with trials employing chemotherapy alone CRT is likely to cause a significant dip in quality of life, at least in the short term.
Table 3.
Chemoradiation in locally advanced nonresectable pancreatic cancer
*Statistically significant.
Abbreviations: 5-FU, 5-fluorouracil; CCNU, lomustine; Cis, cisplatin; EBRT, external-beam radiotherapy; Gem, gemcitabine; SCRT, split-course radiotherapy; SMF, (streptozocin, mitomycin, 5-FU); PVI, peripheral venous infusion.
With the introduction of gemcitabine, the emphasis shifted to the use of chemotherapy alone because it seemed that the survival rate achieved with this drug matched the survival rates seen in earlier trials of CRT using 5-FU. A recent French group trial [64] showed that CRT with concurrent 5-FU and cisplatin followed by maintenance gemcitabine provided a much higher toxicity rate and poorer survival rate than with induction chemotherapy alone with gemcitabine followed by maintenance gemcitabine in a group of 119 patients with LANPC. However, the dose intensities of both the chemotherapy and radiotherapy in the combined-modality arm and the use of large fields of radiation, including uninvolved nodes, are questionable as a strategy. The trial also showed a higher than expected survival duration of 14.3 months in the chemotherapy alone arm. In contrast, the Eastern Cooperative Oncology Group [65] recently reported on a trial comparing CRT with concurrent gemcitabine followed by maintenance gemcitabine with gemcitabine alone. Although the trial was stopped after only 74 of a planned 316 patients were entered as a result of slow accrual, it still showed a significant median survival advantage in favor of the radiotherapy arm. That trial employed more acceptable total doses of radiation and chemotherapy, and the radiation fields were smaller, planned using a conformal technique. As a result, the rates of grade 3 and 4 toxicities were low and these were manageable (Table 3). These data highlight the need for strict quality assurance of radiation techniques and call for the necessity of a uniform approach to radiotherapy of this sensitive anatomical area.
Techniques of Radiation Therapy Planning and Delivery
In order to reduce the toxicity associated with radiotherapy to the pancreas newer techniques have been employed which look at excluding as much normal tissue as feasible and thereby escalate the dose to influence local control and ultimately survival.
IORT
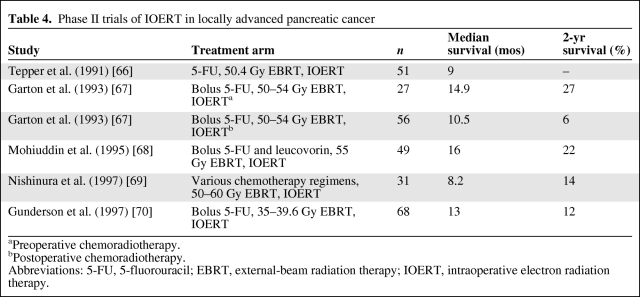
IORT has the advantage of delivering radiotherapy to the tumor/tumor bed under direct vision and reducing toxicity by shielding dose-limiting normal organs. Methods of IORT included either implantation of iodine-125 seeds or intraoperative electron beam radiotherapy (IOERT). IOERT has been the favored approach in most studies. A trial by the National Cancer Institute showed better local control with 20 Gy of IORT following surgical therapy than with observation. Several phase II studies have tried to exploit the radiobiological and anatomical advantages of IOERT (Table 4) [66–70]. A further strategy of brachytherapy used colloidal phosphorus-32 infusion in the tumor interstitial space followed by EBRT with concurrent 5-FU. All five patients treated with this technique showed local control or regression, with three patients surviving for 24 months and one patient surviving for 36 months [71]. Although most have shown better local control, survival was not shown to be superior to that seen with EBRT alone.
Table 4.
Phase II trials of IOERT in locally advanced pancreatic cancer
aPreoperative chemoradiotherapy.
bPostoperative chemoradiotherapy.
Abbreviations: 5-FU, 5-fluorouracil; EBRT, external-beam radiation therapy; IOERT, intraoperative electron radiation therapy.
Stereotactic Radiotherapy
Stereotactic radiotherapy (SRT) aims to deliver one to five high-dose fractions to the area of gross disease, in comparison with conventional EBRT. Such an advantage could potentially be exploited by delivering SRT to the tumor only, preceded or followed by conventional radiation to the tumor volume and at-risk area. Hoyer et al. [72], in a phase II study of SRT, used 45 Gy in three fractions in a space of 5–10 days in 22 patients with LANPC. There were unacceptable acute gastrointestinal toxicities, with 4.5% of patients experiencing gastric perforation. A trial by the Stanford group [73] used a single fraction of SRT delivering 25 Gy to a limited radiation field and demonstrated an 81% local control rate. The same group studied the effect of SRT as a boost to EBRT, yielding a very impressive 94% local control rate [74]. Gastrointestinal toxicity, however, still remained a significant issue, with a 12.5% rate of late duodenal ulceration. Despite a major improvement in local control, no difference in the median survival time was noted in these studies.
Intensity-Modulated Radiotherapy
Intensity-modulated radiotherapy (IMRT) is delivered as conformal radiation but with varying intensities within each radiation field. This has the advantage of mapping the dose to a high-dose volume within the tumor and its vicinity and at the same time keeping the dose low in the regions of at-risk normal structures. It may also allow for dose escalation and a consequent greater tumor control probability. Milano et al. [75], in an efficacy and toxicity finding study of IMRT in pancreatic and bile duct cancer, treated 25 patients with IMRT and concurrent 5-FU. IMRT was well tolerated and reduced the mean dose to the liver, kidneys, stomach, and small bowel, with 80% of patients experiencing grade 2 toxicity only. In a separate study [76], 15 patients with adenocarcinoma of the pancreas were treated with IMRT and concomitant capecitabine. The IMRT was delivered to a dose of 54 Gy to the gross tumor and 45 Gy to the draining lymph nodes in a simultaneous boost method. The study reported a 7% grade 3 toxicity and 0% grade 4 toxicity rate. Thus, with superior planning techniques and better and effective monitoring of toxicities, IMRT is likely to be safely delivered to patients with pancreatic cancer concurrently with systemic chemotherapy. The position of IMRT and that of SRT vis à vis local control and other concurrent or sequential systemic treatment needs RCTs with conventional comparators.
Induction Chemotherapy Prior to CRT
The majority of LANPC patients recur at distant sites. Hence, to improve prognosis in this group, effective systemic chemotherapy is necessary to control micrometastases. Induction chemotherapy is a logical tactic allowing resistant cancer biology to declare itself before offering CRT as a more definitive approach.
At the MD Anderson Cancer Center, in a retrospective analysis of 318 patients [38] with LANPC between 1993 and 2005, 73 patients receiving a median of 2.5 months of induction chemotherapy before proceeding to CRT had a significantly longer overall time to local and distant progression than 245 patients receiving CRT as their first treatment.
A phase I/II study by Brade et al. [77] of induction chemotherapy with gemcitabine followed by concurrent gemcitabine and radiotherapy showed that 22% of patients (six of 27) had disease progression on induction chemotherapy and hence could be spared further treatment with CRT.
A recent audit report [78] showed that patients with LANPC who had stable disease after induction chemotherapy before CRT had a significantly longer survival duration (11.8 months versus 6.6 months; p = .01).
In a recently published nonrandomized series, 181 patients [79] were treated with gemcitabine-based chemotherapy for 3 months, and those with stable disease (128 patients) were treated with CRT or chemotherapy alone. The median survival time was significantly longer in patients receiving CRT (15 months versus 11.7 months). This shows a probable benefit of CRT in patients who have achieved stable disease with induction chemotherapy.
These data as a whole seem promising, but there is a clear need for a RCT designed to test these hypotheses, especially the two strategies of gemcitabine-based CRT followed by gemcitabine versus gemcitabine induction followed by gemcitabine-based CRT.
Conclusion
In terms of the positioning of CRT, our review highlights a number of priority issues that the oncological community needs to address. First, the quality assurance of delivered radiotherapy and agreement on similar standards of what constitutes a radical treatment field in the two settings of an in situ primary and a resected primary are seen as a sine qua non for the success of any trial in this area. Second, the position of CRT in patients with initially resectable disease on first intent ending up with R1 margins needs further study, for example, in an RCT evaluating gemcitabine-based CRT with or without extended adjuvant chemotherapy. Third, the “neoadjuvant” approach using CRT for patients with a borderline resectable primary given the high likelihood of R1 or R2 margins would also benefit from an RCT. Fourth, for LANPC, the two most promising strategies of gemcitabine-based CRT followed by gemcitabine or the reverse need further study in a head-to-head RCT. Finally, the position of IMRT and that of SRT need RCT approaches (e.g., phase IIb trials) with conventional comparators. The failure of biologicals to have an impact on APC treatment means that we cannot at present see a role for these in the CRT setting other than in early-phase (I and II) trial work.
Author Contributions
Conception/Design: Anthony Maraveyas, Rajarshi Roy
Provision of study material or patients: Rajarshi Roy
Collection and/or assembly of data: Anthony Maraveyas, Rajarshi Roy
Data analysis and interpretation: Anthony Maraveyas, Rajarshi Roy
Manuscript writing: Anthony Maraveyas, Rajarshi Roy
Final approval of manuscript: Anthony Maraveyas, Rajarshi Roy
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Research UK. Pancreatic Cancer Statistics—Key Facts. [accessed February 22, 2010]. Available at http://info.cancerresearchuk.org/cancerstats/types/pancreas.
- 3.Evans DB, Varadachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 4.Varadachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 5.Le Scodan R, Mornex F, Partensky C, et al. Histopathological response to preoperative chemoradiation for resectable pancreatic adenocarcinoma: The French phase II FFCD 9704-SFRO trial. Am J Clin Oncol. 2008;31:545–552. doi: 10.1097/COC.0b013e318172d5c5. [DOI] [PubMed] [Google Scholar]
- 6.Takai S, Satoi S, Yanagimoto H, et al. Neoadjuvant chemoradiation in patients with potentially resectable pancreatic cancer. Pancreas. 2008;36:e26–e32. doi: 10.1097/mpa.0b013e31814b229a. [DOI] [PubMed] [Google Scholar]
- 7.Moutardier V, Turrini O, Huiart L, et al. A reappraisal of preoperative chemoradiation for localized pancreatic head ductal adenocarcinoma in a 5-year single-institution experience. J Gastrointest Surg. 2004;8:502–510. doi: 10.1016/j.gassur.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Joensuu TK, Kiviluoto T, Kärkkäainen P, et al. Phase I-II trial of twice weekly gemcitabine and concomitant irradiation in patients undergoing pancreaticoduodenectomy with extended lymphadenectomy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;60:444–452. doi: 10.1016/j.ijrobp.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Calvo FA, Matute R, García-Sabrido JL, et al. Neoadjuvant chemoradiation with tegafur in cancer of the pancreas: Initial analysis of clinical tolerance and outcome. Am J Clin Oncol. 2004;27:343–349. doi: 10.1097/01.coc.0000071462.12769.35. [DOI] [PubMed] [Google Scholar]
- 10.Aristu J, Cañón R, Pardo F, et al. Surgical resection after preoperative chemoradiotherapy benefits selected patients with unresectable pancreatic cancer. Am J Clin Oncol. 2003;26:30–36. doi: 10.1097/00000421-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Wilkowski R, Thoma M, Heineman V, et al. [Radiochemotherapy with gemcitabine and cisplatin in pancreatic cancer—feasible and effective.] Strahlenther Onkol. 2003;179:78–86. doi: 10.1007/s00066-003-1036-x. In German. [DOI] [PubMed] [Google Scholar]
- 12.Mehta VK, Fisher G, Ford JA, et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J Gastrointest Surg. 2001;5:27–35. doi: 10.1016/s1091-255x(01)80010-x. [DOI] [PubMed] [Google Scholar]
- 13.Snady H, Bruckner H, Cooperman A, et al. Survival advantage of combined chemoradiotherapy compared with resection as the initial treatment of patients with regional pancreatic carcinoma. An outcomes trial. Cancer. 2000;89:314–327. doi: 10.1002/1097-0142(20000715)89:2<314::aid-cncr16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Wanebo HJ, Glicksman AS, Vezeridis MP, et al. Preoperative chemotherapy, radiotherapy, and surgical resection of locally advanced pancreatic cancer. Arch Surg. 2000;135:81–87. doi: 10.1001/archsurg.135.1.81. discussion 88. [DOI] [PubMed] [Google Scholar]
- 15.White RR, Hurwitz HI, Morse MA, et al. Neoadjuvant chemoradiation for localized adenocarcinoma of the pancreas. Ann Surg Oncol. 2001;8:758–765. doi: 10.1007/s10434-001-0758-1. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman JP, Lipsitz S, Pisansky T, et al. Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: An Eastern Cooperative Oncology Group study. J Clin Oncol. 1998;16:317–323. doi: 10.1200/JCO.1998.16.1.317. [DOI] [PubMed] [Google Scholar]
- 17.Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997;15:928–937. doi: 10.1200/JCO.1997.15.3.928. [DOI] [PubMed] [Google Scholar]
- 18.Staley CA, Lee JE, Cleary KR, et al. Preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for adenocarcinoma of the pancreatic head. Am J Surg. 1996;171:118–124. doi: 10.1016/S0002-9610(99)80085-3. discussion 124–125. [DOI] [PubMed] [Google Scholar]
- 19.Coia L, Hoffman J, Scher R, et al. Preoperative chemoradiation for adenocarcinoma of the pancreas and duodenum. Int J Radiat Oncol Biol Phys. 1994;30:161–167. doi: 10.1016/0360-3016(94)90531-2. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa O, Ohigashi H, Imaoka S, et al. Is the long-term survival rate improved by preoperative irradiation prior to Whipple's procedure for adenocarcinoma of the pancreatic head? Arch Surg. 1994;129:1075–1080. doi: 10.1001/archsurg.1994.01420340089017. [DOI] [PubMed] [Google Scholar]
- 21.Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–1339. doi: 10.1001/archsurg.1992.01420110083017. [DOI] [PubMed] [Google Scholar]
- 22.Springett GM, Hoffe SE. Borderline resectable pancreatic cancer: On the edge of survival. Cancer Control. 2008;15:295–307. doi: 10.1177/107327480801500404. [DOI] [PubMed] [Google Scholar]
- 23.NCCN practice guidelines for pancreatic cancer. Oncology (Williston Park) 1997;11:41–55. [PubMed] [Google Scholar]
- 24.Lu DS, Reber HA, Krasny RM, et al. Local staging of pancreatic cancer: Criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol. 1997;168:1439–1443. doi: 10.2214/ajr.168.6.9168704. [DOI] [PubMed] [Google Scholar]
- 25.Schima W, Ba-Ssalamah A, Kölblinger C, et al. Pancreatic adenocarcinoma. Eur Radiol. 2007;17:638–649. doi: 10.1007/s00330-006-0435-7. [DOI] [PubMed] [Google Scholar]
- 26.Pilepich MV, Miller HH. Preoperative irradiation in carcinoma of the pancreas. Cancer. 1980;46:1945–1949. doi: 10.1002/1097-0142(19801101)46:9<1945::aid-cncr2820460908>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Roldan GE, Gunderson LL, Nagorney DM, et al. External beam versus intraoperative and external beam irradiation for locally advanced pancreatic cancer. Cancer. 1988;61:1110–1116. doi: 10.1002/1097-0142(19880315)61:6<1110::aid-cncr2820610610>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Raben A, Mychalczak B, Brennan MF, et al. Feasibility study of the treatment of primary unresectable carcinoma of the pancreas with 103Pd brachytherapy. Int J Radiat Oncol Biol Phys. 1996;35:351–356. doi: 10.1016/0360-3016(95)02136-1. [DOI] [PubMed] [Google Scholar]
- 29.Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: A randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil. The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman JP, Weese JL, Solin LJ, et al. A pilot study of preoperative chemoradiation for patients with localized adenocarcinoma of the pancreas. Am J Surg. 1995;169:71–77. doi: 10.1016/s0002-9610(99)80112-3. discussion 77–78. [DOI] [PubMed] [Google Scholar]
- 31.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreatic cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence TS, Chang EY, Hahn TM, et al. Radiosensitization of pancreatic cancer cells by 2`2`-difluoro-2`-deoxycytidine. Int J Radiat Oncol Biol Phys. 1996;34:867–872. doi: 10.1016/0360-3016(95)02134-5. [DOI] [PubMed] [Google Scholar]
- 33.Shewach DS, Lawrence TS. Gemcitabine and radiosensitization in human tumor cells. Invest New Drugs. 1996;14:257–263. doi: 10.1007/BF00194528. [DOI] [PubMed] [Google Scholar]
- 34.McGinn CJ, Zalupski MM. Radiation therapy with once-weekly gemcitabine in pancreatic cancer: Current status of clinical trials. Int J Radiat Oncol Biol Phys. 2003;56(4 suppl):10–15. doi: 10.1016/s0360-3016(03)00449-8. [DOI] [PubMed] [Google Scholar]
- 35.Crane CH, Abbruzzese JL, Evans DB, et al. Is the therapeutic index better with gemcitabine-based chemoradiation than with 5-fluorouracil-based chemoradiation in locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys. 2002;52:1293–1302. doi: 10.1016/s0360-3016(01)02740-7. [DOI] [PubMed] [Google Scholar]
- 36.McGinn CJ, Zalupski MM, Shureiqi I, et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2001;19:4202–4208. doi: 10.1200/JCO.2001.19.22.4202. [DOI] [PubMed] [Google Scholar]
- 37.Safran H, Dipetrillo T, Iannitti D, et al. Gemcitabine, paclitaxel, and radiation for locally advanced pancreatic cancer: A phase I trial. Int J Radiat Oncol Biol Phys. 2002;54:137–141. doi: 10.1016/s0360-3016(02)02902-4. [DOI] [PubMed] [Google Scholar]
- 38.Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47–55. doi: 10.1002/cncr.22735. [DOI] [PubMed] [Google Scholar]
- 39.Fogelman DR, Schreibman S, Sherman W, et al. Neoadjuvant GTX and radiation for unresectable pancreatic cancer: A prospective phase II trial [abstract 143]. Presented at the 4th Annual Gastrointestinal Cancers Symposium; January 19–21, 2007; Orlando, Florida. [Google Scholar]
- 40.Tepper J, Nardi G, Sutt H. Carcinoma of the pancreas: Review of MGH experience from 1963 to 1973. Analysis of surgical failure and implications for radiation therapy. Cancer. 1973;37:1519–1524. doi: 10.1002/1097-0142(197603)37:3<1519::aid-cncr2820370340>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 41.Whittington R, Bryer MP, Haller DG, et al. Adjuvant therapy of resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1991;21:1137–1143. doi: 10.1016/0360-3016(91)90268-9. [DOI] [PubMed] [Google Scholar]
- 42.Kalser MH, Ellenberg SS. Pancreatic cancer: Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 43.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of the cancer of the pancreas and periampullary region: A phase III trial of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Ann Surg. 1999;230:776–782. doi: 10.1097/00000658-199912000-00006. discussion 782–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 45.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 46.Regine WF, Winter KA, Abrams R, et al. RTOG 97-04: A phase III study of adjuvant pre- and post-chemoradiation 5-FU versus gemcitabine for resected pancreatic adenocarcinoma. J Clin Oncol. 2006;24(18 suppl):4007. [Google Scholar]
- 47.Mornex F, Andre T, Louvet C, et al. Postoperative adjuvant gemcitabine plus oxaliplatin (GemOx) chemotherapy followed by chemoradiation in patients with pancreatic carcinoma: A multicenter phase II study. J Clin Oncol. 2007;25(18 suppl):4520. [Google Scholar]
- 48.Wang ML, Foo KF, et al. Adjuvant chemoradiotherapy for high-risk pancreatic cancer. Singapore Med J. 2009;50:43–48. [PubMed] [Google Scholar]
- 49.Gastrointestinal Tumor Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006–2010. doi: 10.1002/1097-0142(19870615)59:12<2006::aid-cncr2820591206>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 50.Garofalo MC, Regine WF, Tan MT. On statistical reanalysis, the EORTC trial is a positive trial for adjuvant chemoradiation in pancreatic cancer. Ann Surg. 2006;244:332–333. doi: 10.1097/01.sla.0000229980.81505.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stocken DD, Büchler MW, Dervenis C, et al. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer. 2005;92:1372–1381. doi: 10.1038/sj.bjc.6602513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butturini G, Stocken DD, Wente MN, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: Meta-analysis of randomized controlled trials. Arch Surg. 2008;143:75–83. doi: 10.1001/archsurg.2007.17. [DOI] [PubMed] [Google Scholar]
- 53.Neoptolemos J, Büchler M, Stocken DD, et al. A multicenter, international, open-label, randomised, controlled phase III trial of adjuvant 5- fluorouracil/folinic acid (5-FU/FA) versus gemcitabine in patients with resected pancreatic ductal adenocarcinoma. J Clin Oncol. 2009;27(18 suppl):LBA4505. [Google Scholar]
- 54.Childs DS, Jr, Moertel CG, Holbrook MA, et al. Treatment of malignant neoplasms of the gastrointestinal tract with a combination of 5-fluorouracil and radiation: A randomized double-blind study. Radiology. 1965;84:843–848. doi: 10.1148/84.5.843. [DOI] [PubMed] [Google Scholar]
- 55.Moertel CG, Childs DS, Jr, Reitemeier RJ, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet. 1969;2:865–867. doi: 10.1016/s0140-6736(69)92326-5. [DOI] [PubMed] [Google Scholar]
- 56.Hazel JJ, Thirlwell MP, Huggins M, et al. Multi-drug chemotherapy with and without radiation for carcinoma of the stomach and pancreas: A prospective randomized trial. J Can Assoc Radiol. 1981;32:164–165. [PubMed] [Google Scholar]
- 57.Klaassen DJ, MacIntyre JM, Catton GE, et al. Treatment of locally unresectable cancer of the stomach and pancreas: A randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil—an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3:373–378. doi: 10.1200/JCO.1985.3.3.373. [DOI] [PubMed] [Google Scholar]
- 58.Gastrointestinal Tumor Study Group. Radiation therapy combined with Adriamycin or 5-fluorouracil for the treatment of locally unresectable pancreatic carcinoma. Cancer. 1985;56:2563–2568. doi: 10.1002/1097-0142(19851201)56:11<2563::aid-cncr2820561104>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 59.Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: Comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst. 1988;80:751–755. [PubMed] [Google Scholar]
- 60.Earle JD, Foley JF, Wieand HS, et al. Evaluation of external-beam radiation therapy plus 5-fluorouracil (5-FU) versus external-beam radiation therapy plus hycanthone (HYC) in confined, unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 1994;28:207–211. doi: 10.1016/0360-3016(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 61.Mehta VK, Poen JC, Ford JM, et al. Protracted venous infusion 5-fluorouracil with concomitant radiotherapy compared with bolus 5-fluorouracil for unresectable pancreatic cancer. Am J Clin Oncol. 2001;24:155–159. doi: 10.1097/00000421-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 62.Shinchi H, Takao S, Noma H, et al. Length and quality of survival after external beam radiotherapy with concurrent continuous 5-fluorouracil infusion for locally unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2002;53:146–150. doi: 10.1016/s0360-3016(01)02806-1. [DOI] [PubMed] [Google Scholar]
- 63.Li CP, Chao Y, Chi KH, et al. Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: Gemcitabine versus 5-fluorouracil, a randomised controlled study. Int J Radiat Oncol Biol Phys. 2003;57:98–104. doi: 10.1016/s0360-3016(03)00435-8. [DOI] [PubMed] [Google Scholar]
- 64.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 65.Loehrer P, Powell ME, Cardenes HR, et al. A randomised phase III study of gemcitabine in combination with radiation therapy versus gemcitabine alone in patients with localised, unresectable pancreatic cancer: E4201. J Clin Oncol. 2008;26(15 Suppl):4506. [Google Scholar]
- 66.Tepper JE, Noyes D, Krall JM, et al. Intraoperative radiation therapy of pancreatic carcinoma: A report of RTOG-8505. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1991;21:1145–1149. doi: 10.1016/0360-3016(91)90269-a. [DOI] [PubMed] [Google Scholar]
- 67.Garton GR, Gunderson LL, Nagorney DM, et al. High-dose preoperative external beam and intraoperative irradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 1993;27:1153–1157. doi: 10.1016/0360-3016(93)90537-6. [DOI] [PubMed] [Google Scholar]
- 68.Mohiuddin M, Regine WF, Stevens J, et al. Combined intraoperative radiation and perioperative chemotherapy for unresectable cancers of the pancreas. J Clin Oncol. 1995;13:2764–2768. doi: 10.1200/JCO.1995.13.11.2764. [DOI] [PubMed] [Google Scholar]
- 69.Nishimura Y, Hosotani R, Shibamoto Y, et al. External and intraoperative radiotherapy for resectable and unresectable pancreatic cancer: Analysis of survival rates and complications. Int J Radiat Oncol Biol Phys. 1997;39:39–49. doi: 10.1016/s0360-3016(97)00295-2. [DOI] [PubMed] [Google Scholar]
- 70.Gunderson LL, Willett CG, Harrison LB, et al. Intraoperative radiation: Current and future status. Semin Oncol. 1997;24:715–731. [PubMed] [Google Scholar]
- 71.DeNittis AS, Stambaugh MD, Lang P, et al. Complete remission of nonresectable pancreatic cancer after infusional colloidal phosphorus-32 brachytherapy, external beam radiation therapy and 5-fluorouracil: A preliminary report. Am J Clin Oncol. 1999;22:355–360. doi: 10.1097/00000421-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 72.Hoyer M, Roed H, Sengelov L, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76:48–53. doi: 10.1016/j.radonc.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 73.Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 74.Koong AC, Christofferson E, Le QT, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320–323. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 75.Milano MT, Chmura SJ, Garofalo MC, et al. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: Toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2004;59:445–453. doi: 10.1016/j.ijrobp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 76.Ben-Josef E, Shields AF, Vaishampayan U, et al. Intensity-modulated radiotherapy (IMRT) and concurrent capecitabine for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;59:454–459. doi: 10.1016/j.ijrobp.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 77.Brade A, Brierley J, Oza A, et al. Concurrent gemcitabine and radiotherapy with and without neoadjuvant gemcitabine for locally advanced unresectable or resected pancreatic cancer: A phase I-II study. Int J Radiat Oncol Biol Phys. 2007;67:1027–1036. doi: 10.1016/j.ijrobp.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 78.Mukherjee S, Hudson E, Reza S, et al. Pancreatic cancer within a UK cancer network with special emphasis on locally advanced non-metastatic pancreatic cancer. Clin Oncol (R Coll Radiol) 2008;20:535–540. doi: 10.1016/j.clon.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 79.Huguet F, André T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]