The study examines three series of de novo stage II–III breast cancer patients treated front line with anthracycline-based regimens of various cyclophosphamide dose intensities. The results strongly suggest that cyclophosphamide dose intensification in estrogen receptor–negative p53-mutated breast cancer patients could significantly improve their response.
Learning Objectives
After completing this course, the reader will be able to:
Analyze the role of p53 mutation in ER-negative tumors in conferring increased sensitivity to high-dose alkylating agents, in order to treat patients with this phenotype using regimens containing high-dose alkylating agents.
Evaluate the role played by dysfunctional p53 in conferring chemosensitivity to anthracyclines, and explore the possibility of using high-dose alkylating agents to overcome the resistance of ER+/p53 mutated tumors.
Examine the mechanism for determining p53 gene function (functional analysis of separated alleles in yeast as opposed to immunohistochemistry) to more precisely determine the role of p53 activation in specific tumors, in order to select appropriate patients for treatment with high-dose alkylating agents.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
The predictive value of p53 for the efficacy of front-line anthracycline-based chemotherapy regimens has been a matter of significant controversy. Anthracyclines are usually combined with widely different doses of alkylating agents, which may significantly modulate tumor response to these combinations. We analyzed three series of de novo stage II–III breast cancer patients treated front line with anthracycline-based regimens of various cyclophosphamide dose intensities: 65 patients with estrogen receptor (ER)− tumors treated with anthracyclines alone (Institut Jules Bordet, Brussels), 51 unselected breast cancer patients treated with intermediate doses of cyclophosphamide (MD Anderson Cancer Center, Houston, TX), and 128 others treated with a dose-dense anthracycline–cyclophosphamide combination (St. Louis, Paris). After chemotherapy and surgery, pathologic complete response (pCR) was evaluated. p53 status was determined by a yeast functional assay on the pretreatment tumor sample. In a multivariate analysis of the pooled results, a lack of ER expression and high-dose cyclophosphamide administration were associated with a higher likelihood of pCR. A sharp statistical interaction was detected between p53 status and cyclophosphamide dose intensity. Indeed, when restricting our analysis to patients with ER− tumors, we confirmed that a mutant p53 status was associated with anthracycline resistance, but found that p53 inactivation was required for response to the dose-intense alkylating regimen. The latter allowed very high levels of pCR in triple-negative tumors. Thus, our data strongly suggest that cyclophosphamide dose intensification in ER− p53-mutated breast cancer patients could significantly improve their response.
Introduction
There is a need to identify drug-specific predictive biomarkers in order to better tailor chemotherapy regimens to individual patients with breast cancer. Several general biomarkers exist, including low estrogen receptor (ER) expression, high proliferative index, or high Oncotype DX recurrence score, that could indicate general chemotherapy sensitivity in early-stage breast cancer [1]. However, there are no established drug-specific biomarkers, although several have been proposed, such as topoisomerase IIa amplification, which may predict the efficacy of anthracyclines [2], or low microtubule-associated protein τ (MAP tau) expression, which could predict paclitaxel efficacy [3].
In cell and animal models, p53 is the critical factor for the outcome of genotoxic stress, such as the one triggered by many cancer therapies [4–8]. Yet the role of p53 mutations in predicting response to anthracylines or other chemotherapy drugs remains controversial. The frequency of p53 mutations is around 30% in breast cancer patients in general [9]. The frequency of p53 mutations is highly variable across breast cancer molecular subclasses. It is rare (0%–5%) in low-grade ER+ breast cancer and can reach 95% in basal-like breast cancer [10]. Lack of p53 function precludes p53-triggered apoptosis or cell-cycle arrest [11, 12]. Some mutations can also exert dominant negative effects on p63 and p73, two related proteins with a key role in apoptosis and differentiation [13, 14]. Recent data have also implicated p53 in mammary stem cell fate determination. Collectively, p53-mutated tumors may not only have an altered response to cellular stress but also have an intrinsically distinct biology [15].
The relationship between p53 mutations and chemotherapy efficacy has been extensively investigated, but no clear consensus has emerged. Some studies found that epirubicin has greater efficacy in patients with wild-type p53 tumors [16–18], whereas other have observed that p53 mutations are associated with much better efficacy of anthracycline-containing chemotherapy regimens [19, 20]. Technologies to assess p53 mutations vary widely among studies and may not detect mutations with the same biological meaning. The distribution of molecular classes of breast cancer is not the same among studies and the predictive value of p53 mutations could differ according to molecular class [20, 21]. The endpoint selected for evaluation of response also differs across studies—clinical response, pathological response, overall survival, or disease-free survival. Most importantly, widely different chemotherapy regimens have been administrated. In particular, widely different doses of alkylating agents have been combined with anthracyclines [16, 19, 20, 22].
Here, we analyzed the predictive value of p53 status for the efficacy of preoperative anthracycline-containing chemotherapy in three different series of breast cancer patients treated with increasing doses of cyclophosphamide. In patients treated with anthracyclines alone, we confirmed that a mutant p53 status predicted resistance. Conversely, p53 inactivation was absolutely required for complete response to a dose-intense alkylating regimen. Thus, in the context of anthracycline therapy, p53 status is a critical biomarker for response to dose-intense cyclophosphamide.
Patients and Methods
Patients and Chemotherapy Regimens
Prospectively collected prechemotherapy specimens from patients with stage II–III breast cancer accrued to three different biomarker studies were included in the present analysis. In one study, conducted in the Saint Louis Hospital in Paris (SIM-HSL), 128 patients were treated with dose-dense epirubicin (75 mg/m2) and cyclophosphamide (1,200 mg/m2) (ddEC) administered every 15 days for six cycles. The efficacy and toxicity of this regimen were reported previously [23]. The second study cohort included 51 patients from the MD Anderson Cancer Center (MDACC) in Houston, Texas treated with 5-fluorouracil (500 mg/m2), doxorubicin (50 mg/m2), and cyclophosphamide (500 mg/m2) (FAC) administered every 21 days for four cycles. The third series included 65 patients treated with single-agent epirubicin (E) (100 mg/m2) given every 21 or 15 days for four or six cycles, respectively, in a clinical trial called TOP (Topoisomerase II alpha gene amplification and protein overexpression predicting efficacy of epirubicin) at the Institut Jules Bordet in Brussels. In contrast to the two other studies, that trial included only ER− breast cancer patients.
Definition of Pathological Variables
ER status was determined by immunohistochemistry (IHC) (cutoff, >10% tumor cells stained positive). Human epidermal growth factor receptor (HER)-2 status was determined by IHC and/or fluorescence in situ hybridization (FISH) according to local procedures. HER-2 overexpression was defined as complete and intense membrane IHC staining in >10% of cells, or 30% of cells for patients treated in SIM-HSL, or an ErbB-2 to centromere 17 ratio >2 when FISH was performed. Grade was defined according to the Scarff–Bloom–Richardson criteria in the SIM-HSL series and TOP trial, and was defined as nuclear grade in the MDACC series. Pathologic complete remission (pCR) was defined as no invasive tumor cells in the primary tumor and axillary lymph nodes after chemotherapy.
p53 Determination
p53 gene functional status was determined by the functional analysis of separated alleles in yeast (FASAY) method [24], which evaluates the transactivation activity of p53 on a p53-responsive promoter stably integrated in the yeast genome. RNA was extracted from pretreatment tumor biopsies and reverse transcribed, and p53 transcripts were amplified by polymerase chain reaction and transfected into yeast. Yeast colonies transformed with wild-type or mutated p53 sequences appear as white and large or red and small, respectively. p53 status was considered mutated when: (a) >10% of the yeast colonies were red, (b) analysis using the split versions of the test could identify the defect in the 5′ or 3′ part of the gene, and (c) sequence analysis from mutant yeast colonies could identify an unambiguous genetic defect.
Statistical Analyses
Associations between molecular and clinical variables and pCR were tested in a univariate analysis using a χ2 or Fisher's exact test and in a multivariate logistic regression. All variables were included in a backward logistic regression model. Odds ratios (ORs), 95% confidence intervals (CIs), and p-values were estimated. Separate analyses were performed for all patients and for patients according to p53 and ER status. Patients with missing values for a particular marker were excluded from the corresponding analysis. A two-sided p < .05 was considered statistically significant. All statistical analyses were done using SPSS 12.0 software (SPSS, Inc., Chicago, IL).
Results
Patient Characteristics
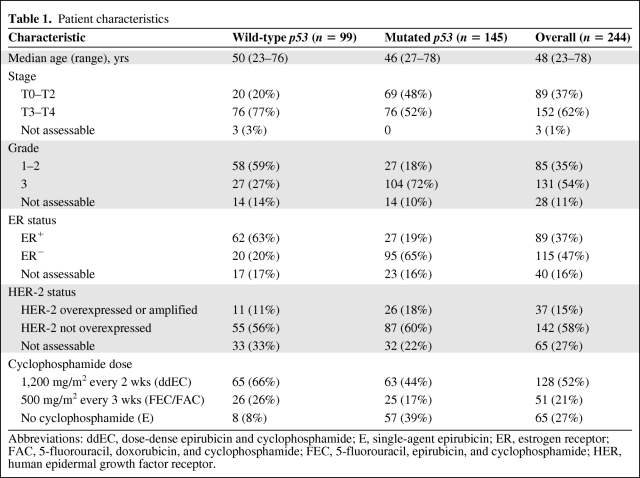
The different clinicopathologic variables (ER, HER-2) and p53 gene functional status (FASAY method) are presented in Table 1. Compared with a series of sporadic breast cancer cases, the present study was enriched in ER− (56%, n = 115 of 204) and high-grade (61%, n = 131 of 216) breast cancers, at least in part because the TOP trial only included ER− tumors. As expected, a much higher incidence of ER− tumors was observed in p53-mutated tumors (78%, n = 95 of 122) than in wild-type p53 tumors (24%, n = 20 of 82; p < .001). As in previous studies, p53-mutated tumors were more likely to present as high grade than wild-type p53 tumors (79%, n = 104 of 131, versus 32%, n = 27 of 85; p < .001).
Table 1.
Patient characteristics
Abbreviations: ddEC, dose-dense epirubicin and cyclophosphamide; E, single-agent epirubicin; ER, estrogen receptor; FAC, 5-fluorouracil, doxorubicin, and cyclophosphamide; FEC, 5-fluorouracil, epirubicin, and cyclophosphamide; HER, human epidermal growth factor receptor.
Predictive Parameters for Chemotherapy Efficacy
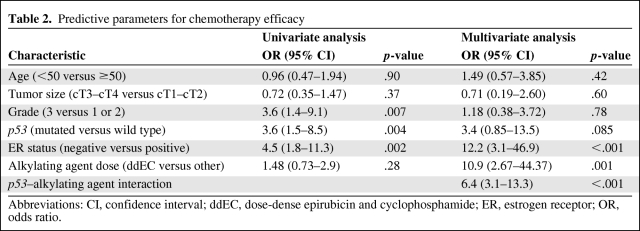
Chemotherapy response was directly assessed by pCR. In a univariate analysis pooling of all three trials (Table 2), high tumor grade (OR, 3.6; 95% CI, 1.4–9.1) and lack of ER expression (OR, 4.5; 95% CI, 1.8–11.3) were associated with a greater likelihood of pCR, in keeping with most previous studies [25]. p53 inactivation (OR, 3.6; 95% CI, 1.5–8.5) was also associated with a higher likelihood of pCR.
Table 2.
Predictive parameters for chemotherapy efficacy
Abbreviations: CI, confidence interval; ddEC, dose-dense epirubicin and cyclophosphamide; ER, estrogen receptor; OR, odds ratio.
In contrast, on multivariate analysis (Table 2), although a lack of ER expression (OR, 12.2; 95% CI, 3.1–46.9; p < .001) remained highly significant (with a higher OR), grade and p53 status were no longer associated with response. Importantly, use of the dose-intense ddEC protocol was associated with a higher likelihood of pCR (OR, 10.9; 95% CI, 2.67–44.37; p = .001). These dramatic differences reflect the tight interconnection among several of the variables under study, including grade and p53 status, as well as cyclophosphamide dose intensity and ER status.
Predictive Value of p53 Inactivation for Efficacy of Increasing Doses of Cyclophosphamide
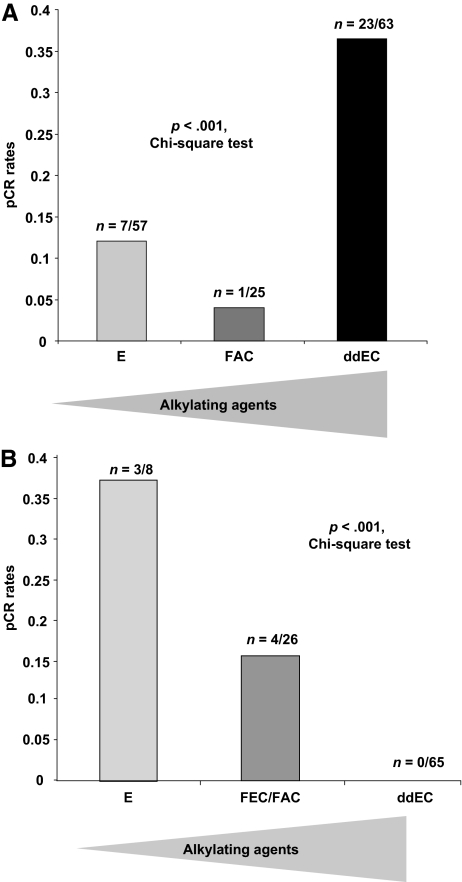
The predictive value of p53 inactivation for chemotherapy response may be intimately linked to the type of regimen. In that respect, a logistic regression model identified a very significant interaction between p53 status and efficacy of the high-dose alkylating agent (OR, 6.4; 95% CI, 3.1–13.3; interaction test p < .001) (Table 2). Indeed, the ORs for pCR and p53 status were highly heterogeneous across the three trials, because the test for heterogeneity yielded a value of 14.39 (p = .0001), implying that the predictive value of p53 for pCR is tightly linked to the type of regimen. As shown in Figure 1A, the pCR rates were 36% (n = 23 of 63), 4% (n = 1 of 25), and 12% (n = 7 of 57) in patients with p53-mutated tumors treated with high-dose (ddEC), standard-dose (FAC), or no (E) cyclophosphamide. Conversely, in patients with wild-type p53 tumors (Fig. 1B), the pCR rates were 0% (n = 0 of 65), 15% (n = 4 of 26), and 37% (n = 3 of 8), respectively. Thus, dose intensification is associated with a much higher rate of complete response in patients with p53-mutated tumors.
Figure 1.
Pathologic complete response (pCR) rate according to alkylating agent dose. (A): Patients with p53-mutated tumors. (B): Patients with wild-type p53 tumors.
Abbreviations: ddEC, dose-dense epirubicin and cyclophosphamide; E, single-agent epirubicin; FAC, 5-fluorouracil, doxorubicin, and cyclophosphamide.
Predictive Value of p53 in ER− Disease
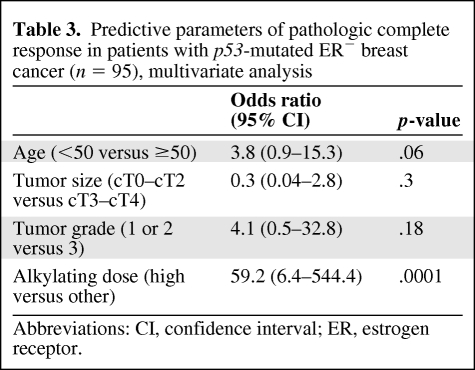
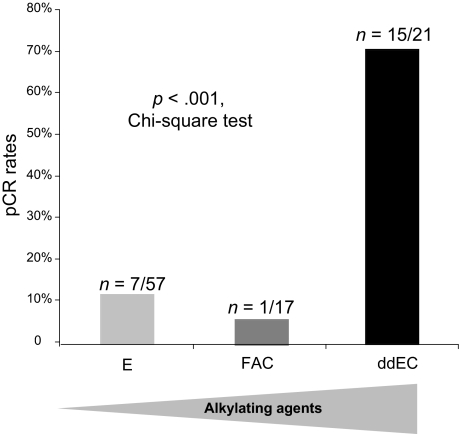
Because one of the trials only recruited patients with ER− tumors, an imbalance in ER expression according to p53 status might have biased our results. We thus compared the efficacies of the three regimens in a more homogeneous subgroup of patients with ER− p53-mutated tumors. The latter is highly enriched in basal breast cancers [10], although it likely also contains some molecular apocrine ones [26, 27]. In patients with ER− p53-mutated tumors, dose-intense cyclophosphamide was associated with a strikingly greater likelihood of a pCR (OR, 59.2; 95% CI, 6.4–544.4; p < .0001) (multivariate analysis in Table 3). Indeed, in patients with ER− p53-mutated tumors, the pCR rates were 71% (n = 15 of 21), 6% (n = 1 of 17), and 12% (n = 7 of 57) (p < .0001, χ2 test) as the cyclophosphamide dose intensity was decreased (Fig. 2). Among the 21 ER− p53-mutated tumors treated by ddEC, 11 could be identified as typically triple negative and 9 of them reached pCR (81.2%). Eight overexpressed HER-2, among which 4 reached pCR. Data were unavailable for the 2 remaining patients, who both responded. The small number of ER+ tumors and the low rate of pCR in this subset (n = 6 of 89 patients) precluded the reverse analysis. Although not randomized, these findings strongly suggest that dose-intense cyclophosphamide/anthracycline therapy is associated with greater efficacy in patients with ER− p53-mutated tumors, notably those with triple-negative tumors.
Table 3.
Predictive parameters of pathologic complete response in patients with p53-mutated ER− breast cancer (n = 95), multivariate analysis
Abbreviations: CI, confidence interval; ER, estrogen receptor.
Figure 2.
Efficacy of high-dose alkylating agents in patients with estrogen receptor–negative p53-mutated tumors.
Abbreviations: ddEC, dose-dense epirubicin and cyclophosphamide; E, single-agent epirubicin; FAC, 5-fluorouracil, doxorubicin, and cyclophosphamide; pCR, pathologic complete response.
Discussion
Use of high-dose alkylating agents in daily practice is a matter of very significant controversy. Two large, randomized trials in the adjuvant setting did not find a significant difference in outcome when increasing the dose of cyclophosphamide [28, 29]. Similarly, a randomized trial that evaluated high-dose alkylating agents with bone marrow transplantation did not report a significant effect on overall survival [30]. However, analysis of those trials was performed considering breast cancer as a single disease, which may have diluted out effects in specific subpopulations. Some previous molecular correlative studies have attempted to define which group could derive benefit from high-dose alkylating agents. In the National Surgical Adjuvant Breast and Bowel Project (NSABP) B25 trial, a subgroup of women aged <50 years or with ER-poor breast cancer (ER, 10–49 fmol/mg) seemed to derive a significant benefit from a higher dose of an alkylating agent [28]. Similarly, in a Dutch trial [31], p53 detection by IHC was associated with a significantly higher efficacy for high-dose cyclophosphamide, carboplatin, and thiotepa combination chemotherapy. Those two studies are fully in line with our findings. Although our study did not have complete annotations (HER-2, PR, and ER) available for all patients and lacked a central review for determination of pCR, it clearly identifies p53-mutant ER− tumors as those most sensitive to high-dose alkylating agents.
The technology used for p53 typing is critical, and the yeast functional assay used here is robust, assesses function (thus excluding passenger mutations), and is more sensitive than direct sequencing [10]. p53 inactivation is very unevenly distributed across the different molecular subclasses of breast cancer, ranging from none (luminal A) to virtually 100% (basal). This could suggest that p53 inactivation contributes to the basic biology of those tumors, as well as to their treatment response. p53 status critically regulates response to DNA-damaging agents, by promoting either cell-cycle arrest or apoptosis, with the opposite effect on tumor outcome [32, 33]. Our findings confirm the observations that dysfunctional p53 is predictive of chemoresistance to anthracyclines alone [34] and raise the tantalizing prospect that high-dose alkylating agents can actually reverse the intrinsic anthracycline resistance of ER− p53-mutated tumors, a proposal with considerable potential clinical importance.
Many p53-mutated and ER− breast cancers fall into the basal-like molecular subtype [10, 20], although some belong to the molecular apocrine one [26, 27] (data not shown). Future studies should determine the sensitivity of each molecular subclass to this combination. In addition to a defective p53 pathway, basal tumors may also have an impaired BRCA1 repair pathway [35], which may contribute to the high sensitivity of these tumors to alkylating agents. Several clinical trials showed that basal-like breast cancers are very sensitive to combination chemotherapy [36, 37]. Yet the rate of pCR achieved here with the dose-intense regimen in ER− p53-mutated tumors (70%) appears notably higher than in those previous trials. The rate of pCR even reached 80% in triple-negative tumors. Accordingly, in St. Louis Hospital, ER− p53-mutated tumors treated by neoadjuvant therapy are prescribed the ddEC regimen. In patients treated with the ddEC regimen, pCR was the only predictor of 10-year survival [38], suggesting that many of the complete remissions observed here will translate into actual cures. Our results identify p53 as a critical biomarker of high-dose cyclophosphamide sensitivity, in the context of anthracycline combinations. The magnitude of the observed effect and the size of the population under study make it most unlikely that the superior response of ER− p53-mutated tumors may be explained by patient heterogeneity. Thus, although this analysis carries several intrinsic limitations, our results warrant prospective randomized trials to directly test the hypothesis that dose-intense alkylating agents actually reverse anthracycline resistance in ER− p53-mutated breast cancers.
Acknowledgments
We thank all the technicians at the Biochemistry Department, Saint Louis Hospital (Evelyne Wittmer, Catherine Brunin, Dominique Chapelin, Martine Legrand, Claire Bocquet) for p53 status determination.
F.A. is supported by an ASCO career development award. L.P. is supported by the Breast Cancer Research Foundation. C.S. is supported by the Fonds National de la Recherche Scientifique. The TOP trial has received support from the Fondation Luxembourgeoise Contre le Cancer, the Brussels region, and the Fonds National de la Recherche Scientifique. Hd.T. is supported by Ligue Contre le Cancer, Région Ile de France, Programme Hospitalier de Recherche Clinique (PHRC), and INCa.
Author Contributions
Conception/design: Jacqueline Lehmann-Che, Fabrice André, Hugues de Thé
Provision of study materials or patients: Jacqueline Lehmann-Che, Fabrice André, Sylvie Giacchetti, Christine Desmedt, Marc Espié, Michel Marty, Philippe Bertheau, Christos Sotiriou, Martine Piccart, W. Fraser Symmans, Lajos Pusztai, Hugues de Thé
Collection/assembly of data: Jacqueline Lehmann-Che, Fabrice André, Christine Desmedt, Elisabeth Turpin, Louis-François Plassa, Philippe Bertheau, W. Fraser Symmans, Lajos Pusztai, Hugues de Thé
Data analysis and interpretation: Jacqueline Lehmann-Che, Fabrice André, Chafika Mazouni, Hugues de Thé
Manuscript writing: Jacqueline Lehmann-Che, Fabrice André, Chafika Mazouni, Lajos Pusztai, Hugues de Thé
Final approval of manuscript: Jacqueline Lehmann-Che, Fabrice André, Christine Desmedt, Chafika Mazouni, Sylvie Giacchetti, Elisabeth Turpin, Marc Espié, Louis-François Plassa, Michel Marty, Philippe Bertheau, Christos Sotiriou, Martine Piccart, W. Fraser Symmans, Lajos Pusztai, Hugues de Thé
References
- 1.Andre F, Pusztai L. Molecular classification of breast cancer: Implications for selection of adjuvant chemotherapy. Nat Clin Pract Oncol. 2006;3:621–632. doi: 10.1038/ncponc0636. [DOI] [PubMed] [Google Scholar]
- 2.Pritchard KI, Messersmith H, Elavathil L, et al. HER-2 and topoisomerase II as predictors of response to chemotherapy. J Clin Oncol. 2008;26:736–744. doi: 10.1200/JCO.2007.15.4716. [DOI] [PubMed] [Google Scholar]
- 3.Rouzier R, Rajan R, Wagner P, et al. Microtubule-associated protein tau: A marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci U S A. 2005;102:8315–8320. doi: 10.1073/pnas.0408974102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogelstein B, Kinzler KW. Achilles' heel of cancer? Nature. 2001;412:865–866. doi: 10.1038/35091170. [DOI] [PubMed] [Google Scholar]
- 5.Soussi T, Béroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001;1:233–240. doi: 10.1038/35106009. [DOI] [PubMed] [Google Scholar]
- 6.Hawkins DS, Demers GW, Galloway DA. Inactivation of p53 enhances sensitivity to multiple chemotherapeutic agents. Cancer Res. 1996;56:892–898. [PubMed] [Google Scholar]
- 7.Bunz F, Hwang PM, Torrance C, et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berns A. Cancer biology: Can less be more for p53? Nature. 2006;443:153–154. doi: 10.1038/443153a. [DOI] [PubMed] [Google Scholar]
- 9.Pharoah PD, Day NE, Caldas C. Somatic mutations in the p53 gene and prognosis in breast cancer: A meta-analysis. Br J Cancer. 1999;80:1968–1973. doi: 10.1038/sj.bjc.6690628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manié E, Vincent-Salomon A, Lehmann-Che J, et al. High frequency of TP53 mutation in BRCA1 and sporadic basal-like carcinomas but not in BRCA1 luminal breast tumors. Cancer Res. 2009;69:663–671. doi: 10.1158/0008-5472.CAN-08-1560. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Holstege H, van der Gulden H, et al. Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc Natl Acad Sci U S A. 2007;104:12111–12116. doi: 10.1073/pnas.0702969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petitjean A, Achatz MI, Borresen-Dale AL, et al. TP53 mutations in human cancers: Functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157–2165. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- 13.Yang A, McKeon F. P63 and P73: P53 mimics, menaces and more. Nat Rev Mol Cell Biol. 2000;1:199–207. doi: 10.1038/35043127. [DOI] [PubMed] [Google Scholar]
- 14.Irwin MS. Family feud in chemosensitivity: p73 and mutant p53. Cell Cycle. 2004;3:319–323. [PubMed] [Google Scholar]
- 15.Zheng H, Ying H, Yan H, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aas T, Børresen AL, Geisler S, et al. Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nat Med. 1996;2:811–814. doi: 10.1038/nm0796-811. [DOI] [PubMed] [Google Scholar]
- 17.Geisler S, Lonning PE, Aas T, et al. Influence of TP53 gene alterations and c-erbB-2 expression on the response to treatment with doxorubicin in locally advanced breast cancer. Cancer Res. 2001;61:2505–2512. [PubMed] [Google Scholar]
- 18.Di Leo A, Tanner M, Desmedt C, et al. p-53 gene mutations as a predictive marker in a population of advanced breast cancer patients randomly treated with doxorubicin or docetaxel in the context of a phase III clinical trial. Ann Oncol. 2007;18:997–1003. doi: 10.1093/annonc/mdm075. [DOI] [PubMed] [Google Scholar]
- 19.Bertheau P, Plassa F, Espié M, et al. Effect of mutated TP53 on response of advanced breast cancers to high-dose chemotherapy. Lancet. 2002;360:852–854. doi: 10.1016/S0140-6736(02)09969-5. [DOI] [PubMed] [Google Scholar]
- 20.Bertheau P, Turpin E, Rickman DS, et al. Exquisite sensitivity of TP53 mutant and basal breast cancers to a dose-dense epirubicin-cyclophosphamide regimen. PLoS Med. 2007;4:e90. doi: 10.1371/journal.pmed.0040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bidard FC, Matthieu MC, Chollet P, et al. p53 status and efficacy of primary anthracyclines/alkylating agent-based regimen according to breast cancer molecular classes. Ann Oncol. 2008;19:1261–1265. doi: 10.1093/annonc/mdn039. [DOI] [PubMed] [Google Scholar]
- 22.Bonnefoi H, Diebold-Berger S, Therasse P, et al. Locally advanced/inflammatory breast cancers treated with intensive epirubicin-based neoadjuvant chemotherapy: Are there molecular markers in the primary tumour that predict for 5-year clinical outcome? Ann Oncol. 2003;14:406–413. doi: 10.1093/annonc/mdg108. [DOI] [PubMed] [Google Scholar]
- 23.Cottu PH, Zelek L, Extra JM, et al. High-dose epirubicin and cyclophosphamide every two weeks as first-line chemotherapy for relapsing metastatic breast cancer patients. Ann Oncol. 1999;10:795–801. doi: 10.1023/a:1008353904351. [DOI] [PubMed] [Google Scholar]
- 24.Flaman JM, Frebourg T, Moreau V, et al. A simple p53 functional assay for screening cell lines, blood, and tumors. Proc Natl Acad Sci U S A. 1995;92:3963–3967. doi: 10.1073/pnas.92.9.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guarneri V, Broglio K, Kau SW, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24:1037–1044. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 26.Doane AS, Danso M, Lal P, et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25:3994–4008. doi: 10.1038/sj.onc.1209415. [DOI] [PubMed] [Google Scholar]
- 27.Farmer P, Bonnefoi H, Becette V, et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24:4660–4671. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 28.Fisher B, Anderson S, DeCillis A, et al. Further evaluation of intensified and increased total dose of cyclophosphamide for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-25. J Clin Oncol. 1999;17:3374–3388. doi: 10.1200/JCO.1999.17.11.3374. [DOI] [PubMed] [Google Scholar]
- 29.Fisher B, Anderson S, Wickerham DL, et al. Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-22. J Clin Oncol. 1997;15:1858–1869. doi: 10.1200/JCO.1997.15.5.1858. [DOI] [PubMed] [Google Scholar]
- 30.Tallman MS, Gray R, Robert NJ, et al. Conventional adjuvant chemotherapy with or without high-dose chemotherapy and autologous stem-cell transplantation in high-risk breast cancer. N Engl J Med. 2003;349:17–26. doi: 10.1056/NEJMoa030684. [DOI] [PubMed] [Google Scholar]
- 31.Rodenhuis S, Bontenbal M, Beex LV, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for high-risk breast cancer. N Engl J Med. 2003;349:7–16. doi: 10.1056/NEJMoa022794. [DOI] [PubMed] [Google Scholar]
- 32.Lowe SW. Cancer therapy and p53. Curr Opin Oncol. 1995;7:547–553. doi: 10.1097/00001622-199511000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Wallace-Brodeur RR, Lowe SW. Clinical implications of p53 mutations. Cell Mol Life Sci. 1999;55:64–75. doi: 10.1007/s000180050270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahko E, Blanco G, Soini Y, et al. A mutant TP53 gene status is associated with a poor prognosis and anthracycline-resistance in breast cancer patients. Eur J Cancer. 2003;39:447–453. doi: 10.1016/s0959-8049(02)00499-9. [DOI] [PubMed] [Google Scholar]
- 35.Scully R, Livingston DM. In search of the tumour-suppressor functions of BRCA1 and BRCA2. Nature. 2000;408:429–432. doi: 10.1038/35044000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carey LA, Rugo HS, Marcom PK, et al. EGFR inhibition with cetuximab added to carboplatin in metastatic triple negative (basal-like) breast cancer. J Clin Oncol. 2008;26(15 suppl):1009. [Google Scholar]
- 37.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 38.Bertheau P, Lerebours F, Mounier N, et al. Prognostic significance of a combined clinicopathologic score for response to primary systemic therapy in locally advanced breast cancer. Oncol Rep. 2005;14:513–520. [PubMed] [Google Scholar]