The article examines the utility of ultrasound in evaluating thyroid nodules, staging thyroid cancer, determining the extent of surgery needed in thyroid cancer patients, and the surveillance of patients treated for thyroid cancer.
Keywords: Ultrasound, Thyroid cancer, Lymph node metastasis, Ultrasound-guided fine-needle aspiration, Ultrasound staging, Recurrent thyroid cancer
Abstract
The use of ultrasound for thyroid cancer has evolved dramatically over the last few decades. Since the late 1960s, ultrasound has become essential in the examination of the thyroid gland with the increased availability of high-frequency linear array transducers and computer-enhanced imaging capabilities of modern day portable ultrasound equipment in a clinic- or office-based setting. As a noninvasive, rapid, and easily reproducible imaging study, ultrasound has been demonstrated to have a broadened utility beyond the simple confirmation of thyroid nodules and their sizes. Recently, office-based ultrasound has become an integral part of clinical practice, where it has demonstrated overwhelming benefits to patients being evaluated and treated for thyroid cancer. Ultrasound has become useful in the qualitative characterization of thyroid nodules based on benign or malignant features. On the basis of such classifications and the relative risk for thyroid malignancy, the need for ultrasound-guided fine-needle aspiration, preoperative and intraoperative staging, lymph node mapping, and the extent of surgery can subsequently be determined. Furthermore, ultrasound has additional value in the surveillance of patients treated for thyroid cancer.
Introduction
The use of ultrasound in the clinical evaluation of patients with thyroid lesions has expanded from the detection of nonpalpable thyroid nodules to include the delineation of benign and malignant thyroid lesions, the examination of lymph node basins for staging purposes and treatment planning, fine-needle aspiration (FNA) guidance for thyroid nodules or suspicious-appearing lymph nodes, intraoperative localization of thyroid lesions and/or lymph nodes, and postoperative surveillance for recurrent thyroid cancer (Table 1) [1–7]. Clinic- or office-based ultrasound can be an invaluable extension of the clinical examination as this modality can image thyroid lesions and provide additional information in real time with greater clarity that otherwise might have been missed by history and physical examination or by incomplete referral ultrasound [1, 5–7]. The superiority of thyroid ultrasound over physical examination allows for the evaluation and characterization of thyroid nodules, the entire thyroid gland, and cervical lymph nodes, which may change management in >60% of patients who have been referred for a solitary thyroid nodule [4]. From such imaging results, ultrasound-guided FNA of suspicious thyroid nodules can be performed with a low sampling error rate. Ultrasound and FNA can provide an important service to patients and referring physicians who may not have the equipment and means to perform FNA biopsies, or in those who have undergone previous inconclusive biopsies. Ultrasound may also identify coexisting thyroid and lymph node disease that may influence the extent of the intended thyroid operation [1, 5, 6]. Recently, ultrasound was used to preoperatively stage patients with well-differentiated thyroid cancer and to determine the extent of surgical resection, including formal neck dissection, in certain patients [5, 8]. Intraoperative ultrasound has been used to determine the optimal neck incision location and to localize difficult and nonpalpable lesions intraoperatively for surgical removal. Ultrasound can also be used to monitor for recurrent disease in those patients who have undergone surgical resection for thyroid cancer.
Table 1.
Indications for the use of ultrasound in the management of thyroid cancer
Ultrasound of Thyroid Nodules
Thyroid nodules are clinically common and important because they often require further evaluation to exclude the underlying possibility of thyroid cancer that can occur in 5%–15% of individuals depending on several factors, including age, gender, a family history of thyroid cancer, and a history of radiation exposure, among others [9]. Differentiated thyroid cancer, namely, papillary and follicular cancer, comprises the overwhelming majority (90%) of thyroid cancers. In the U.S., its overall increased incidence has been attributed solely to a 2.9-fold increase in papillary thyroid cancer from 1988 to 2002 [10]. This overall trend may be explained, in part, by the increased widespread use of neck ultrasound and earlier management of thyroid nodules.
Thyroid ultrasound is used to evaluate index nodule size, location, characteristics, and the number and presence of additional thyroid nodules (e.g., contralateral lobe), and for the detection of suspicious-appearing lymph nodes. Nodule size has been shown to not be predictive of malignancy. Patients with multiple thyroid nodules also have the same risk for malignancy as those with solitary nodules, and it is recommended that all patients with nodular thyroid glands undergo ultrasound evaluation [2, 4]. Although no single ultrasound feature carries a high sensitivity and positive predictive value for thyroid cancer, there is evidence to suggest that a number of ultrasound characteristics, when occurring together, are associated with a higher risk for malignancy [11, 12]. Recent evidence also suggests that preoperative ultrasound may alter the planned surgical procedure in cases where newly discovered multifocal thyroid cancer with regional metastasis may alert the surgeon to perform a total thyroidectomy with extended neck dissection [1, 5].
Characteristics of Thyroid Nodules
A variety of ultrasound characteristics are thought to differentiate benign from malignant thyroid nodules [2, 3]. These ultrasound features include echogenicity, internal echo pattern, margins, shape/dimension ratio, calcifications, acoustic phenomenon, compressibility, and vascularity (Table 2). Whereas the well-defined and smooth margins of a thyroid nodule may signify benignity, irregular or ill-defined lesions suggest malignancy. Although benign nodules are more likely to be entirely cystic in nature, many malignant lesions are solid and appear hypoechoic relative to adjacent thyroid tissue. Furthermore, thyroid nodules with a spongiform appearance comprised of >50% multiple microcystic components are most likely benign [13, 14]. A thyroid nodule defined as being greater in its anteroposterior than its transverse dimension (i.e., taller greater than wider) has been described as more likely to be malignant than benign [3]. Thyroid nodules with microcalcifications are more strongly associated with malignancy than those with coarse or no calcifications (Fig. 1). Central vascularity is more likely to be characteristic of malignant thyroid nodules, whereas peripheral vascularity is associated with benign lesions [2, 3]. Apart from the index nodule, the presence of suspicious-appearing lymph nodes is also associated with thyroid malignancy. More recently, ultrasound compressibility or elastography was shown to distinguish between benign and malignant nodules, whereby significantly higher stiffness or less compressibility of the thyroid lesion was correlated highly with malignancy [15]. This ultrasonographic technique, however, requires additional validation with prospective studies.
Table 2.
Ultrasound features associated with benign and malignant thyroid nodules
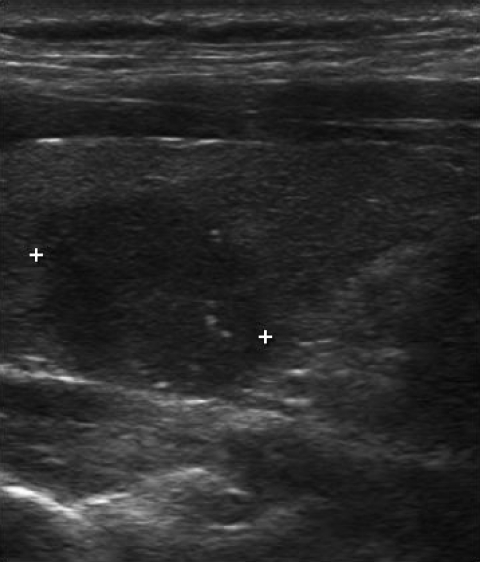
Figure 1.
Malignant thyroid nodule. Longitudinal view of the right thyroid lobe. A combination of hypoechogenicity, irregular borders, and microcalcifications in the same solitary thyroid nodule has a strong correlation with thyroid cancer.
The difficulty in distinguishing between benign and malignant thyroid nodules is, in part, a result of the overlap in ultrasound characteristics with no single feature having the highest accuracy in delineating either diagnosis. Recent evidence, however, suggests that the combination of hypoechogenicity, irregular borders, and microcalcifications in the same solitary thyroid nodule has the strongest correlation with differentiated thyroid cancer with a 30-fold higher risk for malignancy [12]. Furthermore, in patients with indeterminate FNA results, when two or more ultrasound features are present, the likelihood of malignancy increases to >55% [11]. In patients with a thyroid nodule suspicious for papillary thyroid cancer on ultrasound-guided FNA and with ultrasound features associated with malignancy, the risk for thyroid cancer is very high (95%), necessitating thyroidectomy [16]. Such recent findings suggest that a preoperative ultrasound provides additional information that can help surgeons determine the extent of operation needed in patients with suspicious thyroid lesions.
Ultrasound-Guided FNA
Since ultrasound alone cannot reliably predict the benign or malignant nature of thyroid nodules, these lesions should be further evaluated by FNA. Nevertheless, ultrasound allows for more selective FNA of thyroid nodules based on certain sonographic features and combination of features highly associated with malignancy [2–4, 13, 14]. Whereas FNA has traditionally been routinely performed on palpable lesions, ultrasound-guided FNA can be performed more selectively in those patients with nonpalpable or subcentimeter thyroid nodules that have suspicious sonographic features, a previous history of thyroid cancer, a history of radiation exposure, and a family history of thyroid cancer. Ultrasound-guided FNA decreases the percentage of inadequate specimens while maintaining or increasing the sensitivity and specificity [17, 18]. The complementary use of ultrasound and FNA decreases the rate of false-negative results from needle misplacement and reduces the rate of nondiagnostic results. Ultrasound-guided FNA in the clinic setting can be performed with a 91%–93% diagnostic accuracy rate, comparable with or better than those rates reported by radiologists and experienced cytopathologists [19, 20].
Ultrasound in the Staging of Thyroid Cancer
Ultrasound is superior to computed tomography scanning in evaluating the presence of abnormal cervical lymph nodes. Cervical lymph nodes can be involved in 20%–50% of patients with differentiated thyroid cancer; more specifically, in those individuals with papillary thyroid cancer [21, 22]. Preoperative ultrasound can identify suspicious cervical lymphadenopathy in 20%–31% of cases of thyroid cancer, thereby potentially altering the extent of and overall surgical approach in these patients [5–7]. This imaging modality allows for the early detection of nonpalpable cervical lymph node metastasis prior to thyroidectomy in patients with FNA-proven or suspected thyroid cancer that otherwise might have been missed intraoperatively, thereby minimizing the risk for persistent disease [5–7]. Typical ultrasound features of metastatic lymph nodes include hypoechogenicity, rounded appearance, irregular borders, macro- or microcalcifications, loss of the fatty hilus, and increased size (Fig. 2).
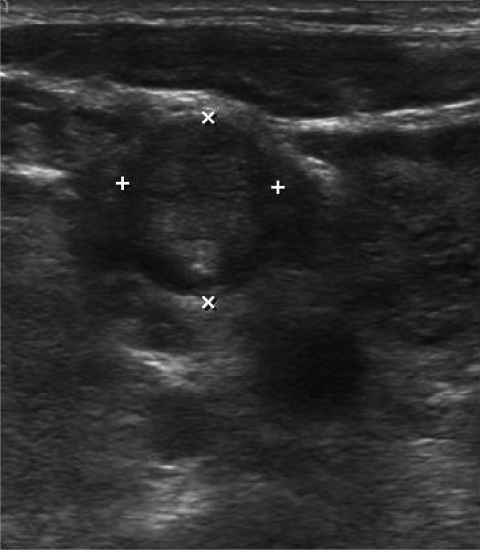
Figure 2.
Ultrasound image of a level 3 lymph node containing metastatic cancer. Transverse view of level 3 lymph node. Abnormal lymph nodes appear as hypoechoic and round lesions with calcifications and the absence of a fatty hilus.
More recently, ultrasound was shown to be useful in the preoperative staging of papillary thyroid cancer in relation to the current tumor–node–metastasis (TNM) classification system [8]. The overall accuracies of ultrasound for T and N staging were 67% and 71.3%, respectively. Ultrasound was also useful for detecting metastatic lateral cervical lymph nodes and evaluating multifocal papillary thyroid cancer [8]. The location of these suspicious lymph nodes may also be useful in surgical decision making. Malignant lymph nodes are more likely to occur in levels III, IV, and VI than in level II [23] (Fig. 3). Furthermore, the presence of >25% contact between papillary thyroid microcarcinoma and the adjacent capsule on preoperative ultrasound can predict the presence of extrathyroidal extension, thus helping the surgeon plan the extent of surgical resection [24]. Finally, ultrasound can be used to guide FNA biopsy of suspicious cervical lymph nodes, whereby measured thyroglobulin levels on cytology specimens can increase the accuracy of the diagnosis. This FNA measurement of thyroglobulin level is valid regardless of the presence of circulating thyroglobulin antibodies [25].
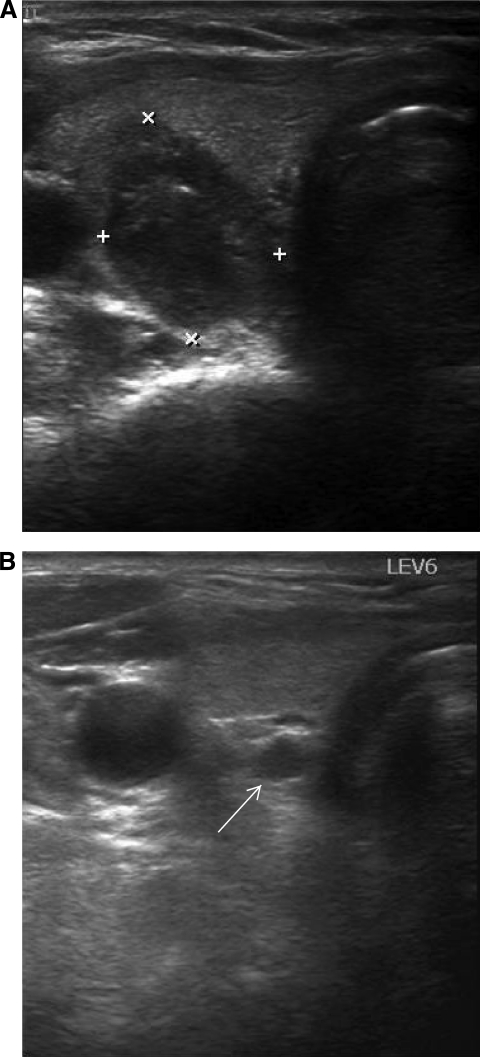
Figure 3.
Ultrasound images of a right malignant nodule and level 6 (central) lymph node. Transverse view of right posteriorly located malignant thyroid nodule (A) and enlarged and hypoechoic level 6 lymph node in the same patient ((B), white arrow).
Intraoperative Thyroid Ultrasound
Intraoperative ultrasound is a useful adjunct in the surgical exploration and resection of thyroid cancer in patients with nonpalpable thyroid lesions, cervical lymph node metastasis, and recurrent disease [6, 26]. Furthermore, ultrasound localization of nonpalpable lesions intraoperatively may also reduce operating room times. Intraoperative ultrasound has been shown to influence the extent of resection by identifying and confirming removal of recurrent thyroid cancer and nonpalpable lymph nodes in 31% of patients [6]. Recently, preincision ultrasound-guided injection of blue dye into abnormal lymph nodes was shown to have additional utility in the safe and efficient removal of lymph node recurrences in the reoperative neck [27]. In these patients, ultrasound is performed in the operating room after the patient is positioned. Abnormal lymph nodes are then identified for blue dye injection prior to incision. This blue dye localization facilitates the identification and removal of abnormal lymph nodes, even in an extensively scarred reoperative neck [27].
Ultrasound for Cancer Surveillance
Currently, ultrasound is the most frequently used imaging study for monitoring and evaluating patients treated for thyroid cancer postoperatively. Ultrasound surveillance can detect recurrent disease in the thyroid bed after total thyroidectomy or lobectomy, in a remaining contralateral lobe, or in lateral neck lymph nodes. In low-risk patients who have undergone total thyroidectomy without subsequent radioiodine therapy or thyroid lobectomy alone, ultrasound may serve as the main surveillance modality postoperatively because this imaging study is highly sensitive in detecting cervical lymph node metastasis [28]. Ultrasound can be performed without discontinuing thyroid hormone therapy, thus avoiding hypothyroidism, and without administration of recombinant thyroid-stimulating hormone, both of which are required for radioiodine imaging. Therefore, low-risk patients with negative thyroglobulin measurements treated for papillary thyroid cancer may only require periodic ultrasound, thus eliminating the need for traditional radioactive iodine imaging. Following surgery and/or radioiodine therapy, ultrasound evaluation of the thyroid bed and central and lateral cervical nodal basins can be performed at 6 and 12 months, and then periodically thereafter, depending on the patient's risk for recurrent disease [5, 28]. Further studies are needed to address the cost-effectiveness of ultrasound in the post-treatment surveillance of certain thyroid cancer patients.
Postoperative ultrasound is also useful in guiding surgical planning for reoperations for recurrent thyroid cancer. Ultrasound is able to detect recurrent thyroid cancer when thyroglobulin levels are elevated but radioiodine or positron emission tomography scans fail to detect it. Localization and mapping of such disease assists in surgical planning, which enables tailored compartmental neck dissection according to the node basins involved [7, 29]. The surgical management of nonpalpable cervical recurrent disease may prove difficult because of extensive scar formation and fibrosis resulting from previous surgery [7, 29]. Ultrasound-guided tattooing of recurrent thyroid cancer in the neck using charcoal has been shown to be a safe and effective method in the localization and removal of nonpalpable recurrent disease in patients after previous neck procedures [30].
Conclusion
Ultrasound has become an indispensible diagnostic modality in the evaluation of thyroid cancer. This imaging study is useful in the diagnosis, staging, and post-treatment surveillance of initial and recurrent thyroid cancer. Future developments in ultrasound usage may lead to further improvements in its diagnostic accuracy and expanded use.
Author Contributions
Conception/Design: John I. Lew
Provision of study material or patients: John I. Lew, Carmen C. Solorzano
Collection and/or assembly of data: John I. Lew, Carmen C. Solorzano
Data analysis and interpretation: John I. Lew, Carmen C. Solorzano
Manuscript writing: John I. Lew
Final approval of manuscript: John I. Lew, Carmen C. Solorzano
References
- 1.Milas M, Stephen A, Berber E, et al. Ultrasonography for the endocrine surgeon: A valuable clinical tool that enhances diagnostic and therapeutic outcomes. Surgery. 2005;138:1193–1201. doi: 10.1016/j.surg.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 2.Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: Predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 3.Kim EK, Park CS, Chung WY, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 4.Marqusee E, Benson CB, Frates MC, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med. 2000;133:696–700. doi: 10.7326/0003-4819-133-9-200011070-00011. [DOI] [PubMed] [Google Scholar]
- 5.Kouvaraki MA, Shapiro SE, Fornage BD, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003;134:946–955. doi: 10.1016/s0039-6060(03)00424-0. [DOI] [PubMed] [Google Scholar]
- 6.Solorzano CC, Carneiro DM, Ramirez M, et al. Surgeon-performed ultrasound in the management of thyroid malignancy. Am Surg. 2004;70:576–580. discussion 580–582. [PubMed] [Google Scholar]
- 7.Stulak JM, Grant CS, Farley DR, et al. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg. 2006;141:489–496. doi: 10.1001/archsurg.141.5.489. [DOI] [PubMed] [Google Scholar]
- 8.Park JS, Son KR, Na DG, et al. Performance of preoperative sonographic staging of papillary thyroid carcinoma based on the sixth edition of the AJCC/UICC TNM classification system. AJR Am J Roentgenol. 2009;192:66–72. doi: 10.2214/AJR.07.3731. [DOI] [PubMed] [Google Scholar]
- 9.Hegedus L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351:1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 10.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 11.Méndez W, Rodgers SE, Lew JI, et al. Role of surgeon-performed ultrasound in predicting malignancy in patients with indeterminate thyroid nodules. Ann Surg Oncol. 2008;15:2487–2492. doi: 10.1245/s10434-008-0052-6. [DOI] [PubMed] [Google Scholar]
- 12.Jabiev AA, Ikeda MH, Reis IM, et al. Surgeon-performed ultrasound can predict differentiated thyroid cancer in patients with solitary thyroid nodules. Ann Surg Oncol. 2009;16:3140–3145. doi: 10.1245/s10434-009-0652-9. [DOI] [PubMed] [Google Scholar]
- 13.Moon WJ, Jung SL, Lee JH, et al. Thyroid Study Group, Korean Society of Neuro- and Head and Neck Radiology. Benign and malignant thyroid nodules: US differentiation—multicenter retrospective study. Radiology. 2008;247:762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- 14.Bonavita JA, Mayo J, Babb J, et al. Pattern recognition of benign nodules at ultrasound of the thyroid: Which nodules can be left alone? AJR Am J Roentgenol. 2009;193:207–213. doi: 10.2214/AJR.08.1820. [DOI] [PubMed] [Google Scholar]
- 15.Asteria C, Giovanardi A, Pizzocaro A, et al. US-elastography in the differential diagnosis of benign and malignant thyroid nodules. Thyroid. 2008;18:523–531. doi: 10.1089/thy.2007.0323. [DOI] [PubMed] [Google Scholar]
- 16.Moon HJ, Kwak JY, Kim EK, et al. The combined role of ultrasound and frozen section in surgical management of thyroid nodules read as suspicious for papillary thyroid carcinoma on fine needle aspiration biopsy: A retrospective study. World J Surg. 2009;33:950–957. doi: 10.1007/s00268-009-9984-7. [DOI] [PubMed] [Google Scholar]
- 17.Danese D, Sciacchitano S, Farsetti A, et al. Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid. 1998;8:15–21. doi: 10.1089/thy.1998.8.15. [DOI] [PubMed] [Google Scholar]
- 18.Carmeci C, Jeffrey RB, McDougall IR, et al. Ultrasound-guided fine-needle aspiration biopsy of thyroid masses. Thyroid. 1998;8:283–289. doi: 10.1089/thy.1998.8.283. [DOI] [PubMed] [Google Scholar]
- 19.Seiberling KA, Dutra JC, Gunn J, et al. Ultrasound-guided fine needle aspiration biopsy of thyroid nodules performed in the office. Laryngoscope. 2008;118:228–231. doi: 10.1097/MLG.0b013e318157465d. [DOI] [PubMed] [Google Scholar]
- 20.Bhatki AM, Brewer B, Robinson-Smith T, et al. Adequacy of surgeon-performed ultrasound-guided thyroid fine-needle aspiration biopsy. Otolaryngol Head Neck Surg. 2008;139:27–31. doi: 10.1016/j.otohns.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Scheumann GF, Gimm O, Wegener G, et al. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg. 1994;18:559–568. doi: 10.1007/BF00353765. [DOI] [PubMed] [Google Scholar]
- 22.Chow SM, Law SC, Chan JK, et al. Papillary microcarcinoma of the thyroid—prognostic significance of lymph node metastasis and multifocality. Cancer. 2003;98:31–40. doi: 10.1002/cncr.11442. [DOI] [PubMed] [Google Scholar]
- 23.Leboulleux S, Girard E, Rose M, et al. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab. 2007;92:3590–3594. doi: 10.1210/jc.2007-0444. [DOI] [PubMed] [Google Scholar]
- 24.Kwak JY, Kim EK, Youk JH, et al. Extrathyroidal extension of well-differentiated papillary thyroid microcarcinoma on US. Thyroid. 2008;18:609–614. doi: 10.1089/thy.2007.0345. [DOI] [PubMed] [Google Scholar]
- 25.Boi F, Baghino G, Atzeni F, et al. The diagnostic value for differentiated thyroid carcinoma metastases of thyroglobulin (Tg) measurement in washout fluid from fine-needle aspiration biopsy of neck lymph nodes is maintained in the presence of circulating anti-Tg antibodies. J Clin Endocrinol Metab. 2006;91:1364–1369. doi: 10.1210/jc.2005-1705. [DOI] [PubMed] [Google Scholar]
- 26.Karwowski JK, Jeffrey RB, McDougall IR, et al. Intraoperative ultrasonography improves identification of recurrent thyroid cancer. Surgery. 2002;132:924–929. doi: 10.1067/msy.2002.128478. [DOI] [PubMed] [Google Scholar]
- 27.Sippel RS, Elaraj DM, Poder L, et al. Localization of recurrent thyroid cancer using intraoperative ultrasound-guided dye injection. World J Surg. 2009;33:434–439. doi: 10.1007/s00268-008-9797-0. [DOI] [PubMed] [Google Scholar]
- 28.Torlontano M, Crocetti U, Augello G, et al. Comparative evaluation of recombinant human thyrotropin-stimulated thyroglobulin levels, 131I whole-body scintigraphy, and neck ultrasonography in the follow-up of patients with papillary thyroid microcarcinoma who have not undergone radioiodine therapy. J Clin Endocrinol Metab. 2006;91:60–63. doi: 10.1210/jc.2005-1185. [DOI] [PubMed] [Google Scholar]
- 29.Lee L, Steward DL. Sonographically-directed neck dissection for recurrent thyroid carcinoma. Laryngoscope. 2008;118:991–994. doi: 10.1097/MLG.0b013e31816b873a. [DOI] [PubMed] [Google Scholar]
- 30.Kang TW, Shin JH, Han BK, et al. Preoperative ultrasound-guided tattooing localization of recurrences after thyroidectomy: Safety and effectiveness. Ann Surg Oncol. 2009;16:1655–1659. doi: 10.1245/s10434-009-0431-7. [DOI] [PubMed] [Google Scholar]