The present study describes the results of a systematic review conducted to objectively assess the clinical effectiveness and toxicity of isolated limb perfusion for the treatment of patients with locally advanced melanoma of the limbs. The technique was found to be safe and efficacious in these patients.
Keywords: Malignant melanoma, Chemotherapy, Isolated limb perfusion, Melphalan, Tumor necrosis factor
Learning Objectives
After completing this course, the reader will be able to:
Compare the response rate of ILP with melphalan and TNF to the response rate of ILP with single-agent melphalan in patients with unresectable locally advanced melanoma of the limbs.
Compare the clinical response rates of repeated ILP after a recurrence or PR to a first ILP to clinical response rates after first ILP in patients with unresectable locally advanced melanoma of the limbs.
In patients with unresectable malignant melanoma of the limbs, consider use of ILP to avoid amputation.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
Isolated limb perfusion (ILP) involves the administration of chemotherapy drugs directly into a limb involved by locoregional metastases. Unresectable locally advanced melanoma of the limbs represents one of the clinical settings in which ILP has demonstrated benefits.
Methods.
A systematic review of the literature on ILP for patients with unresectable locally advanced melanoma of the limbs was conducted. MEDLINE, EMBASE, and Cochrane database searches were conducted to identify studies fulfilling the following inclusion criteria: hyper- or normothermic ILP with melphalan with or without tumor necrosis factor (TNF) or other drugs providing valid data on clinical response, survival, or toxicity. To allocate levels of evidence and grades of recommendation the Scottish Intercollegiate Guidelines Network system was used.
Results.
Twenty-two studies including 2,018 ILPs were selected with a clear predominance of observational studies (90.90%) against experimental studies (9.10%). The median complete response rate to ILP was of 58.20%, with a median overall response rate of 90.35%. ILP with melphalan yielded a median complete response rate of 46.50%, against a 68.90% median complete response rate for melphalan plus TNF ILP. The median 5-year overall-survival rate was 36.50%, with a median overall survival interval of 36.70 months. The Wieberdink IV and V regional toxicity rates were 2.00% and 0.65%, respectively.
Conclusions.
ILP is effective in achieving clinical responses in patients with unresectable locally advanced melanoma of the limbs. The disease-free and overall survival rates provided by ILP are acceptable. ILP is safe, with a low incidence of severe regional and systemic toxicity.
Background
Isolated limb perfusion (ILP) was designed by Creech and Krementz in 1956 to achieve high concentrations of a chemotherapy drug in a limb affected by an unresectable tumor, especially soft tissue sarcoma and melanoma, and to minimize the toxicity related to systemic chemotherapy [1, 2]. With these aims, the circulation of the involved limb is isolated from the systemic circulation and connected to an extracorporeal system. Once high temperatures are reached, the chemotherapy drugs, mainly melphalan and tumor necrosis factor (TNF), are then administered to the patient through the perfusion circuit [3, 4].
After >50 years of experience with ILP, many studies published by a limited number of oncology research centers in the U.S. and Europe have yielded results generally favorable to ILP. However, the evidence available is based mainly on studies that are methodologically heterogeneous and with no appropriate control populations, which hampers determination of the real benefits gained by ILP for particular subsets of melanoma patients.
The present study describes the results of a systematic review conducted to objectively assess the clinical effectiveness and toxicity of ILP for the treatment of patients with locally advanced melanoma of the limbs.
Methods
A systematic review of the literature available on ILP for malignant melanoma (MM) patients was conducted to answer the following research questions: Is ILP effective for the treatment of unresectable locally advanced melanoma of the limbs? Is ILP a safe technique for the treatment of unresectable locally advanced MM of the limbs?
MEDLINE and EMBASE searches were performed following a pre-established keyword list (intraarterial chemotherapy, intraarterial perfusion, isolated limb perfusion, cutaneous melanoma, MM, in-transit metastases, satellitosis, loco-regional metastases, melphalan, interferon-alpha, doxorubicin, cisplatin, tumor necrosis factor-alpha, normothermia, normothermic ILP, hyperthermia, hyperthermic ILP, mild hyperthermia, borderline hyperthermia, true hyperthermia, complete response, partial response, global response, survival, overall survival, disease-free survival, toxicity, regional toxicity, systemic toxicity) defined by consensus among the clinical participants (D.M.R., L.C.M., L.F.) related to the efficacy, clinical effectiveness, and toxicity of ILP in patients with locally advanced melanoma of the limbs. The Cochrane database as well as the reference lists of previous systematic reviews were also searched.
Inclusion and Exclusion Criteria
Eligible studies had to fulfill the following inclusion criteria: (a) studies published in 1990–2008, (b) studies enrolling subjects having unresectable MM of the limbs (stage IIIB and stage IIIC of the American Joint Committee on Cancer [5]) treated with any regimen of ILP regardless of the temperature level (hyperthermia, normothermia) or the chemotherapy drug administered (melphalan, melphalan and TNF, others), (c) studies analyzing efficacy or effectiveness endpoints (clinical response, survival, recurrence rate, limb salvage rate), (d) studies analyzing safety endpoints in terms of regional toxicity and/or systemic toxicity, and (e) studies with eligible study designs: randomized clinical trials (RCT), cohort studies, case–control studies, and case series. Systematic reviews were included for reference list revision.
Studies in which the perfusion methodology (chemotherapeutic drug, temperature regimen, etc.) was not clearly described, studies not reporting valid results on clinical effectiveness or toxicity, letters to the editor, nonsystematic reviews, studies applying obsolete clinical guidelines (i.e., elective lymphadenectomy, etc.), and original studies in languages other than English were excluded from this systematic review.
In order to rule out studies with low methodological quality, the following aspects were also required: detailed description of the ILP regimen applied, clinical setting, follow-up periods, clinical endpoints analyzed, and number of ILPs analyzed.
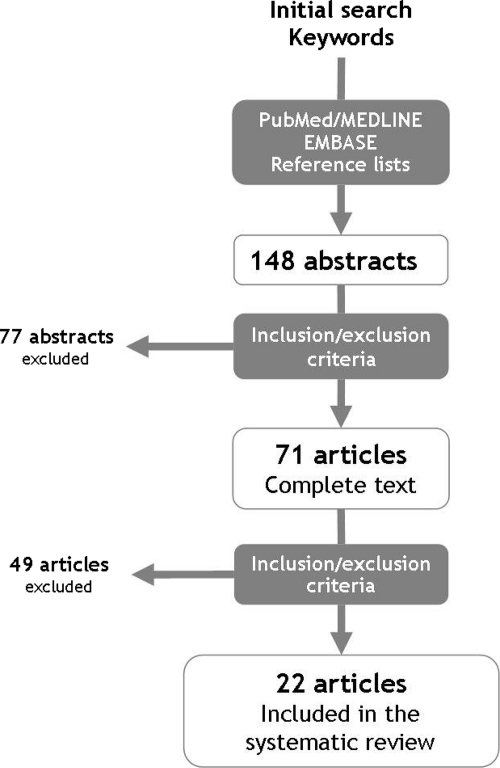
Following the inclusion and exclusion criteria described above, three independent investigators (D.M.R., L.C.M., L.F.) reviewed the abstracts initially retrieved without masking to select articles included in the systematic review after a three-step procedure (Fig. 1). In cases of disagreement among investigators about the inclusion or exclusion of studies, consensus was reached by discussion. After the first step, 148 abstracts were initially identified from MEDLINE and EMBASE (Fig. 1). No studies from the Cochrane Database fulfilling the inclusion criteria were found. Screening of the references cited in retrieved articles and textbooks identified no other eligible studies. After reading their titles and abstracts, 71 full-text articles were assessed further, from which 22 studies were finally included in the systematic review.
Figure 1.
Procedure for the selection of studies included in the systematic review.
Outcome Measures
The Response Evaluation Criteria in Solid Tumors (RECIST) and World Health Organization (WHO) criteria for evaluating tumoral response to nonsurgical treatments were applied to extract data on the objective clinical response to ILP [6, 7]. Thus, the percentages of patients achieving a complete response (CR), partial response (PR), and overall response (OR) were the effectiveness endpoints analyzed. Studies not providing direct information on these measures were also included if they could be calculated from the data available. In that respect, OR was calculated as the sum of CR and PR.
Survival after ILP was also a primary endpoint analyzed; thus, data on disease-free survival (DFS) and overall survival (OS) in terms of the 5-year DFS percentage and interval and 5-year OS percentage and interval were extracted. Other secondary endpoints extracted from the studies analyzed were the recurrence rate and the limb salvage rate.
For the regional toxicity evaluation, studies describing results according to the Wieberdink classification system for regional toxicity were included in the review [8] (Table 1). For the systemic toxicity analysis, the Common Terminology Criteria for Adverse Events, version 3.0 (December 2003) and the WHO classification of chemotherapy toxicity were accepted [9, 10].
Table 1.
Studies of ILP for unresectable locally advanced melanoma of the limbs included in the systematic review
Abbreviations: CC, case–control study; Cis, cisplatin; CS, case series; Dac, dacarbazine; ILP, isolated limb perfusion; Mel, melphalan; PC, prospective cohorts; QE, quasiexperimental; RC, retrospective cohorts; RCT, randomized clinical trial; TNF, tumoral necrosis factor-α.
The Scottish Intercollegiate Guidelines Network system criteria were applied for the assignment of levels of evidence and strength of recommendations [11] (Table 2).
Table 2.
Scottish Intercollegiate Guidelines Network grading system for evidence levels and strength of recommendations
Descriptive statistics to obtain median and average results in those homogeneous subsets from which synthetic data could be obtained were carried out using SPSS 15.0® software (SPSS, Inc., Chicago, IL).
This systematic review was conducted on behalf of the Agencia de Evaluación de Tecnologías Sanitarias de Andalucía (AETSA) with the funding of the Spanish Health Ministry in the framework of the National Program of Health Technologies Evaluation (AETSA 2007/10).
Results
Twenty-two (n = 22) studies on ILP for MM analyzing 2,018 ILPs were included in this systematic review, with a predominance of observational studies (90.90%, n = 20) and with two randomized clinical trials (9.10%) in which different ILP regimens were compared (Table 1). The average age of patients treated with ILP in the studies reviewed was 60.79 years (95% confidence interval [CI], 58.72–62.87 years) [12–33].
Clinical Response
Valid data on the effectiveness of ILP in terms of clinical response were yielded by 20 studies analyzing 1,587 ILPs, reporting a median OR rate of 90.35% (range, 64.00%–100.00%) with a median CR rate of 58.20% (range, 25.00%–89.00%) [12–31] (Table 3).
Table 3.
Clinical response to ILP in studies included in the systematic review
Abbreviations: Cis, cisplatin; CR, complete response; Dac, dacarbazine; H, hyperthermia; ILP, isolated limb perfusion; MD, MD Anderson staging classification system for malignant melanoma; Mel, melphalan; N, normothermia; NR, no response; OR, overall response; PR, partial response; T, temperature regimen; TNF, tumor necrosis factor; ULAM, unresectable locally advanced melanoma.
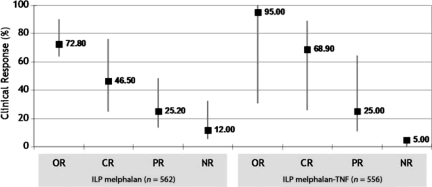
Valid results on the clinical response to ILP with melphalan were available from 562 perfusions (n = 6 studies), with a median CR rate of 46.50% (range, 25.00%–76.00%), versus a 68.90% (range, 26.00%–89.00%) median CR rate for ILP with melphalan and TNF, as obtained from 556 perfusions analyzed from 12 studies (Fig. 2). However, two comparative studies selected failed to demonstrate a statistically significant difference between the CR rate obtained using ILP with single-agent melphalan and ILP with melphalan plus TNF (25.00% versus 26.00%; p = .890 and 59.00% versus 45.00%; p = .14) [13, 20].
Figure 2.
Clinical effectiveness of isolated limb perfusion with melphalan and melphalan plus TNF. Median values of response are indicated by black squares, and ranges are indicated by grey bars.
Abbreviations: CR, complete response; ILP, isolated limb perfusion; NR, no response; OR, overall response; PR, partial response; TNF, tumor necrosis factor.
Other chemotherapy regimens analyzed in isolated studies were ILP with double-agent cisplatin and melphalan (n = 54; CR, 60%; OR, 94%) [31] or dactinomicin and melphalan (n = 100; CR, 45%–65%) [16] and ILP with single-agent TNF (n = 19; CR, 53%; OR, 100%) [12].
In 13 studies (n = 847 ILPs), valid data on the clinical response to different temperature regimens could be obtained [14, 16–19, 22, 25–31]. In 11 studies analyzing 544 hyperthermic ILPs, the median CR rate was 61.80% (range, 36.00%–89.00%) [14, 16–19, 22, 25, 26, 29–31]; 61.03% of the hyperthermic perfusions (n = 332 perfusions) were applied with the double-agent melphalan plus TNF regimen [14, 18, 19, 22, 25, 26, 29, 30]. The median CR rate to normothermic ILP, as shown in two studies analyzing 303 normothermic regimens with melphalan, was 47.00% (range, 42.00%–76.00%). No separate data from each of the hyperthermic regimens (mild hyperthermia, borderline hyperthermia, and true hyperthermia) could be obtained from the studies selected.
Regarding the influence of gender on the effectiveness of ILP, no adjusted data were provided in the selected studies. Moreover, two studies failed to identify gender as an independent response predictor on both univariate and multivariate analysis [19, 20]. In relation to other procedures and clinical factors with a potential role in response to ILP (i.e., time on ILP pump, vascular access taken, and lower versus upper extremities treated), the studies analyzed did not provide separate results adjusted for these variables.
Clinical response to repeated ILP was evaluated in an observational retrospective study of patients developing locoregional recurrence after a first perfusion [15]. The clinical response to repeated ILP (n = 21 ILPs) resulted in response rates similar to those of the first ILP (n = 17 ILPs) in terms of the OR rate (72.00% versus 77.00%), CR rate (62.00% versus 65.00%), and PR rate (10.00% versus 12.00%), with no statistically significant difference (p = .90).
Survival
OS or DFS of melanoma patients treated through ILP was addressed in 14 studies in this systematic review (n = 1,321) [12, 14–20, 22–24, 27, 28, 34]. Five-year OS was analyzed in eight studies, yielding a median OS rate of 36.50% (range, 19.00%–50.00%) and with a median OS interval of 36.70 months (range, 23.50–69.60 months) [15, 16, 18, 19, 20, 23, 28, 34] (Table 4). Regarding the 5-year DFS rate, valid data for this endpoint were reported in four studies under different ILP regimens, with a median survival rate of 39.45% (range, 16.00%–53.40%) [20, 23, 28, 34] and a median DFS interval of 16.00 months (range, 6.00–26.00 months) (Table 4).
Table 4.
Survival results of ILP for unresectable locally advanced melanoma
Abbreviations: Dac, dacarbazine; DFS, disease-free survival; H, hyperthermia; IFN, interferon; ILP, isolated limb perfusion; MD, MD Anderson staging classification system for malignant melanoma; Mel, melphalan; N, normothermia; OS, overall survival; T, temperature regimen; TNF, tumor necrosis factor; ULAM, unresectable locally advanced melanoma.
As for the impact of different chemotherapy ILP regimens on patient survival (melphalan ILP versus melphalan and TNF ILP), the heterogeneity of the outcome measures used hampered the possibility of obtaining synthetic results for this endpoint (Table 4). Even though the melphalan and TNF regimen has been demonstrated to be an independent predictor of clinical response on multivariate analysis, it has not been identified as a survival predictor [35]. A multivariate analysis performed in another study identified the addition of interferon as an independent predictor of longer OS after ILP [12].
Regarding the relation between clinical stage at the time of ILP and survival, most studies did not provide separate survival results for each clinical stage. One study analyzing the effectiveness of dactinomicin and melphalan ILP reported a higher 5-year OS rate for patients having MD Anderson stage IIIA disease, compared with stage IIIAB and stage IV patients (47%, 35%, and 34%, respectively) [16]. Multivariate analyses completed in two studies also showed clinical stage to be an independent predictor of OS and DFS, with stage IIIAB–IV patients (nodal and/or distance disease) showing shorter OS (stage IIIAB: hazard ratio [HR], 2.00; p = 0.011; stage IV: HR, 11.65; p < .001) than patients with stage IIIA disease [19]. In terms of DFS, stage IIIA disease was proven to be the strongest predictive factor on multivariate analysis (odds ratio, 0.3; p = .02) [20]. Tumor burden at presentation was also tested as a prognosis predictor in both univariate and multivariate models. Lesions >4 cm in size were associated with a shorter OS duration than lesions <4 cm on univariate analysis (p = 0.005) [19]. The presence of more than one lesion, versus a single lesion, was also identified as a predictor of a shorter DFS interval on multivariate analysis (p = .047) [35].
Secondary Effectiveness Endpoints
The rate of local recurrence after CR to ILP was evaluated in 10 studies, yielding a median recurrence rate of 40.50% (range, 15.00%–56.30%). The interval for the development of recurrence after ILP was measured in seven studies, yielding a median time to local recurrence of 10.5 months (range, 6.00–30.00 months) [15, 17, 18, 20–23, 25, 29, 30].
The limb salvage rate was analyzed in two studies, including 48 patients with unresectable locally advanced melanoma for which the only alternative therapy was amputation [14, 15]. In those studies, the amputation of the limb was avoided in 95% (median follow-up, 51 months) and 100% (median follow-up, 14 months) of the patients, with 19% of them dying from the disease regardless of the limb salvage [14, 15].
Regarding the impact of patient age on the effectiveness of ILP, the only adjusted analysis available was completed in a comparative study including patients >75 and <75 years old [23]. No statistically significant differences were observed in the CR rate, recurrence rate, DFS rate, and OS rate between the two age groups [23]. A multivariate analysis completed in one study identified advanced age as an independent predictor of a worse prognosis for patients treated with ILP, with shorter OS and DFS times (p = .0162 and p = .038, respectively) [36].
Toxicity of ILP
Fifteen studies (n = 1,483 ILPs) included in this systematic review yielded valid results on regional toxicity of ILP [12–16, 18–23, 30–33]. Data from those studies revealed a median rate of grade II regional toxicity of 73.53%; the rate was 17.10% for grade III regional toxicity, and 2.0% of ILPs resulted in grade IV regional toxicity. Toxic amputation of the treated limb (grade V regional toxicity) was described in 0.65% of treated patients (eight toxic amputations in n = 1,223 ILPs) (Table 5).
Table 5.
Regional toxicity of ILP
aWieberdink grade for locoregional toxicity evaluation: I, no subjective or objective evidence of reaction; II, slight erythema or edema; III, considerable erythema or edema with some blistering, slightly disturbed motility posible; IV, extensive epidermolysis or evident damage to the deep tissues causing definite functional disturbances, threatening or manifest compartmental syndrome. V, reaction that may need amputation.
Abbreviations: Cis, cisplatin; Dac, dacarbazine; H, hyperthermia; ILP, isolated limb perfusion; MD, MD Anderson staging classification system for malignant melanoma; Mel, melphalan; N, normothermia; T, temperature regimen; TNF, tumor necrosis factor; ULAM, unresectable locally advanced melanoma.
Regarding the type of chemotherapy, 10 studies yielded valid results on the regional toxicity of ILP with melphalan and TNF (n = 498), and three studies analyzed the regional toxicity of the single-agent ILP with melphalan (n = 463). Other chemotherapy drugs with a toxicity analysis completed were dacarbazine and melphalan and cisplatin and melphalan (n = 213 perfusions) [16, 31] (Table 5). A multivariate analysis identified hyperthermic ILP with melphalan and TNF as an independent predictor of acute severe regional toxicity, versus normothermic and hyperthermic ILP with melphalan alone (odds ratio, 2.7; p = .013) [32]. No valid data on toxicity adjusted to each temperature regimen were provided by the studies analyzed.
Gender, with a higher toxicity risk for women, was also identified as an independent predictor of regional toxicity after ILP [32]. As for patient age, regional toxicity of ILP in elderly patients was addressed in two studies comparing melanoma patients aged >75 years with those aged <75 years, with no significant difference in the incidence of severe toxic events between the two age groups [23, 33]. A univariate analysis of toxicity predictors did not identify age as an independent factor [32].
As for systemic toxicity, despite the number of studies analyzing this endpoint, the heterogeneous expression of the results allowed for the extraction of valid data from only seven studies, from which the percentage of patients having WHO classification grade III and grade IV toxicity were recorded [13, 18–20, 30, 31, 33] (Table 6). From all studies of ILP for MM reviewed, one case of death was recorded afterward, but it was not directly related to the ILP.
Table 6.
Systemic toxicity of ILP
Abbreviations: Cis, cisplatin; H, hyperthermia; III, IV: World Health Organization classification grade III and grade IV toxicity; ILP, isolated limb perfusion; Mel, melphalan; N, normothermia; T, temperature regimen; TNF, tumor necrosis factor.
Regarding the potential impact of vascular access (femoral versus iliac level) on regional toxicity, one study failed to demonstrate greater toxicity at the iliac level than at the femoral isolation level [23]. No studies addressed the association between the time on ILP pump and the development of regional and systemic side effects. Among the studies included in the systematic review, no series gave separate analyses of toxicity in relation to the anatomic area treated (lower versus upper limbs).
Discussion
In patients with MM, the development of satellitosis or in-transit regional metastases represents a clinical setting with a great impact on quality of life. From a therapeutic point of view, surgical removal of the metastases represents, in most cases, the only treatment able to ameliorate the symptoms and functional impairment related to the disease [35]. However, an unknown percentage of patients with locally advanced melanoma develop bulky metastases (i.e., large-sized metastases, >5–10 lesions) or neurovascular involvement of the limb, which makes surgical removal unfeasible [22]. In patients with unresectable locally advanced melanoma of the limbs, ILP has gained increasing interest in the last decades, with many studies addressing both the effectiveness and toxicity of the technique [37]. However, the paucity of RCTs as well as the lack of control groups and comparative studies with other therapeutic alternatives explain why the conclusions from these studies are not based on the highest levels of evidence.
As often occurs with palliative treatments in oncology, the relatively low incidence of this clinical entity (i.e., unresectable locally advanced melanoma of the limbs) together with the ethical limitations derived from the available alternatives (i.e., limb amputation) account, in part, for this lack of high-quality experimental studies. This was also shown in this systematic review, with only three eligible RCTs and a clear predominance of observational studies. This scarcity of phase III studies, along with the heterogeneity of patient subsets, drug regimens, and outcome measures applied, did not allow the completion of a meta-analysis, which provides the highest strength to the results obtained. Another heterogeneity factor worthy of consideration is the different criteria used in the studies to define CR and PR. In an attempt to minimize this source of heterogeneity, the application of the RECIST or WHO criteria for response definition was required for a study to be included in this systematic review [6, 7].
In this systematic review, ILP yielded a median OR rate of 90%. This figure deserves consideration because it largely improves upon the response rates obtained with other therapeutic options in this clinical setting (i.e., systemic chemotherapy, radiotherapy). Thus, systemic chemotherapy for metastatic melanoma provides response rates in the range of 15%–46% [38–40], with no impact on OS. Regarding palliative radiotherapy, despite the lack of studies on the benefit of radiotherapy in locally advanced melanoma, hypofractionated regimens obtain CR rates of up to 59% in stage I–III patients, including cases of in-transit metastases [41, 42]. Other locoregional therapeutic options tested in locally advanced melanoma patients (intralesional interleukin-2, perilesional GM-CSF, electrochemotherapy) need to be investigated in larger comparative series before discussing their role in the management of this clinical entity [43–47].
As for survival, the multivariate analysis completed in several studies also rendered interesting results. Thus, the use of interferon, the absence of nodal or distant disease, and a lower tumor burden were identified as independent predictors of longer OS. Moreover, two recent studies published after the completion of this review confirmed these findings, with a longer OS duration in women treated with ILP (p = .027; male versus female HR, 1.82; 95% CI, 1.07–3.09) [48, 49].
Locoregional recurrence was a secondary endpoint analyzed in several studies in this systematic review, with a median rate of 40.50%. Again, no direct comparison with the recurrence rate of other therapeutic alternatives was possible. However, even after surgical resection of in-transit melanoma metastases, further locoregional recurrences were described in up to 58% of patients [50].
To date, no locoregional approach has been demonstrated to have an impact on the OS duration of melanoma patients. This systematic review showed a median 5-year DFS rate of 39%, with 36% of patients being alive 5 years after perfusion. Again, because comparative trials are not available, it is not possible to objectively establish whether these results represent any real survival advantage. This figure is under the 5-year OS rate reported for stage IIIB melanoma patients by the American Joint Committee on Cancer in the collaborative 2008 database (69.20% for N2c and 38.70% for N3 patients) [51]. It should be stressed that patients treated with ILP have, by definition, unresectable disease and thus a greater tumor burden with an initially worse prognosis.
Lastly, toxicity data yielded a low incidence of severe regional and systemic toxicity, with a higher incidence of moderate and mild regional and systemic toxic events. The main goal of avoiding toxicity derived from systemic chemotherapy was therefore accomplished. These data support the consideration of ILP as a safe and feasible technique in this clinical setting. Moreover, a recent study addressing patients' long-term health-related quality of life after ILP reported better quality of life scores than in the general population, especially in relation to general health perceptions [52].
Conclusions and Recommendations
According to the results obtained from the studies available on ILP for unresectable locally advanced melanoma of the limbs, the research questions posed may be answered as follows:
-
ILP is effective in achieving objective therapeutic responses in patients with unresectable locally advanced melanoma of the limbs.
Level of evidence, IIa; strength of recommendation, B.
-
ILP provides appropriate DFS and OS rates for patients with unresectable locally advanced melanoma of the limbs.
Level of evidence, IIa; strength of recommendation, B.
-
ILP is a safe technique for the treatment of patients with unresectable locally advanced melanoma of the limbs, with a low incidence of severe regional toxicity.
Level of evidence, Ia; strength of recommendation, A.
-
ILP is a safe technique for the treatment of patients with unresectable locally advanced melanoma of the limbs, with a low incidence of severe systemic toxicity.
Level of evidence, IIb; strength of recommendation, B.
Secondary Recommendations
-
ILP with melphalan and TNF provides a better response rate than ILP with single-agent melphalan in patients with unresectable locally advanced melanoma of the limbs.
Level of evidence, IIa; strength of recommendation, B.
-
Repeated ILP after a recurrence or PR to a first ILP results in clinical response rates similar to those after first ILP in patients with unresectable locally advance melanoma of the limbs.
Level of evidence, III; strength of recommendation, B.
-
ILP results in similar clinical response and regional toxicity rates in elderly patients with unresectable locally advanced melanoma of the limbs.
Level of evidence, IIb; strength of recommendation, B.
-
In patients with unresectable MM of the limbs, ILP may avoid the amputation of the involved limb.
Level of evidence, III; strength of recommendation, B.
Acknowledgments
This study was supported by a grant from the Agencia de Evaluación de Tecnologías Sanitarias de Andalucía (AETSA 2007/10).
The authors thank Prof. Francisco M. Camacho, Prof. Carlos Ferrandiz, Dr. Rafael Botella, Dr. Pedro Redondo, and Dr. Josep Malvehy for the external critical review of this technical report.
Author Contributions
Conception/Design: David Moreno-Ramirez, Luis de la Cruz-Merino, Lara Ferrandiz, Roman Villegas-Portero, Adoracion Nieto-Garcia
Provision of study material or patients: David Moreno-Ramirez, Luis de la Cruz-Merino, Lara Ferrandiz, Roman Villegas-Portero
Collection and/or assembly of data: David Moreno-Ramirez, Luis de la Cruz-Merino, Lara Ferrandiz, Adoracion Nieto-Garcia
Data analysis and interpretation: David Moreno-Ramirez, Luis de la Cruz-Merino, Lara Ferrandiz, Adoracion Nieto-Garcia
Manuscript writing: David Moreno-Ramirez
Final approval of manuscript: David Moreno-Ramirez, Luis de la Cruz-Merino, Lara Ferrandiz, Roman Villegas-Portero, Adoracion Nieto-Garcia
References
- 1.Creech JO, Krementz ET, Ryan RF, et al. Chemotherapy of cancer: Regional perfusion utilizing an extracorporeal circuit. Ann Surg. 1958;148:616–632. doi: 10.1097/00000658-195810000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Creech JO, Krementz ET, Ryan RF, et al. Experiences with isolation-perfusion technics in the treatment of cancer. Ann Surg. 1959;149:627–639. doi: 10.1097/00000658-195905000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vrouenraets BC, Nieweg OE, Kroon BBR. Thirty-five years of isolated limb perfusion for melanoma: Indications and results. Br J Surg. 1996;83:1319–1328. doi: 10.1002/bjs.1800831004. [DOI] [PubMed] [Google Scholar]
- 4.Benckhuijsen C, Kroon BB, van Geel AN, et al. Regional perfusion treatment with melphalan for melanoma in a limb: An evaluation of drug kinetics. Eur J Surg Oncol. 1988;14:157–163. [PubMed] [Google Scholar]
- 5.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 6.Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Wieberdink J, Benckhuysen C, Braat RP, et al. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18:905–910. doi: 10.1016/0277-5379(82)90235-8. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute. Bethesda, Maryland: DCTD, NCI, NIH, DHHS; 2003. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE) pp. 1–72. [Google Scholar]
- 10.World Health Organisation. Geneva: World Health Organisation; 1979. WHO Handbook for Reporting Results of Cancer Treatment. [Google Scholar]
- 11.Scottish Intercollegiate Guidelines Network (SIGN) Edinburgh, Scotland: SIGN; 1999. SIGN Guidelines: An Introduction to SIGN Methodology for the Development of Evidence-Based Clinical Guidelines. SIGN publication no. 39. [Google Scholar]
- 12.Rossi CR, Russano F, Mocellin S, et al. TNF-based isolated limb perfusion followed by consolidation biotherapy with systemic low-dose interferon alpha 2b in patients with in-transit melanoma metastases: A pilot trial. Ann Surg Oncol. 2008;15:1218–1223. doi: 10.1245/s10434-007-9791-z. [DOI] [PubMed] [Google Scholar]
- 13.Cornett WR, McCall LM, Petersen RP, et al. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol. 2006;24:4196–4201. doi: 10.1200/JCO.2005.05.5152. [DOI] [PubMed] [Google Scholar]
- 14.Hayes AJ, Neuhaus SJ, Clark MA, et al. Isolated limb perfusion with melphalan and tumor necrosis factor alpha for advanced melanoma and soft-tissue sarcoma. Ann Surg Oncol. 2007;14:230–238. doi: 10.1245/s10434-006-9040-x. [DOI] [PubMed] [Google Scholar]
- 15.Noorda EM, Vrouenraets BC, Nieweg OE, et al. Repeat isolated limb perfusion with TNFα and melphalan for recurrent limb melanoma after failure of previous perfusion. Eur J Surg Oncol. 2006;32:318–324. doi: 10.1016/j.ejso.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Knorr C, Meyer T, Janssen T, et al. Hyperthermic isolated limb perfusion (HILP) in malignant melanoma. Experience with 101 patients. Eur J Surg Oncol. 2006;32:224–227. doi: 10.1016/j.ejso.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Aloia TA, Grubbs E, Onaitis M, et al. Predictors of outcome after hyperthermic isolated limb perfusion: Role of tumor response. Arch Surg. 2005;140:1115–1120. doi: 10.1001/archsurg.140.11.1115. [DOI] [PubMed] [Google Scholar]
- 18.Grünhagen DJ, de Wilt JH, van Geel AN, et al. TNF dose reduction in isolated limb perfusion. Eur J Surg Oncol. 2005;31:1011–1019. doi: 10.1016/j.ejso.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Grünhagen DJ, Brunstein F, Graveland WJ, et al. One hundred consecutive isolated limb perfusions with TNF-alpha and melphalan in melanoma patients with multiple in-transit metastases. Ann Surg. 2004;240:939–947. doi: 10.1097/01.sla.0000146147.89667.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noorda EM, Vrouenraets BC, Nieweg OE, et al. Isolated limb perfusion for unresectable melanoma of the extremities. Arch Surg. 2004;139:1237–1242. doi: 10.1001/archsurg.139.11.1237. [DOI] [PubMed] [Google Scholar]
- 21.Noorda EM, Takkenberg B, Vrouenraets BC, et al. Isolated limb perfusion prolongs the limb recurrence-free interval after several episodes of excisional surgery for locoregional recurrent melanoma. Ann Surg Oncol. 2004;11:491–499. doi: 10.1245/ASO.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 22.Rossi CR, Foletto M, Mocellin S, et al. Hyperthermic isolated limb perfusion with low-dose tumor necrosis factor-alpha and melphalan for bulky in-transit melanoma metastases. Ann Surg Oncol. 2004;11:173–177. doi: 10.1245/aso.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Noorda EM, Vrouenraets BC, Nieweg OE, et al. Safety and efficacy of isolated limb perfusion in elderly melanoma patients. Ann Surg Oncol. 2002;9:968–974. doi: 10.1007/BF02574514. [DOI] [PubMed] [Google Scholar]
- 24.Liénard D, Eggermont AM, Koops HS, et al. Isolated limb perfusion with tumour necrosis factor-alpha and melphalan with or without interferon-gamma for the treatment of in-transit melanoma metastases: A multicentre randomized phase II study. Melanoma Res. 1999;9:491–502. doi: 10.1097/00008390-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Feldman AL, Alexander HR, Jr, Bartlett DL, et al. Management of extremity recurrences after complete responses to isolated limb perfusion in patients with melanoma. Ann Surg Oncol. 1999;6:562–567. doi: 10.1007/s10434-999-0562-x. [DOI] [PubMed] [Google Scholar]
- 26.Fraker DL, Alexander HR, Andrich M, et al. Treatment of patients with melanoma of the extremity using hyperthermic isolated limb perfusion with melphalan, tumor necrosis factor, and interferon gamma: Results of a tumor necrosis factor dose-escalation study. J Clin Oncol. 1996;14:479–489. doi: 10.1200/JCO.1996.14.2.479. [DOI] [PubMed] [Google Scholar]
- 27.Klaase JM, Kroon BB, van Geel AN, et al. Is there an indication for a double perfusion schedule with melphalan for patients with recurrent melanoma of the limbs? Melanoma Res. 1994;4(suppl 1):13–16. [PubMed] [Google Scholar]
- 28.Klaase JM, Kroon BB, van Geel AN, et al. Limb recurrence-free interval and survival in patients with recurrent melanoma of the extremities treated with normothermic isolated perfusion. J Am Coll Surg. 1994;178:564–572. [PubMed] [Google Scholar]
- 29.Vaglini M, Santinami M, Manzi R, et al. Treatment of in-transit metastases from cutaneous melanoma by isolation perfusion with tumour necrosis factor-alpha (TNF-α), melphalan and interferon-gamma (IFN-γ). Dose-finding experience at the National Cancer Institute of Milan. Melanoma Res. 1994;4(suppl 1):35–38. [PubMed] [Google Scholar]
- 30.Lienard D, Ewalenko P, Delmotte JJ, et al. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992;10:52–60. doi: 10.1200/JCO.1992.10.1.52. [DOI] [PubMed] [Google Scholar]
- 31.Kettelhack C, Kraus T, Hupp T, et al. Hyperthermic limb perfusion for malignant melanoma and soft tissue sarcoma. Eur J Surg Oncol. 1990;16:370–375. [PubMed] [Google Scholar]
- 32.Vrouenraets BC, Eggermont AMM, Hart AAM, et al. Regional toxicity after isolated limb perfusion with melphalan and tumour necrosis factor-α versus toxicity after melphalan alone. Eur J Surg Oncol. 2001;27:390–395. doi: 10.1053/ejso.2001.1124. [DOI] [PubMed] [Google Scholar]
- 33.Van Etten B, van Geel AN, de Wilt JHW, et al. Fifty tumor necrosis factor-based isolated limb perfusions for limb salvage in patients older than 75 years with limb-threatening soft tissue sarcomas and other extremity tumors. Ann Surg Oncol. 2003;10:32–37. doi: 10.1245/aso.2003.03.076. [DOI] [PubMed] [Google Scholar]
- 34.Zogakis TG, Bartlett DL, Libutti SK, et al. Factors affecting survival after complete response to isolated limb perfusion in patients with in-transit melanoma. Ann Surg Oncol. 2001;8:771–778. doi: 10.1007/s10434-001-0771-4. [DOI] [PubMed] [Google Scholar]
- 35.Vrouenraets BC, Hart GAM, Eggermont AMM, et al. Relation between limb toxicity and treatment outcomes after isolated limb perfusion for recurrent melanoma. J Am Coll Surg. 1999;188:522–530. doi: 10.1016/s1072-7515(99)00018-6. [DOI] [PubMed] [Google Scholar]
- 36.Brobeil A, Berman C, Cruse CW, et al. Efficacy of hyperthermic isolated limb perfusion for extremity-confined recurrent melanoma. Ann Surg Oncol. 1998;5:376–383. doi: 10.1007/BF02303503. [DOI] [PubMed] [Google Scholar]
- 37.Moreno-Ramirez D, de la Cruz-Merino L, Ferrandiz L, et al. Study and treatment of locally advanced melanoma. Actas Dermosifiliogr. 2009;100:767–779. [PubMed] [Google Scholar]
- 38.Li Y, McClay EF. Systemic chemotherapy for the treatment of metastatic melanoma. Semin Oncol. 2002;29:413–426. doi: 10.1053/sonc.2002.35237. [DOI] [PubMed] [Google Scholar]
- 39.Eggermont AMM, Kirkwood JM. Re-evaluating of the role of dacarbazine in metastatic melanoma: What have we learned in 30 years? Eur J Cancer. 2004;40:1825–1836. doi: 10.1016/j.ejca.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 40.Gogas HJ, Kirkwood JM, Sondak VK. Chemotherapy for metastatic melanoma: Time for a change? Cancer. 2007;109:455–464. doi: 10.1002/cncr.22427. [DOI] [PubMed] [Google Scholar]
- 41.Stevens G, McKay MJ. Dispelling the myths surrounding radiotherapy for treatment of cutaneous melanoma. Lancet Oncol. 2006;7:575–583. doi: 10.1016/S1470-2045(06)70758-6. [DOI] [PubMed] [Google Scholar]
- 42.Sause WT, Cooper JS, Rush S, et al. Fraction size in external beam radiation therapy in the treatment of melanoma. Int J Radiat Oncol Biol Phys. 1991;20:429–432. doi: 10.1016/0360-3016(91)90053-7. [DOI] [PubMed] [Google Scholar]
- 43.Green DS, Bodman-Smith MD, Dalgleish AG, et al. Phase I/II study of topical imiquimod and intralesional interleukin-2 in the treatment of accessible metastases in malignant melanoma. Br J Dermatol. 2007;156:337–345. doi: 10.1111/j.1365-2133.2006.07664.x. [DOI] [PubMed] [Google Scholar]
- 44.Radny P, Carola UM, Bauer J, et al. Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases. Br J Cancer. 2003;89:1620–1626. doi: 10.1038/sj.bjc.6601320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoeller C, Jansen B, Heere-Ress E, et al. Perilesional injection of r-GM-CSF in patients with cutaneous melanoma metastases. J Invest Dermatol. 2001;117:371–374. doi: 10.1046/j.0022-202x.2001.01427.x. [DOI] [PubMed] [Google Scholar]
- 46.Byrne CM, Thompson JF, Johnston H, et al. Treatment of metastatic melanoma using electroporation therapy with bleomycin (electrochemotherapy) Melanoma Res. 2005;15:45–51. doi: 10.1097/00008390-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Wolf IH, Smolle J, Binder B, et al. Topical imiquimod in the treatment of metastatic melanoma to skin. Arch Dermatol. 2003;139:273–276. doi: 10.1001/archderm.139.3.273. [DOI] [PubMed] [Google Scholar]
- 48.Alexander HR, Jr, Fraker DL, Bartlett DL, et al. Analysis of factors influencing outcome in patients with in-transit malignant melanoma undergoing isolated limb perfusion using modern treatment parameters. J Clin Oncol. 2010;28:114–118. doi: 10.1200/JCO.2009.23.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Filippo F, Giacomini P, Rossi CR, et al. Prognostic factors influencing tumor response, locoregional control and survival, in melanoma patients with multiple limb in-transit metastases treated with TNFα-based isolated limb perfusion. In Vivo. 2009;23:347–352. [PubMed] [Google Scholar]
- 50.Dong XD, Tyler D, Jonson JL, et al. Analysis of prognosis and disease progression alter local recurrence of melanoma. Cancer. 2000;88:1063–1071. doi: 10.1002/(sici)1097-0142(20000301)88:5<1063::aid-cncr17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 51.Balch CM, Gershenwald JE, Soong SJ, et al. Melanoma staging and classification. In: Balch CM, Houghton N, Sober AJ, et al., editors. Cutaneous Melanoma. Fifth Edition. St. Louis, MO: Quality Medical Publishing Inc.; 2009. pp. 65–85. [Google Scholar]
- 52.Noorda EM, van Kreij RHJ, Vrouenraets BC, et al. The health-related quality of life of long-term survivors of melanoma treated with isolated limb perfusion. Eur J Surg Oncol. 2007;33:776–782. doi: 10.1016/j.ejso.2006.03.024. [DOI] [PubMed] [Google Scholar]