Evidence supporting the critical role of transforming growth factor β1 in the development of normal tissue injury after cancer therapy is reviewed and the results of recent research aimed at preventing normal tissue injury by targeting the transforming growth factor β1 pathway are presented.
Keywords: Transforming growth factor β, Complications, Fibrosis, Lung Cancer
Abstract
With >10,000,000 cancer survivors in the U.S. alone, the late effects of cancer treatment are a significant public health issue. Over the past 15 years, much work has been done that has led to an improvement in our understanding of the molecular mechanisms underlying the development of normal tissue injury after cancer therapy. In many cases, these injuries are characterized at the histologic level by loss of parenchymal cells, excessive fibrosis, and tissue atrophy. Among the many cytokines involved in this process, transforming growth factor (TGF)-β1 is thought to play a pivotal role. TGF-β1 has a multitude of functions, including both promoting the formation and inhibiting the breakdown of connective tissue. It also inhibits epithelial cell proliferation. TGF-β1 is overexpressed at sites of injury after radiation and chemotherapy. Thus, TGF-β1 represents a logical target for molecular therapies designed to prevent or reduce normal tissue injury after cancer therapy. Herein, the evidence supporting the critical role of TGF-ß1 in the development of normal tissue injury after cancer therapy is reviewed and the results of recent research aimed at preventing normal tissue injury by targeting the TGF-ß1 pathway are presented.
Introduction
The tolerance of normal tissues and organs limits the amount of chemotherapy and radiation therapy that can be administered to a patient undergoing cancer treatment [1, 2]. In many cases, these doses may be less than what one would give under ideal circumstances in an effort to control a tumor. Consequently, cure of the cancer might not be achievable as a result of limitations imposed by normal tissue tolerance. This is particularly true in circumstances requiring high doses of radiation for tumors located within or adjacent to sensitive organs, such as the lung.
Traditionally, radiation oncologists have been forced to deal with normal tissue tolerance by limiting the dose and/or volume of tissue receiving radiation [3, 4]. With recent advances in imaging technology, these dose–volume relationships have received additional attention in an attempt to more precisely correlate dose, volume, and the risk for normal tissue injury [2]. These dose–volume relationships, however, disregard the spatial distribution of dose within an organ and also do not account for biologic heterogeneity, genetic factors, and underlying comorbidities that could impact an individual patient's risk for treatment-related complications.
Research over the past 15 years has led to a better understanding of the underlying molecular events responsible for the development of normal tissue injury after cancer therapy [5–8]. Of the many cytokines and growth factors shown to contribute to the injury process, transforming growth factor TGF)-β1 is among the most important [9]. TGF-β1 belongs to a family of secreted polypeptide growth factors subcategorized by function, including its three mammalian isoforms (TGF-β1, TGF-β2, and TGF-β3), bone morphogenic proteins, activins, inhibins, and Mullerian inhibiting substance [10, 11]. These proteins have important functions in both normal and disease-related processes, such as cell growth, differentiation, migration, adhesion, angiogenesis, immunity, extracellular matrix synthesis, and epithelial–mesenchymal transition [12–15]. The TGF-β family signals through a complex interactome [16] involving multiple pathways. Disruption of the TGF-β signaling pathway has been implicated in the cellular acquisition of the hallmarks of malignancy [17], that is, failure to respond to growth inhibitory factors, proliferation in the absence of exogenous cues, invasion, metastasis, immortality, loss of apoptosis, stimulation of angiogenesis, and evasion of the immune system. Thus, under normal circumstances, a number of genes coding for proteins in the TGF-β signaling pathway would be considered tumor suppressor genes. In particular, most malignant cells are resistant to the growth inhibitory effects of TGF-β through a variety of different mechanisms [18–20], and, in fact, TGF-β has been shown to stimulate the growth of malignant cell lines [21]. Thus, overexpression of TGF-β within the tumor microenvironment could promote the local growth and metastatic potential of many solid tumors [22]. That TGF-β can function as both a tumor suppressor and a tumor promotor, depending on the environment in which it operates, points to the complexity of the processes in which these proteins participate.
The most widely studied of this family of molecules is TGF-β1. A detailed discussion of its role in tumor promotion, progression, and metastasis is beyond the scope of this review. Rather, herein, the role of TGF-β1 in normal tissue injury, particularly the lung, is reviewed and strategies to target TGF-β1 to reduce the risk for normal tissue injury are discussed.
Evidence for a Role for TGF-β1 in Chronic Normal Tissue Injury After Cancer Therapy
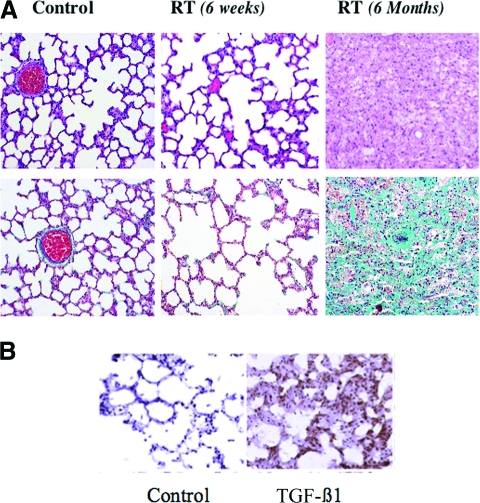
Loss of parenchymal cells and an excess of fibrous tissue often characterize late injury after cancer therapy (Fig. 1A, 1B). TGF-β1 plays an important role in both normal and abnormal wound healing [23, 24]. This cytokine is produced in a biologically inert form, bound to a latency associate peptide to form the small latent TGF-β complex, which is secreted from cells and can be activated by several methods, including through exposure to proteases, integrins, and free radicals [25–30]. The small latent complex may also be covalently bound to one of several latent TGF-β binding proteins to form the large latent complex, which may be sequestered in the extracellular matrix [31]. TGF-β1 is found in many different cell types, but its highest concentration is in platelets. As a result, TGF-β1 is normally released in large quantities from platelets at the site of a wound. Among the wound-healing properties of this cytokine are recruitment of monocytes and macrophages to an injury site [32], inhibition of the proliferation of epithelial cells [33], enhancement of the maturation of fibroblasts into postmitotic fibrocytes that increase production of fibrous tissue [34], promotion of angiogenesis [35, 36], and inhibition of the breakdown of extracellular matrix [23, 37]. Despite its role in normal wound healing, increased expression of TGF-β1 has been demonstrated in a number of conditions characterized by excessive fibrosis, including chronic hepatitis and glomerulosclerosis [24, 38–41]. In addition, effective treatments for these conditions have been shown to reduce the development of fibrosis in the affected organ, with a corresponding decrease in the expression of TGF-β1 [42–53].
Figure 1.
TGF-β expression in radiation-induced lung injury. (A): Radiation (RT)-induced lung injury in a rat model. Fischer 344 rats were irradiated to 28 Gy in one fraction to the right hemithorax and sacrificed at 6 weeks or 6 months after RT. Slides were stained with either hematoxylin & eosin (top row) or Masson's trichrome (bottom row). A nonirradiated control lung (left column) demonstrates the normal honeycomb architecture of the lung. At 6 weeks after irradiation (middle column), one begins to see thickening of the alveolar walls resulting from edema, corresponding to the inflammatory phase, but with little fibrosis. At 6 months (right column), there is complete loss of normal alveolar architecture, with extensive fibrosis (green staining in bottom right panel). Reprinted from Stone HB, Coleman CN, Anscher MS et al. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol 2003;4:529–536, with permission from Elsevier. (B): Fischer 344 rats were irradiated to 28 Gy in one fraction to the right hemithorax, sacrificed 6 months after radiation, and stained for transforming growth factor (TGF)-β1. There is very little staining for TGF-β1 in the control lung (nonirradiated) in contrast to the irradiated lung, which demonstrates greater expression of TGF-β1 in regions of fibrosis.
Following exposure to ionizing radiation, the expression of TGF-β1 increased in a dose-dependent manner in the rat liver [54]. Similarly, in a rat model of radiation-induced lung injury, fibrosis developed, which was accompanied by an increase in TGF-β1 expression and activation of the TGF-β1 signal transduction pathway [55, 56]. Furthermore, TGF-β1 activation by radiation has been shown to occur at doses <10 cGy and is roughly proportional to dose in the range of 10 cGy to 5 Gy [57, 58]. Although genetically engineered loss of TGF-β1 expression is a lethal mutation, knockout mice missing a component of the TGF-β1 signaling pathway (Smad3) are viable, and have been shown to be resistant to radiation-induced soft tissue fibrosis [59]. In contrast, humans expressing certain single nucleotide polymorphisms in the TGF-β1 gene have been found to be at a higher risk for normal tissue injury [60]. In animal models of pulmonary fibrosis induced by bleomycin [52] and in humans exposed to high doses of chemotherapy in preparation for bone marrow transplantation who develop pulmonary drug toxicity or hepatic veno-occlusive disease (Fig. 2), the associated fibrosis is accompanied by increased expression of TGF-β1 in affected tissues. Thus, there is substantial evidence on the importance of TGF-β1 in the development of excessive fibrosis following exposure to radiation and/or chemotherapy in both animals and humans.
Figure 2.
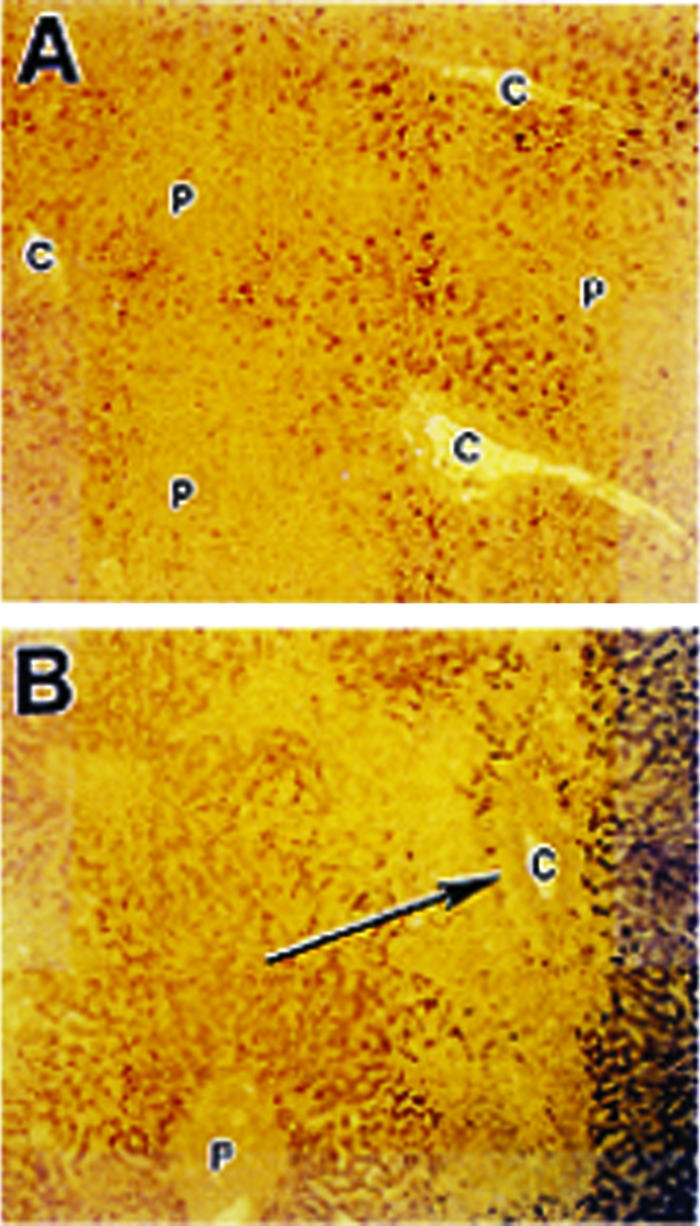
TGF-β expression in chemotherapy-induced liver injury. Section of normal liver (A) and liver from patient that died of hepatic veno-occlusive disease after high-dose chemotherapy (B). In (B), evidence of extensive fibrosis around the central veins (C) is noted (arrow) and there is greater staining for transforming growth factor β1 (reddish brown areas) immediately adjacent to regions of fibrosis than in the normal liver in (A). P, portal vein. Reprinted from Anscher MS, Kong FM, Jirtle RL. The relevance of transforming growth factor beta 1 in pulmonary injury after radiation therapy. Lung Cancer 1998;19:109–120, with permission from Elsevier.
TGF-β1 as a Predictor of Normal Tissue Injury Risk
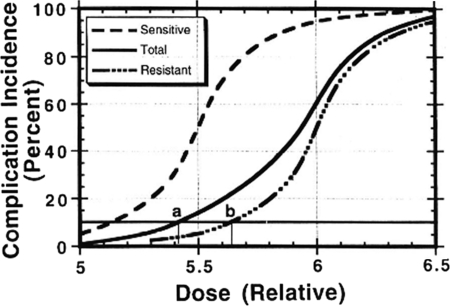
Dosing of chemotherapy or radiation is generally based either on a prospectively determined maximum-tolerated dose or, more commonly, in the case of radiation, an empirically determined estimate of the risk for a particular injury developing in a certain percentage of patients within a defined period of time [1, 2]. In either case, the accepted tolerance dose of radiation or chemotherapy produces dose-limiting toxicity in a minority of patients, yet it is precisely these patients that dictate the dose of chemotherapy or radiation for an entire population, when in reality any given population of patients is likely to contain people who may be more or less likely to experience toxicity than the “average” population used to determine dosing guidelines (Fig. 3). Thus, the ability to determine the likelihood of toxicity for an individual patient, rather than rely on dosing based on the average sensitivity of a population, is a desirable goal that should improve the therapeutic ratio (i.e., reduce the likelihood of toxicity, increase the likelihood of cure, or both).
Figure 3.
Altering dose on the basis of an individual patient's sensitivity to toxicity can affect the therapeutic ratio. Dosing of chemotherapy or radiation is based on sensitivity to toxicity that is based on population averages (solid line). In reality, any population also contains individuals that will be either more sensitive (dashed line) or more resistant (dot-dash line) to treatment toxicity than the average population. The sensitive patients, which probably comprise ≤5%–10% of the overall population [1, 2], nonetheless drive the dosing schemes. In this example, by holding the acceptable complication incidence at 10%, a resistant patient (b) could receive an approximately 5% greater dose than an average patient (a) and a 10% greater dose than a sensitive patient. Dose differences of this magnitude have been associated with differences in outcome.
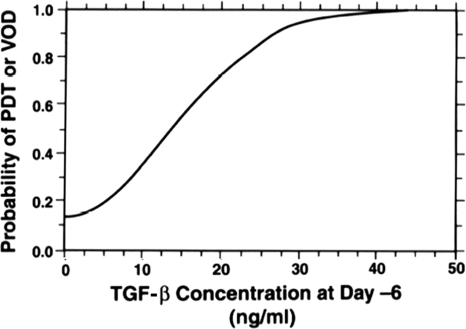
In addition to being widely produced throughout the body, TGF-β1 can also be measured in the blood [61]. Increased circulating levels of TGF-β1 have been found in patients with many diseases, including various types of cancer [62, 63]. Because of the fact that TGF-β1 expression in tissue also increases in response to radiation or chemotherapy exposure, several researchers have sought to measure circulating TGF-β1 levels in an attempt to predict which patients might be at an increased or a decreased risk for normal tissue injury after exposure to chemotherapy or radiation. The first reported study using circulating TGF-β1 levels to predict the risk for normal tissue injury was by Anscher et al. [61]. Those researchers found, in a group of patients treated with induction chemotherapy, high-dose chemotherapy, autologous bone marrow transplant, and involved field radiation for high-risk breast cancer, that plasma TGF-β1 measured after induction chemotherapy but before high-dose chemotherapy, radiation, or marrow transplant was strongly correlated with the risk of developing either pulmonary drug toxicity or hepatic veno-occlusive disease (Fig. 4). Subsequent studies by Anscher and colleagues, as well as others, have focused on determining whether TGF-β1 could be used in a similar manner to predict the risk of developing pulmonary injury after radiation therapy. The results of these studies have been mixed, with some, but not all, authors finding that TGF-β1 levels could be used to predict the risk for lung injury [64–68]. However, most of these studies contained small numbers of patients with relatively few events, thus having little power to detect differences between study groups [9]. Also, toxicity endpoints differed (e.g., some considered only symptomatic lung injury, whereas others included asymptomatic radiographic changes), as did techniques for measuring TGF-β1 in the blood. A more recent larger pooled analysis supports the contention that plasma TGF-β1 levels can be used to predict the risk for radiation-induced lung injury [69]. Additional work is needed to confirm this finding.
Figure 4.
Correlation of plasma transforming growth factor (TGF)-β1 concentration and the risk for pulmonary drug toxicity (PDT) or hepatic veno-occlusive disease (VOD) after high-dose chemotherapy for advanced breast cancer. Blood samples were taken from patients after induction chemotherapy but prior to administration of high-dose chemotherapy (day −6) and bone marrow transplant. There is a strong correlation between the plasma TGF-β1 concentration at day −6 and the risk for either PDT or VOD developing after subsequent high-dose chemotherapy. Reprinted from Anscher MS, Kong FM, Jirtle RL. The relevance of transforming growth factor beta 1 in pulmonary injury after radiation therapy. Lung Cancer 1998;19:109–120, with permission from Elsevier.
In reality, however, because radiation oncologists are skilled at limiting the volume of normal lung receiving a high dose, the incidence of significant symptomatic lung injury (grade ≥2) in most series is low, and plasma TGF-β1 measurements may actually be better suited to determine patients at low risk for lung injury rather than identifying those at very high risk [70]. This observation suggests that plasma TGF-β1 measurements might be useful to identify patients with lung cancer who might be candidates for radiation dose escalation. Anscher et al. [71] tested this hypothesis in a small clinical trial designed to assess the maximum-tolerated dose of radiation that could be delivered to the lung using TGF-β1 to estimate the risk for dose-limiting lung toxicity. Patients with locally advanced or medically inoperable non-small cell lung cancer were treated with the institutional standard high-dose radiation approach at the time (1.6 Gy twice daily using a concurrent boost technique) to a dose of 73.6 Gy. Concurrent chemotherapy was not allowed, and the technique included elective nodal irradiation. TGF-β1 was measured at baseline and after 73.6 Gy. If the TGF-β1 level normalized by 73.6 Gy, then the dose was escalated in increments of 6.4 Gy to successive patients until a dose-limiting toxicity was reached. In total, 38 patients were enrolled. In 24, the dose could not be escalated beyond 73.6 Gy based on TGF-β1 criteria. In the remaining patients, eight received 80 Gy and six received 86.4 Gy. The latter dose was determined to be the maximally tolerated dose. Thus, this study determined that it was feasible to use TGF-β1 to guide radiation dose selection for patients with lung cancer. Given the changes in the treatment approach to lung cancer since that study was conducted (i.e., ubiquitous use of combined chemoradiotherapy and increased use of stereotactic radiation therapy for smaller tumors), the study needs to be repeated using a more current treatment regimen. In a follow-up report, the authors noted that serious long-term toxicity (grade 3–5) occurred only in the control arm, further supporting the potential of this approach to treatment individualization [72]. It should be noted, however, that these higher doses of radiation did not translate into longer survival. The study was not designed to test this endpoint, and further work is needed before any conclusions can be drawn in this regard.
Why Is TGF-β1 Elevated in Cancer Patients Even Before Treatment Begins?
As hinted at above, circulating TGF-β1 levels in many cancer patients are elevated at the time of diagnosis, before treatment, as compared with people without cancer, and the tumor is thought to be the source of TGF-β1 [63, 73]. In addition to contributing to the risk for normal tissue injury from cancer treatment, TGF-β1 also promotes metastasis [74] and suppresses the immune system [75], so that the presence of high levels of TGF-β1 in cancer patients may have an adverse impact on prognosis [76–78]. Animal studies support this conclusion. For example, in transgenic mouse models, induction of active TGF-β increased the incidence of lung metastases from breast cancer [79], whereas blocking TGF-β activity reduced the metastatic potential [80]. Recent evidence has also suggested that receptor tyrosine kinase inhibitors that block the binding of TGF-β to its transmembrane receptors prevent the activation of the TGF-β signaling pathway and reduce the incidence of metastases in a breast cancer model [81]. Also, tumors that express TGF-β1 are more resistant to chemotherapy, and sensitivity to cisplatin can be restored with TGF-β1 inhibition [82, 83]. These studies support the rationale for targeting the TGF-β pathway as a potential anticancer therapy.
The reason for the presence of higher levels of TGF-β1 in these cancer patients appears to be related to both greater production and altered bioavailability of this cytokine [84–86]. Kong et al. [62] studied a group of women suspected to have breast cancer on the basis of an abnormal mammogram. In these patients, blood was obtained for cytokine measurements before and after removal of the tumors by lumpectomy. Following surgery, there was a significant reduction in plasma TGF-β1 levels in these patients, suggesting that the tumor was the source of the TGF-β1 production. Those authors also studied the tumor specimens using immunohistochemistry to stain for TGF-β1 expression and in situ hybridization to look for the message to produce the TGF-β1 protein. In comparison with regions of normal breast tissue in the biopsy specimens, there was significantly greater expression of both messenger RNA and TGF-β1 in the tumor stroma, but not in epithelial cells. As noted above, many malignant epithelial cells lose the ability to respond to the growth inhibitory effects of TGF-β1, and it appears that the loss of this growth inhibitory feedback loop leads to increased production of TGF-β1 in a futile attempt to stop the proliferation of these malignant epithelial cells [33, 47, 74, 87, 88]. In terms of bioavailability, the mannose-6-phosphate/insulin-like growth factor receptor type II (M6P/IGF2R) plays a critical role in this process [89]. The gene coding for this receptor has been shown to be a tumor suppressor gene [90]. Binding of latent TGF-β1 to M6P/IGF2R facilitates its activation [85], but it also enables the molecule to be presented to lysosomes for degradation [91]. Thus, loss of function of this tumor suppressor gene could lead to a decreased ability to dispose of latent TGF-β1, which could be activated by other mechanisms (e.g., free radicals) and lead to an increased risk for normal tissue injury. Kong et al. [92] studied a group of lung cancer patients to test this hypothesis. They found that if the patients had loss of heterozygosity in M6P/IGF2R, and consequently a nonfunctioning receptor, they were significantly more likely to have higher plasma TGF-β1 levels and to develop radiation-induced lung injury than those without loss of heterozygosity in the receptor [93]. Thus, it appears that bioavailability of TGF-β1 plays an important role in the risk for normal tissue injury in at least a subset of cancer patients.
Sustaining the Phenotype of Normal Tissue Injury After Treatment Is Completed
The development of normal tissue injury results from a complex interplay of contributions to risk from the treatment, the tumor, and the patient (Figure 5). Radiation contributes numerous insults beyond merely dose and volume, although these two factors no doubt impact the risk for injury. For example, radiation produces free radicals, damages vasculature, creates a cascade of local and systemic cytokine and chemokine expression, elicits an inflammatory response, and causes loss of parenchymal cells. Chemotherapy may increase radiosensitization through various mechanisms, lead to parenchymal cell loss, affect DNA repair, suppress the immune system, or cause inflammation. The tumor itself may lead to architectural destruction, release its own cytokines, affect the immune system, and alter vascular permeability. The patient may have underlying medical conditions predisposing to injury (e.g., diabetes, poor underlying pulmonary function, or certain collagen vascular diseases) or have an unknown genetic susceptibility. All these factors may contribute to a varying degree in any given patient, and their relative importance cannot as yet be reliably determined. Nonetheless, it is becoming clear that abnormal microenvironmental conditions exist that are sustained long after the beam is turned off, the drugs are discontinued, and the tumor is eradicated, which appear to be responsible for the perpetuation of the tissue atrophy, loss of parenchymal cells, and excessive fibrosis characteristic of late normal tissue injury after cancer therapy.
Figure 5.
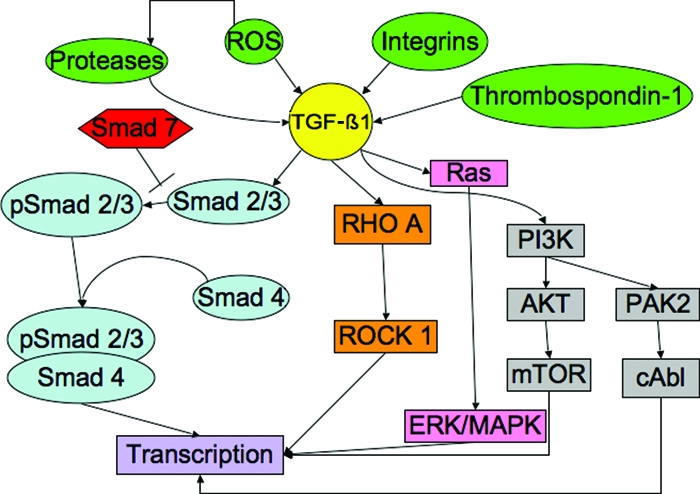
Potential targets for intervention in the TGF-β1 pathway (partial list) [17, 74, 114]. The inactive form of TGF-β1 can be activated through the action of several factors (green ovals), including ROS, proteases, integrins, and thrombospondin-1. The active form of TGF-β1 (yellow) can then signal through either Smad-dependent (light blue) or Smad-independent pathways (orange, pink, gray). Any point along these pathways might be targeted by potential inhibitors.
Abbreviations: ERK, extracellular signal–related kinase; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3′ kinase; ROS, reactive oxygen species; TGF, transforming growth factor.
There is increasing evidence that chronic normal tissue hypoxia develops after exposure to radiation therapy, and that this phenomenon may be, in part, responsible for sustaining the normal tissue injury phenotype [94–96]. Hypoxia has been shown to promote the production of profibrotic cytokines and stimulate collagen deposition through the action of TGF-β1 [97, 98]. Hypoxia has been demonstrated after radiation in the lung, central nervous system, and kidney. It is thought that hypoxia develops as a result of decreased oxygen delivery resulting from vascular injury plus greater consumption by activated macrophages recruited to the site of injury in response to reactive oxygen species–mediated tissue damage. In the lung, evidence of sustained oxidative stress and tissue hypoxia have been demonstrated 6 months after radiation in a rat model, and this finding was correlated with greater expression and activation of TGF-β1 and its signaling pathway [95]. Westbury et al. [99] evaluated a group of patients undergoing salvage mastectomy for recurrent breast cancer following radiation therapy. The patients received an injection of the hypoxic marker pimonidazole prior to surgery. Following surgery, the normal breast tissue in the specimens was evaluated for the presence of pimonidazole staining, and for the presence of the hypoxic marker carbonic anhydrase IX (CA IX). The authors also looked for the presence of radiation damage histologically. In the only breast sample (of 12) that showed marked histologic changes consistent with a radiation effect, there was greater staining for pimonidazole, but not CA IX, suggesting an oxygen tension of <10 mmHg in this tissue. This is the first in vivo evidence demonstrating hypoxia in normal irradiated human tissue using this approach. Given the relationship noted above between hypoxia and TGF-β1, these data further support the central role of TGF-β1 in the development of chronic radiation injury.
The Impact of TGF-β1 Blockade on Normal Tissue Injury
The TGF-β1 interactome is extremely complex in that many proteins interact with its transmembrane receptors and signaling proteins (Smads) within the cytoplasm and the nucleus, affecting signaling crosstalk and protein transcription [16]. In addition, there is increasing evidence that some forms of radiation injury may develop via Smad-independent TGF-β1 signaling [100, 101]. The dominant pathway at this time seems to involve the binding of TGF-β1 to its type II transmembrane receptor. This interaction recruits the type I transmembrane receptor to form a complex with the type II receptor. This complex is phosphorylated and activates the signaling proteins Smad2 and Smad3. These activated Smads bind with Smad4 and are translocated to the nucleus, where they bind to promoters and modulate transcription [74]. Given the vast number of potential targets in this pathway, most work has focused on interfering with the binding of TGF-β1 to its receptors, thus preventing initiation of the multitude of potential downstream interactions. Several approaches have been taken to prevent the binding of TGF-β1 to its receptor, including the use of anti-TGF-β1 antibodies, small molecule receptor tyrosine kinase inhibitors, and gene therapy approaches designed to induce production of a soluble TGF-β1 type II receptor. Anscher et al. [96] administered a single dose of anti-TGF-β1 antibody immediately after delivering the final fraction of right hemithorax irradiation (40 Gy in five fractions over 5 days) to Fischer rats. Compared with animals receiving radiation without antibody, there was significantly less fibrosis, TGF-β1 expression, and TGF-β1-induced signaling in the animals treated with the antibody. A higher dose of antibody (1 mg/kg) afforded better protection than did a lower dose (0.1 mg/kg). The same group also tested daily administration of a type I receptor tyrosine kinase inhibitor administered daily to a group of Sprague-Dawley rats irradiated to the right lung [102]. The drug was administered beginning 7 days prior to irradiation and continued either for 3 weeks or until sacrifice. Compared with irradiated animals not receiving the drug, there was significantly less fibrosis, TGF-β1 expression and signaling, and chronic oxidative stress in the treated groups, with a better response seen in the high-dose group (0.15 g/kg versus 0.07 g/kg) and in those treated with a long course versus a short course of therapy. Neither compound has been tested in the clinic for this purpose.
As stated previously, there is increasing evidence that TGF-β1 signaling through Smad-independent pathways may stimulate overproduction of fibrous tissue in response to radiation or chemotherapy. One such pathway signals through cAbl. The drug imatinib, an inhibitor of the cAbl pathway in use in the clinic for the treatment of gastrointestinal stromal tumors, was shown to reduce the development of pulmonary fibrosis in response to bleomycin in an animal model [53]. TGF-β1 was also recently shown to signal through the Rho/Rock pathway, independently of the Smad signal transduction cascade [100, 101]. This pathway has been implicated in the development of radiation-induced small intestinal injury. Inhibition of this signaling pathway has been shown to protect against radiation enteritis.
Other potential approaches target TGF-β1 more indirectly, for example, by reducing the stimulus for TGF-β1 activation (Fig. 5). Following exposure to radiation, reactive oxygen species are produced [103], and these have been shown to be capable of activating latent TGF-β1 [30]. Mice engineered to overexpress one of the isoforms of superoxide dismutase, an endogenous free radical scavenger, have been demonstrated to be resistant to radiation-induced lung injury [104]. Similarly, administration of superoxide dismutase mimetics has been shown to reduce the severity of lung injury in an animal model [105]. Both approaches result in lower expression and activation of TGF-β1 as well as lower activiation of the Smad-dependent TGF-β1 signaling pathway.
As is the case with radiation therapy, targeting the TGF-β1 pathway may be an effective means to prevent or ameliorate chemotherapy-induced lung injury. Bleomycin-induced lung injury (Fig. 2) is the classic example of this toxicity, although newer agents, such as gefitinib, may be associated with the development of lung injury and fibrosis [106]. Susceptibility to both radiation- and bleomycin-induced lung injury in mice may have a common genetic basis [107]. Bleomycin-induced lung injury in the acute phases is mediated through the epidermal growth factor receptor (EGFR) family, most likely human epidermal growth factor receptor (HER)-2/HER-3 [108]. Blockade of HER-2/HER-3 signaling ameliorates the development of pulmonary fibrosis from bleomycin [109]. Because TGF-β may upregulate profibrotic protein expression, in part, through an EGFR-mediated mechanism [110], it should not be surprising that inhibiting the TGF-β pathway also ameliorates the development of this toxicity in animals [50, 111, 112].
In summary, TGF-β1 is a critical cytokine responsible for the development of late normal tissue injury after cancer therapy. Monitoring TGF-β1 in the plasma and/or screening for TGF-β1 polymorphisms may help to identify patients at greater or lesser risk for normal tissue injury, but more work is needed to determine the optimal situations in which to apply this molecular and genetic information in the clinic. Strategies to target TGF-β1 clearly reduce the severity of normal tissue injury in animal models. This approach needs to be tested prospectively in the clinic to determine if inhibiting the TGF-β1 pathway is safe and effective in humans.
References
- 1.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 2.Milano MT, Constine LS, Okunieff P. Normal tissue tolerance dose metrics for radiation therapy of major organs. Semin Radiat Oncol. 2007;17:131–140. doi: 10.1016/j.semradonc.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: Pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys. 2005;63:5–24. doi: 10.1016/j.ijrobp.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 4.Kong FM, Pan C, Eisbruch A, et al. Physical models and simpler dosimetric descriptors of radiation late toxicity. Semin Radiat Oncol. 2007;17:108–120. doi: 10.1016/j.semradonc.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: Mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006;66:1281–1293. doi: 10.1016/j.ijrobp.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 6.Brush J, Lipnick SL, Phillips T, et al. Molecular mechanisms of late normal tissue injury. Semin Radiat Oncol. 2007;17:121–130. doi: 10.1016/j.semradonc.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Rodemann HP, Blaese MA. Responses of normal cells to ionizing radiation. Semin Radiat Oncol. 2007;17:81–88. doi: 10.1016/j.semradonc.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Travis EL. Genetic susceptibility to late normal tissue injury. Semin Radiat Oncol. 2007;17:149–155. doi: 10.1016/j.semradonc.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Anscher MS, Chen L, Rabbani Z, et al. Recent progress in defining mechanisms and potential targets for prevention of normal tissue injury after radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62:255–259. doi: 10.1016/j.ijrobp.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 10.Laiho M, Keski-Oja J. Transforming growth factors-beta as regulators of cellular growth and phenotype. Crit Rev Oncog. 1992;3:1–26. [PubMed] [Google Scholar]
- 11.Massagué J, Cheifetz S, Laiho M, et al. Transforming growth factor-beta. Cancer Surv. 1992;12:81–103. [PubMed] [Google Scholar]
- 12.Massagué J, Blain SW, Lo RS. TGFβ signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 13.Lamouille S, Derynck R. Cell size and invasion in TGF-β-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;178:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derynck R, Akhurst RJ. Differentiation plasticity regulated by TGF-β family proteins in development and disease. Nat Cell Biol. 2007;9:1000–1004. doi: 10.1038/ncb434. [DOI] [PubMed] [Google Scholar]
- 15.Massagué J. TGFβ in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor IW, Wrana JL. SnapShot: The TGFβ pathway interactome. Cell. 2008;133:378.e1. doi: 10.1016/j.cell.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Elliott RL, Blobe GC. Role of transforming growth factor beta in human cancer. J Clin Oncol. 2005;23:2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Wang XF, Sun L. Expression of transforming growth factor beta (TGFβ) type III receptor restores autocrine TGFβ1 activity in human breast cancer MCF-7 cells. J Biol Chem. 1997;272:12862–12867. doi: 10.1074/jbc.272.19.12862. [DOI] [PubMed] [Google Scholar]
- 19.Grady WM, Myeroff LL, Swinler SE, et al. Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res. 1999;59:320–324. [PubMed] [Google Scholar]
- 20.Villanueva A, Garcia C, Paules AB, et al. Disruption of the antiproliferative TGF-β signaling pathways in human pancreatic cancer cells. Oncogene. 1998;17:1969–1978. doi: 10.1038/sj.onc.1202118. [DOI] [PubMed] [Google Scholar]
- 21.Yan Z, Deng X, Friedman E. Oncogenic Ki-ras confers a more aggressive colon cancer phenotype through modification of transforming growth factor-beta receptor III. J Biol Chem. 2001;276:1555–1563. doi: 10.1074/jbc.M004553200. [DOI] [PubMed] [Google Scholar]
- 22.Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 23.Branton MH, Kopp JB. TGF-β and fibrosis. Microbes Infect. 1999;1:1349–1365. doi: 10.1016/s1286-4579(99)00250-6. [DOI] [PubMed] [Google Scholar]
- 24.Border WA, Brees D, Noble NA. Transforming growth factor-beta and extracellular matrix deposition in the kidney. Contrib Nephrol. 1994;107:140–145. doi: 10.1159/000422972. [DOI] [PubMed] [Google Scholar]
- 25.Munger J, Huang X, Kawakatsu H, et al. The integrin αvß6 binds and activates latent TGFβ1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence D. Identification and activation of latent transforming growth factor β. Methods Enzymol. 1991;198:327–336. doi: 10.1016/0076-6879(91)98033-3. [DOI] [PubMed] [Google Scholar]
- 27.Koli K, Saharinen J, Hyytiäinen M, et al. Latency, activation, and binding proteins of TGF-β. Microsc Res Tech. 2001;52:354–362. doi: 10.1002/1097-0029(20010215)52:4<354::AID-JEMT1020>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Godár S, Horejsi V, Weidle UH, et al. M6P/IGFII-receptor complexes urokinase receptor and plasminogen for activation of transforming growth factor-ß1. Eur J Immunol. 1999;29:1004–1013. doi: 10.1002/(SICI)1521-4141(199903)29:03<1004::AID-IMMU1004>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 29.Blakytny R, Ludlow A, Martin GE, et al. Latent TGF-β1 activation by platelets. J Cell Physiol. 2004;199:67–76. doi: 10.1002/jcp.10454. [DOI] [PubMed] [Google Scholar]
- 30.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-β1. Mol Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 31.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFβ activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 32.Ashcroft GS. Bidirectional regulation of macrophage function by TGF-β. Microbes Infect. 1999;1:1275–1282. doi: 10.1016/s1286-4579(99)00257-9. [DOI] [PubMed] [Google Scholar]
- 33.Boyd F, Massagué J. Transforming growth factor-β inhibition of epithelial cell proliferation linked to the expression of a 53-kDa membrane receptor. J Biol Chem. 1989;264:2272–2278. [PubMed] [Google Scholar]
- 34.Hakenjos L, Bamberg M, Rodemann H. TGF-ß1-mediated alterations of rat lung fibroblast differentiation resulting in the radiation-induced fibrotic phenotype. Int J Radiat Biol. 2000;76:503–509. doi: 10.1080/095530000138501. [DOI] [PubMed] [Google Scholar]
- 35.Fajardo LF, Prionas SD, Kwan HH, et al. Transforming growth factor β1 induces angiogenesis in vivo with a threshold pattern. Lab Invest. 1996;74:600–608. [PubMed] [Google Scholar]
- 36.Phillips GD, Whitehead RA, Stone AM, et al. Transforming growth factor beta (TGF-B) stimulation of angiogenesis: An electron microscopic study. J Submicroscop Cytol Pathol. 1993;25:149–155. [PubMed] [Google Scholar]
- 37.Roberts AB, McCune BK, Sporn MB. TGFβ regulation of extracellular matrix. Kidney Int. 1992;41:557–559. doi: 10.1038/ki.1992.81. [DOI] [PubMed] [Google Scholar]
- 38.Peters H, Border WA, Noble NA. Targeting TGF-β overexpression in renal disease: Maximizing the antifibrotic action of angiotensin II blockade. Kidney Int. 1998;54:1570–1580. doi: 10.1046/j.1523-1755.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 39.Border WA, Ruoslahti E. Transforming growth factor-ß in disease: The dark side of tissue repair. J Clin Invest. 1992;90:1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest. 2004;125:754–765. doi: 10.1378/chest.125.2.754. [DOI] [PubMed] [Google Scholar]
- 41.Anscher MS, Kong FM, Jirtle RL. The relevance of transforming growth factor beta 1 in pulmonary injury after radiation therapy. Lung Cancer. 1998;19:109–120. doi: 10.1016/s0169-5002(97)00076-7. [DOI] [PubMed] [Google Scholar]
- 42.Isaka Y, Brees DK, Ikegaya K, et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med. 1996;2:418–423. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- 43.Akagi Y, Isaka Y, Arai M, et al. Inhibition of TGF-β 1 expression by antisense oligonucleotides suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int. 1996;50:148–155. doi: 10.1038/ki.1996.297. [DOI] [PubMed] [Google Scholar]
- 44.Tsushima H, Kawata S, Tamura S, et al. Reduced plasma transforming growth factor-ß1 levels in patients with chronic hepatitis C after interferon-α therapy: Association with regression of hepatic fibrosis. J Hepatol. 1999;30:1–7. doi: 10.1016/s0168-8278(99)80001-4. [DOI] [PubMed] [Google Scholar]
- 45.Border W, Okuda S, Languino LR, et al. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990;346:371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- 46.Rabbani ZN, Batinic-Haberle I, Anscher MS, et al. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67:573–580. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabbani ZN, Anscher MS, Zhang X, et al. Soluble TGFβ type II receptor gene therapy ameliorates acute radiation-induced pulmonary injury in rats. Int J Radiat Oncol Biol Phys. 2003;57:563–572. doi: 10.1016/s0360-3016(03)00639-4. [DOI] [PubMed] [Google Scholar]
- 48.Rabbani ZN, Anscher MS, Golson ML, et al. Overexpression of extracellular superoxide dismutase reduces severity of radiation-induced lung toxicity through downregulation of the TGF-β signal transduction pathway. Int J Radiat Oncol Biol Phys. 2003;57:S158–S159. doi: 10.1016/s0360-3016(03)01369-5. [DOI] [PubMed] [Google Scholar]
- 49.Nishioka A, Ogawa Y, Mima T, et al. Histopathologic amelioration of fibroproliferative change in rat irradiated lung using soluble transforming growth factor-beta (TGF-β) receptor mediated by adenoviral vector. Int J Radiat Oncol Biol Phys. 2004;58:1235–1241. doi: 10.1016/j.ijrobp.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Nakao A, Fujii M, Matsumura R, et al. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J Clin Invest. 1999;104:5–11. doi: 10.1172/JCI6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang S, Rabbani Z, Folz R, et al. Overexpression of extracellular superoxide dismutase protects mice from radiation induced lung injury. Int J Radiat Oncol Biol Phys. 2002;54:78. doi: 10.1016/s0360-3016(03)01369-5. [DOI] [PubMed] [Google Scholar]
- 52.Giri SN, Hyde DM, Hollinger MA. Effect of antibody to transforming growth factor ß on bleomycin induced accumulation of lung collagen in mice. Thorax. 1993;48:959–966. doi: 10.1136/thx.48.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daniels CE, Wilkes MC, Edens M, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-β and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anscher MS, Crocker IR, Jirtle RL. Transforming growth factor-beta 1 expression in irradiated liver. Radiat Res. 1990;122:77–85. [PubMed] [Google Scholar]
- 55.Franko AJ, Sharplin J, Ghahary A, et al. Immunohistochemical localization of transforming growth factor beta and tumor necrosis factor alpha in the lungs of fibrosis-prone and “non-fibrosing” mice during the latent period and early phase after irradiation. Radiat Res. 1997;147:245–256. [PubMed] [Google Scholar]
- 56.Liguang C, Larrier N, Rabbani ZN, et al. Assessment of the protective effect of keratinocyte growth factor on radiation-induced pulmonary toxicity in rats. Int J Radiat Oncol Biol Phys. 2003;57:S162. doi: 10.1016/j.ijrobp.2004.07.729. [DOI] [PubMed] [Google Scholar]
- 57.Andarawewa KL, Paupert J, Pal A, et al. New rationales for using TGFβ inhibitors in radiotherapy. Int J Radiat Biol. 2007;83:803–811. doi: 10.1080/09553000701711063. [DOI] [PubMed] [Google Scholar]
- 58.Portess DI, Bauer G, Hill MA, et al. Low-dose irradiation of nontransformed cells stimulates the selective removal of precancerous cells via intercellular induction of apoptosis. Cancer Res. 2007;67:1246–1253. doi: 10.1158/0008-5472.CAN-06-2985. [DOI] [PubMed] [Google Scholar]
- 59.Flanders KC, Sullivan CD, Fujii M, et al. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol. 2002;160:1057–1068. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters CA, Stock RG, Cesaretti JA, et al. TGFB1 single nucleotide polymorphisms are associated with adverse quality of life in prostate cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:752–759. doi: 10.1016/j.ijrobp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 61.Anscher MS, Peters WP, Reisenbichler H, et al. Transforming growth factor beta as a predictor of liver and lung fibrosis after autologous bone marrow transplantation for advanced breast cancer. N Engl J Med. 1993;328:1592–1598. doi: 10.1056/NEJM199306033282203. [DOI] [PubMed] [Google Scholar]
- 62.Kong FM, Anscher MS, Murase T, et al. Elevated plasma transforming growth factor beta 1 levels in breast cancer patients decrease after surgical removal of the tumor. Ann Surg. 1995;222:155–162. doi: 10.1097/00000658-199508000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kong F-M, Anscher M, Xiong Z, et al. Elevated circulating transforming growth factor β1 levels decreased after radiotherapy in patients with lung cancer, cervical cancer and Hodgkin's disease: A possible tumor marker. Int J Radiat Oncol Biol Phys. 1995;32(suppl 1):239. [Google Scholar]
- 64.Novakova-Jiresova A, Van Gameren MM, Coppes RP, et al. Transforming growth factor-beta plasma dynamics and post-irradiation lung injury in lung cancer patients. Radiother Oncol. 2004;71:183–189. doi: 10.1016/j.radonc.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 65.De Jaeger K, Seppenwoolde Y, Kampinga HH, et al. Significance of plasma transforming growth factor-beta levels in radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1378–1387. doi: 10.1016/j.ijrobp.2003.09.078. [DOI] [PubMed] [Google Scholar]
- 66.Anscher MS, Kong FM, Andrews K, et al. Plasma transforming growth factor β1 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1998;41:1029–1035. doi: 10.1016/s0360-3016(98)00154-0. [DOI] [PubMed] [Google Scholar]
- 67.Anscher MS, Kong FM, Marks LB, et al. Changes in plasma transforming growth factor beta during radiotherapy and the risk of symptomatic radiation-induced pneumonitis. Int J Radiat Oncol Biol Phys. 1997;37:253–258. doi: 10.1016/s0360-3016(96)00529-9. [DOI] [PubMed] [Google Scholar]
- 68.Anscher MS, Murase T, Prescott DM, et al. Changes in plasma TGF beta levels during pulmonary radiotherapy as a predictor of the risk of developing radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1994;30:671–676. doi: 10.1016/0360-3016(92)90954-g. [DOI] [PubMed] [Google Scholar]
- 69.Kong FM, Ao X, Wang L, et al. The use of blood biomarkers to predict radiation lung toxicity: A potential strategy to individualize thoracic radiation therapy. Cancer Control. 2008;15:140–150. doi: 10.1177/107327480801500206. [DOI] [PubMed] [Google Scholar]
- 70.Fu XL, Huang H, Bentel G, et al. Predicting the risk of symptomatic radiation-induced lung injury using both the physical and biologic parameters V(30) and transforming growth factor beta. Int J Radiat Oncol Biol Phys. 2001;50:899–908. doi: 10.1016/s0360-3016(01)01524-3. [DOI] [PubMed] [Google Scholar]
- 71.Anscher MS, Marks LB, Shafman TD, et al. Using plasma transforming growth factor beta-1 during radiotherapy to select patients for dose escalation. J Clin Oncol. 2001;19:3758–3765. doi: 10.1200/JCO.2001.19.17.3758. [DOI] [PubMed] [Google Scholar]
- 72.Anscher MS, Marks LB, Shafman TD, et al. Risk of long-term complications after TGF-β1-guided very-high-dose thoracic radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:988–995. doi: 10.1016/s0360-3016(03)00184-6. [DOI] [PubMed] [Google Scholar]
- 73.Teicher BA. Malignant cells, directors of the malignant process: Role of transforming growth factor-beta. Cancer Metastasis Rev. 2001;20:133–143. doi: 10.1023/a:1013177011767. [DOI] [PubMed] [Google Scholar]
- 74.Bierie B, Moses HL. Tumour microenvironment: TGFβ: The molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 75.Beck C, Schreiber H, Rowley D. Role of TGF-β in immune-evasion of cancer. Microsc Res Tech. 2001;52:387–395. doi: 10.1002/1097-0029(20010215)52:4<387::AID-JEMT1023>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 76.Shariat SF, Kattan MW, Traxel E, et al. Association of pre- and postoperative plasma levels of transforming growth factor beta(1) and interleukin 6 and its soluble receptor with prostate cancer progression. Clin Cancer Res. 2004;10:1992–1999. doi: 10.1158/1078-0432.ccr-0768-03. [DOI] [PubMed] [Google Scholar]
- 77.Shariat SF, Walz J, Roehrborn CG, et al. Early postoperative plasma transforming growth factor-β1 is a strong predictor of biochemical progression after radical prostatectomy. J Urol. 2008;179:1593–1597. doi: 10.1016/j.juro.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 78.Wikström P, Damber J, Bergh A. Role of transforming growth factor-β1 in prostate cancer. Microsc Res Tech. 2001;52:411–419. doi: 10.1002/1097-0029(20010215)52:4<411::AID-JEMT1026>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 79.Muraoka-Cook RS, Kurokawa H, Koh Y, et al. Conditional overexpression of active transforming growth factor β1 in vivo accelerates metastases of transgenic mammary tumors. Cancer Res. 2004;64:9002–9011. doi: 10.1158/0008-5472.CAN-04-2111. [DOI] [PubMed] [Google Scholar]
- 80.Muraoka RS, Dumont N, Ritter CA, et al. Blockade of TGF-β inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109:1551–1559. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bandyopadhyay A, Agyin JK, Wang L, et al. Inhibition of pulmonary and skeletal metastasis by a transforming growth factor-beta type I receptor kinase inhibitor. Cancer Res. 2006;66:6714–6721. doi: 10.1158/0008-5472.CAN-05-3565. [DOI] [PubMed] [Google Scholar]
- 82.Teicher BA, Ikebe M, Ara G, et al. Transforming growth factor-beta 1 overexpression produces drug resistance in vivo: Reversal by decorin. In Vivo. 1997;11:463–472. [PubMed] [Google Scholar]
- 83.Teicher BA, Holden SA, Ara G, et al. Transforming growth factor-beta in in vivo resistance. Cancer Chemother Pharmacol. 1996;37:601–609. doi: 10.1007/s002800050435. [DOI] [PubMed] [Google Scholar]
- 84.Kong F, Anscher M, Sporn T, et al. Loss of mannose 6-phosphate/insulin-like growth factor receptor (M6P/IGF2R) may lead to an elevated transforming growth factor β1 (TGFβ1) in circulation. Presented at the 45th Annual Meeting of the Radiation Research Society; May 3–7, 1997; Providence, RI. [Google Scholar]
- 85.Dennis PA, Rifkin DB. Cellular activation of latent transforming growth factor ß requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci U S A. 1991;88:580–584. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anscher M, Kong F, Sporn T, et al. Loss of a normally functioning mannose 6-phosphate/insulin-like growth factor 2 receptor may lead to an elevated plasma transforming growth factor ß1 level: Clinical implications. Presented at the 9th Lorne Cancer Conference; February 13–16, 1997; Victoria, Australia. [Google Scholar]
- 87.Wrana JL, Attisano L, Cárcamo J, et al. TGF β signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 88.Chen RH, Ebner R, Derynck R. Inactivation of the type II receptor reveals two receptor pathways for the diverse TGF-β activities. Science. 1993;260:1335–1338. doi: 10.1126/science.8388126. [DOI] [PubMed] [Google Scholar]
- 89.Yang L, Tredget EE, Ghahary A. Activation of latent transforming growth factor-β1 is induced by mannose 6-phosphate/insulin-like growth factor-II receptor. Wound Repair Regen. 2000;8:538–546. doi: 10.1046/j.1524-475x.2000.00538.x. [DOI] [PubMed] [Google Scholar]
- 90.Hankins GR, De Souza AT, Bentley RC, et al. M6P/IGF2 receptor: A candidate breast tumor suppressor gene. Oncogene. 1996;12:2003–2009. [PubMed] [Google Scholar]
- 91.Dahms NM, Lobel P, Kornfeld S. Mannose-6 phosphate receptors and lysosomal enzyme targeting. J Biol Chem. 1989;264:12115–12118. [PubMed] [Google Scholar]
- 92.Kong F, Pulford D, Bandera M, et al. M6P/IGF2R tumor suppressor gene is mutated in lung cancer. Proc Am Assoc Cancer Res. 1999;40:733. [Google Scholar]
- 93.Kong FM, Anscher MS, Sporn TA, et al. Loss of heterozygosity at the mannose 6-phosphate insulin-like growth factor 2 receptor (M6P/IGF2R) locus predisposes patients to radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2001;49:35–41. doi: 10.1016/s0360-3016(00)01377-8. [DOI] [PubMed] [Google Scholar]
- 94.Li Y-Q, Ballinger JR, Nordal RA, et al. Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Res. 2001;61:3348–3354. [PubMed] [Google Scholar]
- 95.Vujaskovic Z, Anscher MS, Feng Q-F, et al. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys. 2001;50:851–855. doi: 10.1016/s0360-3016(01)01593-0. [DOI] [PubMed] [Google Scholar]
- 96.Anscher MS, Thrasher B, Rabbani Z, et al. Antitransforming growth factor-beta antibody 1D11 ameliorates normal tissue damage caused by high-dose radiation. Int J Radiat Oncol Biol Phys. 2006;65:876–881. doi: 10.1016/j.ijrobp.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 97.Falanga V, Zhou L, Yufit T. Low oxygen tension stimulates collagen synthesis and COL1A1 transcription through the action of TGF-β1. J Cell Physiol. 2002;191:42–50. doi: 10.1002/jcp.10065. [DOI] [PubMed] [Google Scholar]
- 98.Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000;58:2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- 99.Westbury CB, Pearson A, Nerurkar A, et al. Hypoxia can be detected in irradiated normal human tissue: A study using the hypoxic marker pimonidazole hydrochloride. Br J Radiol. 2007;80:934–938. doi: 10.1259/bjr/25046649. [DOI] [PubMed] [Google Scholar]
- 100.Haydont V, Mathe D, Bourgier C, et al. Induction of CTGF by TGF-β1 in normal and radiation enteritis human smooth muscle cells: Smad/Rho balance and therapeutic perspectives. Radiother Oncol. 2005;76:219–225. doi: 10.1016/j.radonc.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 101.Haydont V, Riser BL, Aigueperse J, et al. Specific signals involved in the long-term maintenance of radiation-induced fibrogenic differentiation: A role for CCN2 and low concentration of TGF-β1. Am J Physiol Cell Physiol. 2008;294:C1332–C1341. doi: 10.1152/ajpcell.90626.2007. [DOI] [PubMed] [Google Scholar]
- 102.Anscher MS, Thrasher B, Zgonjanin L, et al. Small molecular inhibitor of transforming growth factor-beta protects against development of radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2008;71:829–837. doi: 10.1016/j.ijrobp.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 103.Riley PA. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 104.Kang SK, Rabbani ZN, Folz RJ, et al. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2003;57:1056–1066. doi: 10.1016/s0360-3016(03)01369-5. [DOI] [PubMed] [Google Scholar]
- 105.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, et al. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33:857–863. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 106.Inomata S, Takahashi H, Nagata M, et al. Acute lung injury as an adverse event of gefitinib. Anticancer Drugs. 2004;15:461–467. doi: 10.1097/01.cad.0000127666.12215.7b. [DOI] [PubMed] [Google Scholar]
- 107.Haston CK, Travis EL. Murine susceptibility to radiation-induced pulmonary fibrosis is influenced by a genetic factor implicated in susceptibility to bleomycin-induced pulmonary fibrosis. Cancer Res. 1997;57:5286–5291. [PubMed] [Google Scholar]
- 108.Nethery DE, Moore BB, Minowada G, et al. Expression of mutant human epidermal receptor 3 attenuates lung fibrosis and improves survival in mice. J Appl Physiol. 2005;99:298–307. doi: 10.1152/japplphysiol.01360.2004. [DOI] [PubMed] [Google Scholar]
- 109.Faress JA, Nethery DE, Kern EF, et al. Bleomycin-induced pulmonary fibrosis is attenuated by a monoclonal antibody targeting HER2. J Appl Physiol. 2007;103:2077–2083. doi: 10.1152/japplphysiol.00239.2007. [DOI] [PubMed] [Google Scholar]
- 110.Cho HJ, Kang JH, Kim T, et al. Suppression of PAI-1 expression through inhibition of the EGFR-mediated signaling cascade in rat kidney fibroblast by ascofuranone. J Cell Biochem. 2009;107:335–344. doi: 10.1002/jcb.22130. [DOI] [PubMed] [Google Scholar]
- 111.Wang Q, Wang Y, Hyde DM, et al. Reduction of bleomycin induced lung fibrosis by transforming growth factor beta soluble receptor in hamsters. Thorax. 1999;54:805–812. doi: 10.1136/thx.54.9.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kolb M, Margetts PJ, Galt T, et al. Transient transgene expression of decorin in the lung reduces the fibrotic response to bleomycin. Am J Respir Crit Care Med. 2001;163:770–777. doi: 10.1164/ajrccm.163.3.2006084. [DOI] [PubMed] [Google Scholar]
- 113.Stone HB, Coleman CN, Anscher MS, et al. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol. 2003;4:529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 114.Koli K, Myllärniemi M, Keski-Oja J, et al. Transforming growth factor-beta activation in the lung: Focus on fibrosis and reactive oxygen species. Antioxid Redox Signal. 2008;10:333–342. doi: 10.1089/ars.2007.1914. [DOI] [PubMed] [Google Scholar]