The study evaluates the prognostic factors and morbidities of patients undergoing completion surgery for locally advanced-stage cervical cancer after initial chemoradiation therapy.
Keywords: Chemoradiation therapy, Completion surgery, Locally advanced cervical cancer, Morbidities, Nodal involvement, Prognostic factors, Residual disease, Survival
Learning Objectives
After completing this course, the reader will be able to:
Rate the prognostic factors for overall survival in patients undergoing completion surgery after initial chemoradiation therapy (CRT) for locally advanced cervical cancer.
In cervical cancer patients undergoing completion surgery, consider using laparoscopy to decrease the morbidity of the surgery.
In cervical cancer patients undergoing completion surgery, use PET-CT imaging to improve detection of para-aortic involvement.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Purpose.
The aim of this study was to evaluate the prognostic factors and morbidities of patients undergoing completion surgery for locally advanced-stage cervical cancer after initial chemoradiation therapy (CRT).
Patients and Methods.
Patients fulfilling the following inclusion criteria were studied: stage IB2–IVA cervical carcinoma, tumor initially confined to the pelvic cavity on conventional imaging, pelvic external radiation therapy with delivery of 45 Gy to the pelvic cavity and concomitant chemotherapy (cisplatin, 40 mg/m2 per week) followed by uterovaginal brachytherapy, and completion surgery after the end of radiation therapy including at least a hysterectomy.
Results.
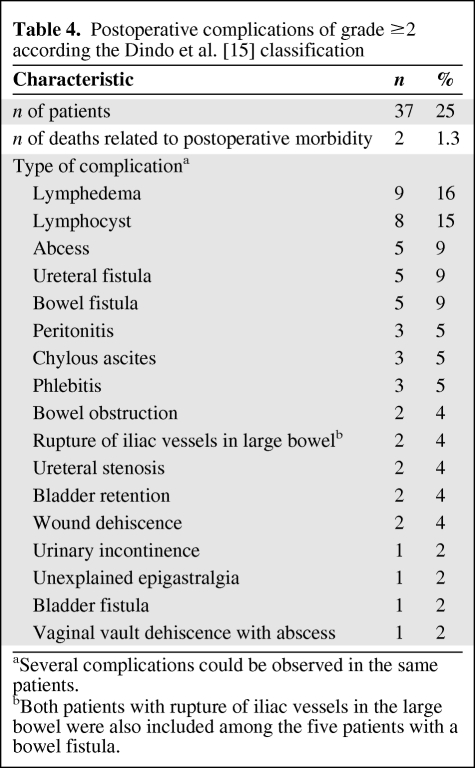
One-hundred fifty patients treated in 1998–2007 fulfilled the inclusion criteria. Prognostic factors for overall survival in the multivariate analysis were the presence and level of nodal spread (positive pelvic nodes alone: hazard ratio [HR], 2.03; positive para-aortic nodes: HR, 5.46; p < .001) and the presence and size of residual disease (RD) in the cervix (p = .02). Thirty-seven (25%) patients had 55 postoperative complications. The risk for complications was higher with a radical hysterectomy (p = .04) and the presence of cervical RD (p = .01).
Conclusion.
In this series, the presence and size of RD and histologic nodal involvement were the strongest prognostic factors. Such results suggest that the survival of patients treated using CRT for locally advanced cervical cancer could potentially be enhanced by improving the rate of complete response in the irradiated area (cervix or pelvic nodes) and by initially detecting patients with para-aortic spread so that treatment could be adapted in such patients. The morbidity of completion surgery is high in this context.
Introduction
Chemoradiation therapy (CRT) is considered the standard treatment for bulky cervical cancer (stage ≥IB2 according to the Fédération Internationale de Gynécologie et d'Obstetrique [FIGO] classification) by many American and European teams [1, 2]. In such patients, the role and modalities of completion surgery (after CRT) continue to be debated. In the literature, very few data are available on the results of completion surgery in patients treated with CRT [3–10]. Nevertheless, even if the therapeutic impact of completion hysterectomy continues to fuel debate, the analysis of prognostic factors (and mainly histologic factors) in hysterectomy and lymphadenectomy specimens could add interesting data in order to improve local and distant control for future patients undergoing CRT. This is the aim of this study. Morbidities of completion surgery in this context were also studied.
Patients and Methods
Patients treated at our institution from January 1998 to December 2007 fulfilling the following inclusion criteria were retrospectively reviewed and included in the present study.
Patients had to have a diagnosis of stage IB2–IVA cervical carcinoma according to the 1995 FIGO classification [11]). The pathologic subtypes included were squamous cell carcinoma, adenocarcinoma, adenosquamous carcinoma, glassy cell carcinoma, and small cell carcinoma. Tumors had to be radiologically confined to the pelvic cavity (no para-aortic nodes >1 cm) on initial abdominopelvic magnetic resonance imaging (MRI) or CT scan and pelvic MRI.
Patients were treated with pelvic external radiation therapy delivering 45 Gy to the pelvic cavity and concurrent chemotherapy (cisplatin, 40 mg/m2 per week). CRT was followed by uterovaginal brachytherapy performed at our institute. The brachytherapy dose was 15 Gy according to the International Commission on Radiation Units recommendations [12]. Since 2004, some patients were subjected to three-dimensional (3-D) MRI-guided brachytherapy according to the recommendations of the GEC-ESTRO group in order to deliver pulsed-dose-rate (PDR) brachytherapy [13]. Some patients with parametrial spread and/or bulky pelvic nodes on initial imaging received a pelvic lateral boost of 10–15 Gy.
The use of completion surgeries performed at our institution included at least a (radical) hysterectomy. During the study period, completion surgery after CRT was systematically used in patients with stage IB2–II cervical cancer (except for a few patients included in a randomized trial assessing the value of completion surgery). Surgery wasn't performed in patients with distant metastasis confirmed histologically. Surgery was conventionally performed 8–10 weeks after completion of brachytherapy. In 1996–2001, the type of radical hysterectomy performed was a Piver II or III procedure [14]. In order to decrease urinary tract morbidity, since 2001, a simple extrafascial hysterectomy was performed in patients who achieved a clinical and radiological complete response after brachytherapy.
During this pelvic surgery, nodal dissection could be added. Most of the patients had undergone complete para-aortic lymphadenectomy up to the left renal vessels. In terms of the surgical policy regarding pelvic nodes, all patients treated in 1998–2001 underwent a complete pelvic lymphadenectomy. Since 2001, in order to decrease the rate of postoperative morbidity related to this complete lymphadenectomy in a previously irradiated area (particularly the risk for permanent lymphedema), a selective lymphadenectomy was performed in patients with residual lymphadenomegaly detected during the surgical procedure.
Statistical Analysis
The statistical analysis was performed with SAS version 9.1 software (SAS Institute, Inc., Cary, NC). Association between factors was assessed by χ2 tests or Fisher's exact tests. Postoperative complications were extracted from medical charts. We took into account all complications up to 90 days following surgery. The rate of lymphedema was studied without a time limit. Morbidities were classified according to the classification of Dindo et al. [15].
The median follow-up duration was estimated using Schemper's test [16]. Survival differences were compared using the log-rank test. To determine the independent prognostic significance of factors for survival, a multivariate analysis was conducted using the Cox proportional hazards regression method. Variables attaining significance at a p-value of .25 in the univariate analysis were retained for the multivariate analysis. Variables with a p-value < .05 in the multivariate analysis were considered significant prognostic factors for survival. The overall survival time was defined as the time between surgery and death from any cause, or the last follow-up for patients still alive. The event-free survival time was defined as the time between surgery and the first event (local or distant recurrence or death), or the last follow-up for patients free from recurrence.
Results
One hundred fifty patients fulfilled the inclusion criteria. The median age of the patients was 47 years (range, 19–77 years). The distribution of disease stages was as follows: stage IB2, n = 48 (32%); stage II, n = 91 (61%); stage III, n = 10 (7%), and stage IV, n = 1. The distribution of histologic subtypes was as follows: squamous cell carcinoma, n = 108 (72%); adenocarcinoma, n = 26 (17%); and other (and/or mixed) subtypes, n = 16 (11%).
Initial Imaging
At the pretherapeutic abdominopelvic MRI or CT scan, 49 patients were found to have enlarged pelvic lymph nodes. Six patients had enlarged para-aortic nodes, but all were <1 cm (five of them had suspicious nodes in the pelvic area). Twenty-four patients underwent initial positron emission tomography (PET)-CT imaging. Ten of those had pelvic node uptake and one also had uptake in the para-aortic area.
Treatment Modalities
All patients received CRT in the pelvic cavity (45 Gy) and brachytherapy. CRT modalities, including brachytherapy, are reported in Table 1.
Table 1.
Radiochemotherapy modalities (n = 150 patients)
Abbreviations: LDR, low dose rate; PDR, pulsed dose rate.
Details concerning surgical treatments are given in Table 2. Among the 54 patients who underwent a pelvic lymph node resection, 34 had a complete lymphadenectomy and 20 had a selective adenectomy. All patients treated laparoscopically underwent an extrafascial hysterectomy (with para-aortic lymphadenectomy in 22 patients).
Table 2.
Patient surgical and pathological characteristics
aRate not determined because pelvic lymphadenectomy was performed mainly in cases of suspicious lymph nodes during surgery.
Histologic Results at the Time of Completion Surgery
Seventy-eight (52%) patients had histologic residual disease (RD) in the cervix. The sizes of the cervical RD were ≤1 cm in 38 cases (25%)—32 were <2 mm and six were 2–10 mm—and >1 cm in 34 patients (23%); size was missing in four cases. Involvement of the surgical margins was observed in nine pathological specimens (7%). Lymphatic node involvement was diagnosed in 28 cases (19%). Table 3 shows the association among nodal spread, surgical margin status, and the size of the RD.
Table 3.
Association between nodal status and status of surgical margins and the presence and size of cervical residual disease (RD) on the hysterectomy specimen (144 patients)
aOne patient without residual disease in the cervix but one single lymphovascular space involvement in the vagina.
Among six patients with enlarged (but <1 cm) para-aortic lymph nodes on initial conventional imaging (MRI or CT scan), four underwent a para-aortic lymphadenectomy and two had positive nodes.
Among the 29 patients with enlarged pelvic lymph nodes on initial imaging who received a lateropelvic boost (10–15 Gy), eight had nodal disease after a pelvic lymphadenectomy at the time of completion surgery. In the group of 19 patients with enlarged pelvic lymph nodes on initial imaging who did not receive a lateropelvic boost, five had involved nodes after the pelvic lymphadenectomy.
Complications
No major intraoperative morbidity (urinary tract, bowel, or vascular injuries) was observed. Thirty-seven patients (25%) had 55 postoperative complications. Details of these complications are shown in Table 4. The factors increasing the risk for postoperative complications were a radical hysterectomy, compared with an extrafascial hysterectomy (odds ratio, [OR], 2.4; p = .04), and the presence of cervical RD ≤1 cm (OR, 4.3) or >1 cm (OR, 2.5), compared with no RD (p = .01) (Table 5).
Table 4.
Postoperative complications of grade ≥2 according the Dindo et al. [15] classification
aSeveral complications could be observed in the same patients.
bBoth patients with rupture of iliac vessels in the large bowel were also included among the five patients with a bowel fistula.
Table 5.
Risk factor for postoperative morbidity
Results in italics are results of the variable added to the final model (containing pelvic surgery and residual cervical disease).
Abbreviations: CI, confidence interval; MD, missing data; OR, odds ratio.
Recurrences
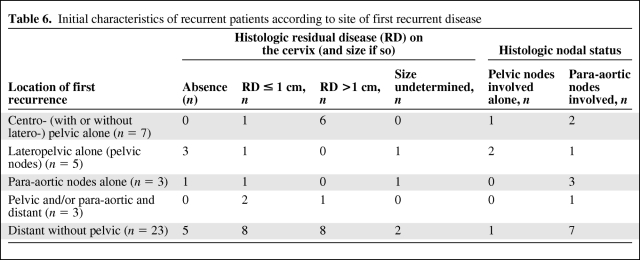
The median follow-up duration was 3.6 years (range, 0.2–10.6 years). Four patients were lost to follow-up postoperatively. During follow-up, 41 patients (27%) developed a recurrence. Thirty-seven patients died of recurrent disease. Table 6 shows the distribution of patients according to the location of the first recurrence, the nodal status, and the presence and size of RD in the cervix.
Table 6.
Initial characteristics of recurrent patients according to site of first recurrent disease
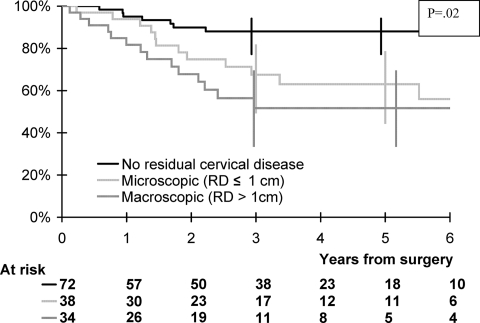
Among the 14 patients who received PDR brachytherapy, only one developed a pelvic recurrence. The overall survival rates at 1 year and 5 years were 91% (95% confidence interval [CI], 85%–95%) and 71% (95% CI, 61%–78%), respectively (Fig. 1). The event-free survival rates at 1 year and 5 years were 83% (95% CI, 77%–89%) and 66% (95% CI, 57%–75%), respectively.
Figure 1.
Overall survival according to the size of residual disease (RD) on the hysterectomy specimen.
Prognostic Factors
Overall survival was found to be associated with pathological response and lymph node involvement in the multivariate analysis. Patients with millimetric RD or RD ≤1 cm and patients with RD >1 cm had a higher risk for death than patients without RD (HR, 1.92 and 3.85, respectively; p = .02). Patients with positive pelvic nodes and positive para-aortic nodes had a higher risk for death than patients without nodal involvement (HR, 2.03 and 5.46, respectively; p < .001) (Table 7). Pathological response (HR, 2.18 for millimetric RD or RD ≤1 cm and 3.99 for RD >1 cm; p = .008) and lymph node involvement (HR, 1.76 for pelvic nodes and 4.81 for para-aortic nodes; p < .001) were associated with a higher risk for events. Overall survival curves according to the presence and size (if so) of RD are shown in Figure 1.
Table 7.
Prognostic factors for overall survival
aItalic results are results of the variable adding to final model (containing residual cervical disease and nodal status).
bReference class.
cNot included in the multivariate analysis because of the association with residual cervical disease (correlation = −0.31).
dLymph node status determined after histologic analysis of lymphadenectomy.
Abbreviations: CI, confidence interval; FIGO, Fédération Internationale de Gynécologie et d'Obstetrique; HR, hazard ratio; MD, missing data; SE, standard error.
Discussion
Several teams consider CRT as a “neoadjuvant” therapy, adding a hysterectomy at the end of treatment [17]. A number of retrospective studies have been published concerning the results of this surgical procedure in this context. Those studies demonstrated that such surgery is “feasible” and “beneficial” in terms of removing RD [3–10, 17]. Nevertheless, those papers were unable to demonstrate any survival advantage in patients subjected to completion surgery because they all reported on their experience of patients treated surgically without comparing them with a control group of patients exclusively managed with CRT. Furthermore, in most of those papers, the CRT modalities were heterogeneous. This is why our study focused on a population of patients with very strict inclusion criteria concerning CRT in order to improve the reliability of the results observed.
Survival rates reported in the present series seem to be very close or similar to those reported by teams who manage patients exclusively with definitive CRT [1, 2, 18]. Nevertheless, the aim of this study was not to try to demonstrate the therapeutic value of completion surgery after CRT in locally advanced cervical cancer, because only a randomized trial could adequately explore this crucial question. A trial was opened in France 6 years ago (randomizing patients with a macroscopic and radiologic complete response after CRT between extrafascial hysterectomy and no hysterectomy), but it was closed because of insufficient accrual. Since the closure of that trial, at our institution, completion surgery is exclusively considered in the group of patients with persistent disease 8–10 weeks after brachytherapy. Our strategy is therefore to perform clinical and MRI evaluation to diagnose any RD. In cases of a clinical and radiological complete response, no surgery is performed. In cases of RD, a simple extrafascial hysterectomy (type A from Querleu and Morrow's classification [19]) is performed, or radical hysterectomy fitting the disease when possible.
Data concerning prognostic factors are important because histological results concerning the lymph nodes and cervix after CRT could help us to understand the natural history of treatment failure. We could then attempt to improve the modalities of CRT and local and distant control of disease for future patients.
The first important prognostic factor in the multivariate analysis was the presence of RD in the cervix. The rate of RD we observed is very close to that of other different series, which was in the range of 20%–50% [17]. In theory, this could be a strong plea for completion surgery. Nevertheless, finding histologic RD and removing it does not necessarily imply a survival improvement. One half to two thirds of the patients with RD had millimetric RD, and many of them would have had total surgical sterilization of the cervix if surgery had been performed later. In patients with larger RD (>1 cm in the current series or >2 cm in other series [6, 10, 20]), surgery has a theoretical major therapeutic impact. But given the greater risk for extracervical disease (nodal spread or distant disease) in patients with RD, the real impact on survival of completion surgery in this subgroup remains unproven and is still debated [6, 20].
Given the frequency of histologic RD, the burning question is how to improve local control of the disease without significantly increasing morbidity (as we observed after completion surgery). The ideal solution is to improve the delivery of radiation therapy, particularly at the time of brachytherapy. The new modalities of this treatment (with 3-D MRI-guided procedures [13]) that are now becoming the new standard for this approach seem to clearly reduce the rate of local failure in this context [13, 21]. Fourteen patients received such treatment in this series and only one had (pelvic) failure.
The second major prognostic factor in the current series is histological nodal status. The rate of patients with positive para-aortic nodes was very high in the current series: 14% of patients had nodal involvement above the level of radiation therapy fields. In this series, among the 127 patients without enlarged para-aortic lymph nodes on conventional imaging who had undergone para-aortic lymphadenectomy, 17 (13%) had positive para-aortic nodes. Some of them could have experienced disease “progression” during CRT because this para-aortic spread may not have been present initially. However, most of them probably would have had such spread that was not visible during conventional imaging at the time of initial management.
This rate is high but is similar to that reported in a previous study [22]. Furthermore, the survival of patients with para-aortic nodal involvement at the time of completion surgery is very poor [22]. Lymphadenectomy at the time of completion surgery is probably pointless or of very limited value in terms of improving the survival of patients with para-aortic spread [22]. Thus, the next step is to improve the detection of para-aortic involvement. PET-CT imaging is a major asset in this context [23, 24]. Several excellent papers have clearly suggested longer survival in patients undergoing treatment based on PET-CT imaging [23, 24]. Over the last 3 years, this imaging modality has been systematically performed at our institution. However, although PET-CT imaging fails to exhibit uptake in the para-aortic area, we know that nearly 10% of patients have nodal involvement [25, 26]. This is the rationale behind the inclusion of laparoscopic para-aortic staging surgery in such patients to extend radiation fields in cases of positive para-aortic nodes [27, 28]. Even if the value of such management is still under debate [29], several papers suggest longer survival in patients undergoing surgical staging [30]. This surgery is now systematically performed at our institution in “operable” patients without uptake in the para-aortic area [25]. Such para-aortic lymphadenectomy is performed up to the level of the left vein [31]. The objective of this strategy is to extend the external radiation therapy field to the para-aortic region in cases of para-aortic node disease.
The most “problematic” result of the current analysis concerns the number of patients with positive residual nodes in an irradiated area (19 patients). Houvenaeghel et al. [32] and Ferrandina et al. [33] previously reported on residual pelvic lymph nodes after CRT, having observed 18% and 11% positive pelvic nodes, respectively. Such important data do not plead for us in favor of adding a pelvic lymphadenectomy at the time of completion surgery in this context because the lymphatic morbidity incurred by such a procedure is high in irradiated patients (lymphedema) and its usefulness in terms of optimizing survival is not proven.
However, this important observation raises the question of the optimization of pelvic nodal control in this context [34, 35]. The most appropriate procedure for optimizing complete nodal sterilization is the use of a lateropelvic boost of 10–15 Gy in patients exhibiting enlarged nodes on conventional imaging. Yet in this series of 29 patients who underwent lateropelvic boost, eight still had positive pelvic lymph nodes at the time of surgery, which means that the boost was probably not sufficient to completely sterilize bulky pelvic lymph nodes [34]. A new regimen of concurrent chemotherapy and/or image-guided intensity-modulated radiation therapy would probably increase the rate of complete sterilization [36]. In the present series, six patients had histologic nodal involvement, although no suspicious pelvic lymph nodes were observed on conventional imaging (and so no lateropelvic boost was performed). Furthermore, in the subgroup of five patients who had lateropelvic recurrence (considered pelvic nodal recurrence, Table 6), two had positive lymph nodes after lymphadenectomy, and three other patients did not undergo lymphadenectomy. Three of these five patients had complete sterilization of the cervical tumor (suggesting the absence of disease progression during CRT), and those patients died of their lateropelvic recurrence. Those patients probably had initial spread in the pelvic lymph nodes that was misdiagnosed on conventional imaging. This observation is a strong argument in favor of using a lateropelvic boost in patients without enlarged lymph nodes on initial conventional imaging but exhibiting uptake in pelvic nodes during PET-CT imaging. This strategy is now used at our institution in this context.
The second results from the current series concern the morbidity of completion surgery in this context. We did not investigate the morbidity of combination CRT followed by completion surgery. If this had been the case, the interval of 3 months after the end of treatment would have clearly been too short to accurately evaluate this issue. In an excellent paper by Eifel et al. [37] published before the era of CRT, the rates of major morbidity at 3 and 5 years in a cohort of 1,784 patients treated for stage IB disease using radiation therapy (with completion hysterectomy in 234) were 7.7% and 9.3%, respectively. After 5 years, there was a continuous risk of 0.34% per year for major morbidity, with a 14.4% rate of major complications at 20 years [37]. Thus, a longer follow-up would be required to evaluate the morbidity of the entire treatment. Furthermore, the morbidity of CRT itself is now relatively well evaluated [37, 38]. The aim of the study at a time when the usefulness of completion surgery is being questioned is to evaluate the morbidity directly related to the surgical procedure itself. This is why a period of 3 months after surgery seemed appropriate to answer to this question.
One of the major difficulties of studying morbidities in a retrospective study is the high risk of underreporting real morbidity rates. In the present series, we included morbidity ≥2 (i.e., morbidity requiring specific treatment) according to the Dindo et al. [15] classification. “Minor” morbidities (grade 1 in the Dindo et al. classification [15]) were not reported in this series because the analysis of such complications would probably not have been so accurate.
We also included lymphedema, which is rarely reported in different analyses of morbidity because it can really deeply impair the quality of life of patients and is mainly observed >3 months after surgery. It was included also because the risk for lymphedema exists in patients treated exclusively with radiation therapy, but it is low [37, 38]. However, this risk is clearly higher in patients subjected to combination surgery (particularly lymph node dissection) and radiation therapy [38]. In the randomized trial published by Landoni et al. [38], the rate of lymphedema was 0.6% in patients treated for early-stage cervical cancer using radiation therapy alone and 9% in patients treated with surgery and external radiation therapy. In the paper by Eifel et al. [37], among the seven patients who experienced “leg edema,” six had undergone lymph node surgery combined with radiation therapy, and only one patient was treated with radiation therapy alone. In the series by Perez et al. [39] involving 811 patients treated with radiation therapy, only one case of leg edema was observed.
Even with the potential limit of underreporting in the current study, our series clearly demonstrates a very high morbidity rate after hysterectomy following CRT. Two patients died of postoperative complications and the rate of “major” digestive or urinary tract complications (fistula or stenosis) was close to 15%. Two patients developed major vascular complications.
Three groups of complications were mainly observed: lymphadenectomy-related morbidities, urinary or digestive tract morbidities, and infectious morbidities (peritonitis or a deep abscess) treated using further surgery. The last two groups of complications were strongly correlated because peritonitis or a deep abscess often occurred secondary to a urinary or bowel fistula. In the paper by Eifel et al. [37], the risk for digestive or urinary tract fistula was double in patients who underwent a hysterectomy (and in that series only an extrafascial procedure was performed), compared with patients treated with radiation therapy alone (2.6% versus 5.3%; p = .04). Those complications were strongly correlated with the type of surgery used: the rate of ureteral stenosis or fistula or bowel fistula was greater in cases of more radical hysterectomy (with parametrial dissection). This phenomenon was previously reported at the time of pelvic surgery in patients treated with initial external radiation therapy [40]. We also observed a greater rate of morbidity in patients subjected to parametrial dissection in the present study. Such radical hysterectomies were statistically more frequently used in patients with RD in order to ensure clear surgical margins. These two factors (radical hysterectomy and RD) were correlated (Table 3). This result clearly suggests that systematic radical hysterectomy should be avoided in this context (if completion surgery is considered). This might have been feasible given the low risk for parametrial (7%) or vaginal (9%) involvement in our patients (Table 2).
Basically, if completion surgery is discussed (it has been proposed systematically by several teams) after CRT in patients devoid of macroscopic RD in the cervix, an extrafascial hysterectomy should be considered. A radical hysterectomy is more “logical” in patients with RD to guarantee free margins. However, such a basic proposal would also increase the morbidity of surgery, whereas the therapeutic value of completion surgery in patients with bulky RD (>1 cm or 2 cm according to the series) remains totally unproven because these poor responders also run a higher risk for extrapelvic disease (nodal involvement or distant metastasis) [6, 20]. This point also raises the important question of the evaluation of response (and thus, the potential presence of RD) at the end of CRT (should completion surgery be discussed). Response evaluation is based on a clinical examination and imaging (MRI) performed 6–8 weeks after brachytherapy, but the accuracy of such management is still debated [41]. Perhaps adding diffusion-weighted MRI or PET-CT imaging to predict potential RD could be helpful in this context [42, 43]. However, this last procedure should then be performed at least 3 months after brachytherapy, when the surgical procedure is more difficult (because adhesions and severe fibrosis are more frequent at that time).
A laparoscopic approach could also be a way to decrease the morbidity of the surgery. In the present series, a laparoscopic hysterectomy was used during the last 3 years of the study in a selected group of patients devoid of clinical or radiological RD in the cervix, who had therefore undergone a “simple extrafascial hysterectomy.” This is why we did not find any radical hysterectomies among these laparoscopically treated patients. Logically, no urinary or digestive tract morbidity was observed. Most of the morbidities in laparoscopically treated patients in our series were related to the use of a lymphadenectomy. The differences in the rates of lymphocysts and chylous ascites between the laparoscopic and laparotomic approach were almost of borderline statistical significance. We have no explanation for this higher rate of lymphatic morbidities in the laparoscopy group, but this explains why the use of a laparoscopic approach failed to reduce morbidity in our series. A recent interesting paper published on this topic compared a group of 46 patients undergoing radical hysterectomy by laparoscopy after CRT with a group of 56 patients undergoing similar surgery by a laparoscopic approach [8]. The rate of postoperative complications (particularly urinary fistula) was significantly lower in the laparoscopically treated patients without a higher rate of positive margins [8]. We were unable to conduct such a comparison in our study because no radical hysterectomy was performed laparoscopically.
The morbidity of completion surgery (based on hysterectomy with or without lymphadenectomy) was very high in this group of patients initially treated with CRT for locally advanced cervical cancer. Mortality was observed in 1.3% of cases, and the overall rate of urinary or bowel tract morbidity was close to 12%. The therapeutic value of completion surgery (which remains unproven today) should be weighed against the high morbidity rate in this context. Perhaps a laparoscopic approach could reduce the overall morbidity of completion surgery. Nevertheless, because the therapeutic value of this surgery has not been demonstrated, using an approach that could reduce surgery-related morbidity is not a proof of the usefulness of such surgery in terms of improving survival. Finally, the only certainty about completion surgery after CRT is that it gives rise to a high incidence of morbidity.
Acknowledgment
Lorna Saint Ange edited the manuscript.
Author Contributions
Conception/Design: Philippe Morice, Christine Haie-Meder
Provision of study material or patients: Cyril Touboul, Catherine Uzan, Sebastien Gouy, Patricia Pautier, Catherine Lhommé, Pierre Duvillard, Christine Haie-Meder
Collection and/or assembly of data: Cyril Touboul, Catherine Uzan, Sebastien Gouy, Patricia Pautier, Catherine Lhommé, Pierre Duvillard
Data analysis and interpretation: Philippe Morice, Catherine Uzan, Audrey Mauguen, Sebastien Gouy, Annie Rey, Patricia Pautier, Catherine Lhommé, Pierre Duvillard, Christine Haie-Meder
Manuscript writing: Philippe Morice, Cyril Touboul, Catherine Uzan, Sebastien Gouy, Patricia Pautier, Catherine Lhommé, Pierre Duvillard, Christine Haie-Meder
Final approval of manuscript: Philippe Morice, Cyril Touboul, Catherine Uzan, Audrey Mauguen, Sebastien Gouy, Annie Rey, Patricia Pautier, Catherine Lhommé, Pierre Duvillard, Christine Haie-Meder
References
- 1.Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysis. Lancet. 2001;358:781–786. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 2.Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houvenaeghel G, Lelievre L, Gonzague-Casabianca L, et al. Long-term survival after concomitant chemoradiotherapy prior to surgery in advanced cervical carcinoma. Gynecol Oncol. 2006;100:338–343. doi: 10.1016/j.ygyno.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 4.Ferrandina G, Legge F, Fagotti A, et al. Preoperative concomitant chemoradiotherapy in locally advanced cervical cancer: Safety, outcome, and prognostic measures. Gynecol Oncol. 2007;107(suppl 1):S127–S132. doi: 10.1016/j.ygyno.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Classe JM, Rauch P, Rodier JF, et al. Surgery after concurrent chemoradiotherapy and brachytherapy for the treatment of advanced cervical cancer: Morbidity and outcome: Results of a multicenter study of the GCCLCC (Groupe des Chirurgiens de Centres de Lutte Contre le Cancer) Gynecol Oncol. 2006;102:523–529. doi: 10.1016/j.ygyno.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Azria E, Morice P, Haie-Meder C, et al. Results of hysterectomy in patients with bulky residual disease at the end of chemoradiotherapy for stage IB2/II cervical carcinoma. Ann Surg Oncol. 2005;12:332–337. doi: 10.1245/ASO.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Darus CJ, Callahan MB, Nguyen QN, et al. Chemoradiation with and without adjuvant extrafascial hysterectomy for IB2 cervical carcinoma. Int J Gynecol Cancer. 2008;18:730–735. doi: 10.1111/j.1525-1438.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 8.Colombo PE, Bertrand MM, Gutowski M, et al. Total laparoscopic radical hysterectomy for locally advanced cervical carcinoma (stages IIB, IIA and bulky stages IB) after concurrent chemoradiation therapy: Surgical morbidity and oncological results. Gynecol Oncol. 2009;114:404–409. doi: 10.1016/j.ygyno.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 9.Huguet F, Cojocariu OM, Levy P, et al. Preoperative concurrent radiation therapy and chemotherapy for bulky stage IB2, IIA, and IIB carcinoma of the uterine cervix with proximal parametrial invasion. Int J Radiat Oncol Biol Phys. 2008;72:1508–1515. doi: 10.1016/j.ijrobp.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 10.Ota T, Takeshima N, Tabata T, et al. Adjuvant hysterectomy for treatment of residual disease in patients with cervical cancer treated with radiation therapy. Br J Cancer. 2008;99:1216–1220. doi: 10.1038/sj.bjc.6604619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Federation of Gynecology and Obstetrics. Modifications in the staging for stage I vulvar and stage I cervical cancer. Report of the FIGO Committee on Gynecologic Oncology. International Federation of Gynecology and Obstetrics. Int J Gynaecol Obstet. 1995;50:215–216. [PubMed] [Google Scholar]
- 12.International Commission on Radiation Units (ICRU). Bethesda, MD: ICRU; 1985. Dose and Volume Specification for Reporting Intracavitary Therapy in Gynecology. Report No. 38. [Google Scholar]
- 13.Pôtter R, Haie-Meder C, Van Limbergen E, et al. GEC ESTRO Working Group. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy—3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Piver MS, Rutledge F, Smith JP. Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol. 1974;44:265–272. [PubMed] [Google Scholar]
- 15.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 17.Morice P, Uzan C, Zafrani Y, et al. The role of surgery after chemoradiation therapy and brachytherapy for stage IB2/II cervical cancer. Gynecol Oncol. 2007;107(suppl 1):S122–S124. doi: 10.1016/j.ygyno.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Pötter R, Dimopoulos J, Georg P, et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol. 2007;83:148–155. doi: 10.1016/j.radonc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Querleu D, Morrow CP. Classification of radical hysterectomy. Lancet Oncol. 2008;9:297–303. doi: 10.1016/S1470-2045(08)70074-3. [DOI] [PubMed] [Google Scholar]
- 20.Houvenaeghel G, Lelievre L, Buttarelli M, et al. Contribution of surgery in patients with bulky residual disease after chemoradiation for advanced cervical carcinoma. Eur J Surg Oncol. 2007;33:498–503. doi: 10.1016/j.ejso.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Chargari C, Magné N, Dumas I, et al. Physics contributions and clinical outcome with 3D-MRI-based pulsed-dose-rate intracavitary brachytherapy in cervical cancer patients. Int J Radiat Oncol Biol Phys. 2009;74:133–139. doi: 10.1016/j.ijrobp.2008.06.1912. [DOI] [PubMed] [Google Scholar]
- 22.Delpech Y, Haie-Meder C, Rey A, et al. Para-aortic involvement and interest of para-aortic lymphadenectomy after chemoradiation therapy in patients with stage IB2 and II cervical carcinoma radiologically confined to the pelvic cavity. Ann Surg Oncol. 2007;14:3223–3231. doi: 10.1245/s10434-007-9526-1. [DOI] [PubMed] [Google Scholar]
- 23.Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. 2001;19:3745–3749. doi: 10.1200/JCO.2001.19.17.3745. [DOI] [PubMed] [Google Scholar]
- 24.Wright JD, Dehdashti F, Herzog TJ, et al. Preoperative lymph node staging of early-stage cervical carcinoma by [18F]-fluoro-2-deoxy-D-glucose-positron emission tomography. Cancer. 2005;104:2484–2491. doi: 10.1002/cncr.21527. [DOI] [PubMed] [Google Scholar]
- 25.Boughanim M, Leboulleux S, Rey A, et al. Histologic results of para-aortic lymphadenectomy in patients treated for stage IB2/II cervical cancer with negative [18F] fluorodeoxyglucose positron emission tomography scans in the para-aortic area. J Clin Oncol. 2008;26:2558–2561. doi: 10.1200/JCO.2007.14.3933. [DOI] [PubMed] [Google Scholar]
- 26.Mortier DG, Stroobants S, Amant F, et al. Laparoscopic para-aortic lymphadenectomy and positron emission tomography scan as staging procedures in patients with cervical carcinoma stage IB2-IIIB. Int J Gynecol Cancer. 2008;18:723–729. doi: 10.1111/j.1525-1438.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 27.Leblanc E, Narducci F, Frumovitz M, et al. Therapeutic value of pretherapeutic extraperitoneal laparoscopic staging in locally advanced cervical carcinoma. Gynecol Oncol. 2007;105:304–311. doi: 10.1016/j.ygyno.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Varia MA, Bundy BN, Deppe G, et al. Cervical carcinoma metastatic to para-aortic nodes: Extended field radiation therapy with concomitant 5-fluorouracil and cisplatin chemotherapy: A Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 1998;42:1015–1023. doi: 10.1016/s0360-3016(98)00267-3. [DOI] [PubMed] [Google Scholar]
- 29.Lai CH, Huang KG, Hong JH, et al. Randomized trial of surgical staging (extraperitoneal or laparoscopic) versus clinical staging in locally advanced cervical cancer. Gynecol Oncol. 2003;89:160–167. doi: 10.1016/s0090-8258(03)00064-7. [DOI] [PubMed] [Google Scholar]
- 30.Gold MA, Tian C, Whitney CW, et al. Surgical versus radiographic determination of para-aortic lymph node metastases before chemoradiation for locally advanced cervical carcinoma: A Gynecologic Oncology Group Study. Cancer. 2008;112:1954–1963. doi: 10.1002/cncr.23400. [DOI] [PubMed] [Google Scholar]
- 31.Michel G, Morice P, Castaigne D, et al. Lymphatic spread in stage Ib and II cervical carcinoma: Anatomy and surgical implications. Obstet Gynecol. 1998;91:360–363. doi: 10.1016/s0029-7844(97)00696-0. [DOI] [PubMed] [Google Scholar]
- 32.Houvenaeghel G, Lelievre L, Rigouard AL, et al. Residual pelvic lymph node involvement after concomitant chemoradiation for locally advanced cervical cancer. Gynecol Oncol. 2006;102:74–79. doi: 10.1016/j.ygyno.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 33.Ferrandina G, Distefano M, Ludovisi M, et al. Lymph node involvement in locally advanced cervical cancer patients administered preoperative chemoradiation versus chemotherapy. Ann Surg Oncol. 2007;14:1129–1135. doi: 10.1245/s10434-006-9252-0. [DOI] [PubMed] [Google Scholar]
- 34.Kupets R, Thomas GM, Covens A. Is there a role for pelvic lymph node debulking in advanced cervical cancer? Gynecol Oncol. 2002;87:163–170. doi: 10.1006/gyno.2002.6815. [DOI] [PubMed] [Google Scholar]
- 35.Grigsby PW, Singh AK, Siegel BA, et al. Lymph node control in cervical cancer. Int J Radiat Oncol Biol Phys. 2004;59:706–712. doi: 10.1016/j.ijrobp.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 36.Macdonald DM, Lin LL, Biehl K, et al. Combined intensity-modulated radiation therapy and brachytherapy in the treatment of cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:618–624. doi: 10.1016/j.ijrobp.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Eifel PJ, Levenback C, Wharton JT, et al. Time course and incidence of late complications in patients treated with radiation therapy for FIGO stage IB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1995;32:1289–1300. doi: 10.1016/0360-3016(95)00118-I. [DOI] [PubMed] [Google Scholar]
- 38.Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 39.Perez CA, Breaux S, Bedwinek JM, et al. Radiation therapy alone in the treatment of carcinoma of the uterine cervix. II. Analysis of complications. Cancer. 1984;54:235–246. doi: 10.1002/1097-0142(19840715)54:2<235::aid-cncr2820540210>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 40.Morice P, Le Bouedec G, Pomel C, et al. Complications of primary external radiation therapy followed by radical hysterectomy for bulky stage IB and II cervical cancer. Eur J Cancer. 2001;37(suppl 6):1232. [Google Scholar]
- 41.Vincens E, Balleyguier C, Rey A, et al. Accuracy of magnetic resonance imaging in predicting residual disease in patients treated for stage IB2/II cervical carcinoma with chemoradiation therapy: Correlation of radiologic findings with surgicopathologic results. Cancer. 2008;113:2158–2165. doi: 10.1002/cncr.23817. [DOI] [PubMed] [Google Scholar]
- 42.Schwarz JK, Siegel BA, Dehdashti F, et al. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA. 2007;298:2289–2295. doi: 10.1001/jama.298.19.2289. [DOI] [PubMed] [Google Scholar]
- 43.McVeigh PZ, Syed AM, Milosevic M, et al. Diffusion-weighted MRI in cervical cancer. Eur Radiol. 2008;18:1058–1064. doi: 10.1007/s00330-007-0843-3. [DOI] [PubMed] [Google Scholar]