This article develops a model to evaluate the cost-effectiveness of recurrence score-guided treatment using 21-gene assay as compared with treatment guided by the Adjuvant! Online program; the study concludes that the 21-gene assay appears to be cost effective from a Canadian health care perspective.
Keywords: Cost-effectiveness analysis, 21-gene assay, Early breast cancer, Oncotype DX
Abstract
Purpose.
Most guidelines for hormone receptor (HR)–positive early breast cancer recommend addition of adjuvant chemotherapy for most women, leading to overtreatment, which causes considerable morbidity and cost. There has been recent incorporation of gene expression analysis in aiding decision making. We evaluated the cost-effectiveness of recurrence score (RS)–guided treatment using 21-gene assay as compared with treatment guided by the Adjuvant! Online program (AOL).
Patients and Methods.
A Markov model was developed to compare the cost-effectiveness of treatment guided either by 21-gene assay or by AOL in a 50-year-old woman with lymph node–negative HR-positive breast cancer over a lifetime horizon. We assumed that women classified to be at high risk all received chemotherapy followed by tamoxifen and those classified to be at low risk received tamoxifen only. The model took a health care payer's perspective with results reported in 2008 Canadian dollars ($). Event rates, costs, and utilities were derived from the literature. Both costs and benefits were discounted at 5%. Outcome measures were life years gained, quality-adjusted life years (QALYs), lifetime costs, and incremental cost-effectiveness ratios (ICERs).
Results.
For a 50-year-old woman, RS-guided treatment was associated with an incremental lifetime cost of $4,102 and a gain in 0.065 QALY, with an ICER of $63,064 per QALY compared with AOL-guided treatment. ICER increased with increasing cost of 21-gene assay and increasing age of patients. Results were most sensitive to probabilities relating to risk categorization and recurrence rate.
Conclusions.
The 21-gene assay appears cost-effective from a Canadian health care perspective.
Background
Breast cancer is the most common cancer among Canadian women. It is estimated that 22,700 women will be diagnosed with and 5,400 women will die of breast cancer in 2009 in Canada [1]. Over 50% of women diagnosed with breast cancer have lymph node–negative hormone receptor (HR)–positive disease [2]. Optimizing care for these patients has enormous potential for improving health outcome. Although the incidence of breast cancer is increasing, the overall breast cancer mortality has been decreasing steadily since the early 1990s because of early detection and advances in adjuvant therapy. The use of adjuvant systemic treatment in breast cancer after curative resection of primary tumor is associated with prolonged recurrence-free and overall survival [3].
The National Surgical and Adjuvant Breast and Bowel Project (NSABP) B-14 trial demonstrated that 15% of patients with node-negative HR-positive disease who were treated with tamoxifen alone after surgery developed recurrence in 10 years. The B-20 study demonstrated a further absolute benefit of approximately 4% if chemotherapy was added to hormonal treatment. Therefore, for 85% of this population, hormonal treatment alone was adequate systemic therapy and adverse effects and costs of chemotherapy could potentially have been avoided [4, 5]. The major challenge is the selection of women who would benefit from chemotherapy in addition to hormonal therapy.
There are a number of guidelines for the management of early-stage breast cancer based on standard clinicopathological features including tumor size, grade, and receptor expressions [3, 6]. However, these guidelines recommend adjuvant chemotherapy in addition to hormonal therapy for the majority of women, leading to overtreatment, which causes considerable morbidity and cost. A validated software program Adjuvant! Online (AOL) has been developed that projects outcomes at 10 years based on clinicopathological features and therapy. It is freely available on the Internet and is now widely used to assist in the adjuvant treatment decision-making process [3]. However, this program also tends to overestimate the benefit of chemotherapy and does not help select patients who do not need chemotherapy.
A number of biological and clinical factors have suggested that not all patients derive the same degree of benefit from chemotherapy [7]. Advances in cancer genomics have led us to examine gene expression analysis in individual tumors to define responsiveness to treatment. The only one of these tests that is widely commercially available and can be done on paraffin-embedded tissue is the 21-gene assay, Oncotype DX, developed by Genomic Health Inc., Redwood City, CA. A recurrence score (RS) is generated for each case based on expression of the 21 genes, and patients are assigned to risk groups (low, intermediate, or high) according to prespecified RS categories [8]. Studies have indicated that RS provides additional risk information to the conventional clinical classification of individual high-risk patients by identifying a subset of patients at lower risk of recurrence who would otherwise be recommended to receive chemotherapy and also by identifying a smaller subset of traditionally classified low-risk patients who are reclassified at higher risk of recurrence [9, 10]. There are currently more data validating this 21-gene assay than any other gene signature. Therefore, we aimed to evaluate the cost-effectiveness of RS-guided treatment using 21-gene assay as compared with treatment guided by AOL for women with node-negative HR-positive HER-2–negative early breast cancer to add to current recommendations regarding the use of this assay.
Materials and Methods
A Markov model was developed using TreeAge Pro 2008 Suite (TreeAge, Williamson, MA) to compare the lifetime cost and utility of treatment guided either by RS or by AOL. The base case was a 50-year-old woman with node-negative HR-positive HER-2–negative early-stage breast cancer. Two diagnostic strategies were considered. For strategy 1, patients' risk classification for distant recurrence was performed using AOL followed by reclassification using RS. Treatment decision was made according to RS. For strategy 2, patients' risk classification was performed using AOL alone. In either strategy, patients classified as high risk received chemotherapy followed by tamoxifen; those classified as low risk received tamoxifen alone. The time horizon represented the remaining lifetime of patients up to a maximum age of 100 years. The cycle length of the model was 1 month. The analysis was conducted from a health care payer's perspective. A discount rate of 5% per annum was applied to costs, life years, and quality-adjusted life years (QALYs) as recommended by the Canadian Agency for Drugs and Technologies in Health [11].
Health States
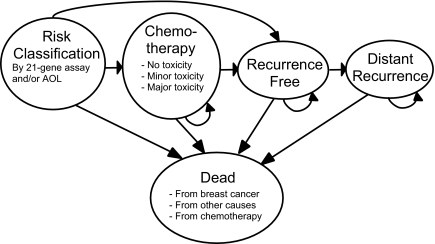
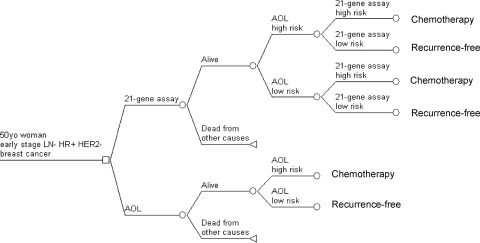
Figure 1 shows a simplified schema representing the different health states in the Markov model. All patients start in the risk classification state where they undergo risk assessment by AOL with or without RS reclassification (Figure 2). Treatment strategies are also determined at this state. Patients receive chemotherapy if they are considered high risk by RS reclassification (strategy 1) or high risk by AOL assessment only (strategy 2). They enter the chemotherapy state for the next 6 months. They may experience no, minor, or major toxicity or may die from complications related to chemotherapy. After 6 months they transit to the recurrence-free state and complete 5 years of tamoxifen. Patients who are deemed low risk by RS reclassification (strategy 1) or low risk by AOL assessment only (strategy 2) proceed directly to the recurrence-free state and complete 5 years of tamoxifen.
Figure 1.
Markov health states.
Figure 2.
Risk classification subtrees. Abbreviation: AOL, Adjuvant! Online.
During each Markov cycle, these patients may remain disease-free or develop distant recurrence. Once patients develop distant recurrence, they remain in the distant recurrence state until they die from breast cancer. During each Markov cycle at any health state, patients may also die from causes other than breast cancer.
Probability of Risk Classification
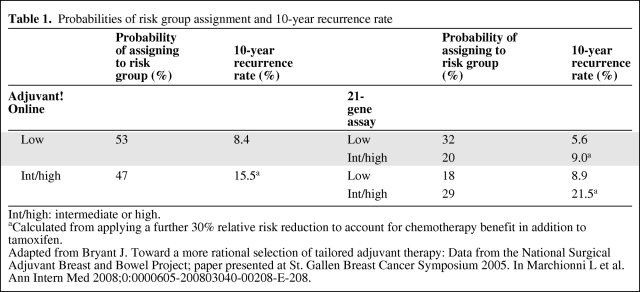
Data on risk classification using RS and/or AOL were adapted from Bryant's analysis of Paik et al.'s [8] 21-gene assay validation study (Table 1) [12]. Paik et al. demonstrated that risk classification using RS was a significant independent prognostic factor for distant recurrence in 668 patients with node-negative HR-positive breast cancer from the NSABP B-14 study who received tamoxifen alone [8]. Bryant et al. undertook further analysis of these results by comparing risk classification using RS with that using AOL. In this analysis, the intermediate-risk patients were grouped together with the high-risk patients in the RS category. Of the 53% patients at low risk according to AOL, 39% were reclassified by RS as having high risk. Of the 47% at high risk according to AOL, 39% were reclassified by RS as low risk.
Table 1.
Probabilities of risk group assignment and 10-year recurrence rate
Int/high: intermediate or high.
aCalculated from applying a further 30% relative risk reduction to account for chemotherapy benefit in addition to tamoxifen.
Adapted from Bryant J. Toward a more rational selection of tailored adjuvant therapy: Data from the National Surgical Adjuvant Breast and Bowel Project; paper presented at St. Gallen Breast Cancer Symposium 2005. In Marchionni L et al. Ann Intern Med 2008;0:0000605-200803040-00208-E-208.
Probability of Toxicity from Chemotherapy
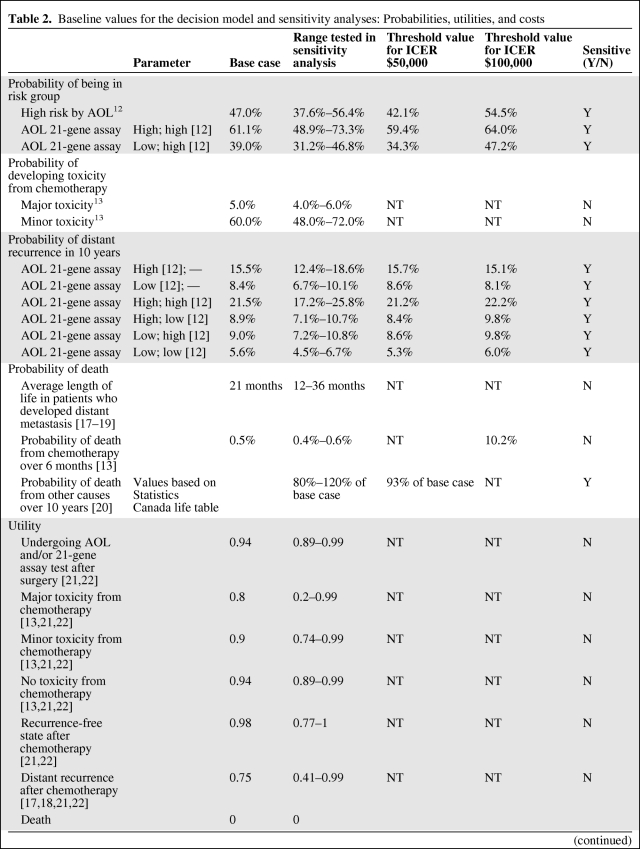
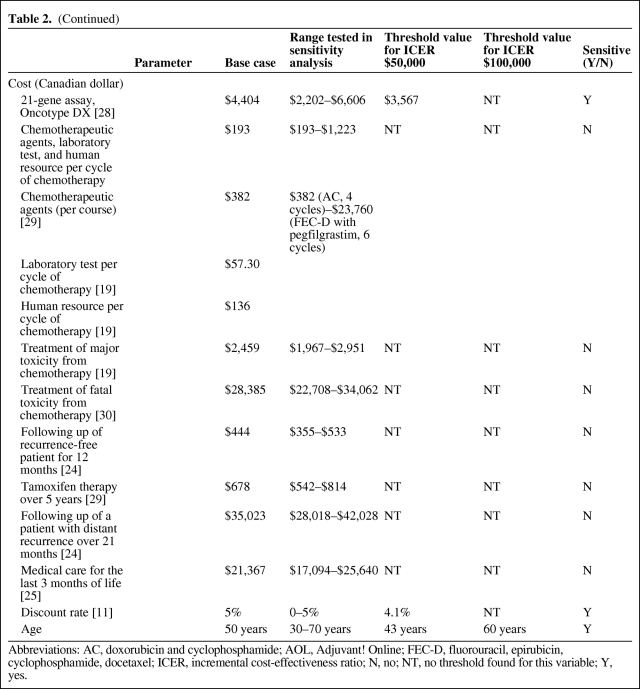
The probabilities of different toxicity profiles from chemotherapy were obtained from the literature (Table 2) [13, 14]. Sensitivity analysis was performed to account for different toxicity potentials from different regimens.
Table 2.
Baseline values for the decision model and sensitivity analyses: Probabilities, utilities, and costs
Table 2.
(Continued)
Abbreviations: AC, doxorubicin and cyclophosphamide; AOL, Adjuvant! Online; FEC-D, fluorouracil, epirubicin, cyclophosphamide, docetaxel; ICER, incremental cost-effectiveness ratio; N, no; NT, no threshold found for this variable; Y, yes.
Risk of Recurrence and Death
Transitional probabilities for recurrence for different risk classifications were derived from the 10-year recurrence rate reported by Bryant et al. (Table 1) [12]. Because the patient cohort analyzed by Bryant et al. received tamoxifen alone, a further 30% relative risk reduction was applied to high-risk patients who received combined chemo-hormonal therapy [15]. Sensitivity analysis was performed to examine variation of this relative risk reduction according to efficacies of different chemotherapy regimens [15, 16]. Once patients developed distant recurrence, it was assumed that the transitional probability for breast cancer death was identical in all patients regardless of the original risk classifications and prior treatment. The median survival for patients with distant recurrence was estimated to be 21 months [17–19]. We assumed that probability of recurrence in recurrence-free patients and probability of death in patients with recurrence followed an exponential distribution. Transitional probability of non-breast cancer deaths was extrapolated from life tables from Statistics Canada 2000–2002 [20].
Utilities
The utility value at baseline, after surgery awaiting treatment allocation, was assumed to be 0.94 [21]. Utility of death was 0. Utilities for chemotherapy, disease-free, and recurrence states were obtained from a number of published sources including a systemic review of cost-utility assessments in oncology and a review of health-related quality-of-life estimates (Table 2) [21, 22]. These utilities were derived from a variety of methods ranging from visual analogue to standard gamble. The quality-adjusted life years (QALYs) were calculated by the length of time spent in a state multiplied by the utility of that state.
Costs
Unit costs were obtained from various sources (Table 2). All costs are reported in 2008 Canadian dollars. Costs from a different base year were inflated by 5% per year using the Canadian consumer price index for health care [23].
Cost of the 21-gene assay, Oncotype DX, was based on the manufacturer's suggested retail price of $3,650 USD ($4,404 Canadian dollar based on 1 USD = 1.2067 CAD conversion rate). Costs of chemotherapy drugs and supportive medications were obtained from the Sunnybrook Odette Cancer Centre pharmacy, Toronto, Ontario. Three commonly used chemotherapy regimens were considered (Table 2): doxorubicin and cyclophosphamide (AC) every 3 weeks × 4 cycles; docetaxel and cyclophosphamide (TC) every 3 weeks × 4 cycles; 5-fluorouracil, epirubicin, and cyclophosphamide every 3 weeks × 3 cycles followed by docetaxel every 3 weeks × 3 cycles (FEC-D). The cost of AC chemotherapy was used for the base-case calculation. Sensitivity analysis was performed using individual costs of these regimens. Costs of chemotherapy included costs of chemotherapeutic agents, supportive medications such as antiemetics, laboratory evaluation, and human resources utilization. The cost of prophylactic growth factor, pegfilgrastim, was included in the FEC-D regimen as the upper limit in sensitivity analysis. The cost of minor toxicity was assumed to have already been incorporated into the cost of supportive medication as part of chemotherapy treatment. The cost of major toxicity incorporated management of febrile neutropenic complications and growth factor support. We adapted the cost of septicemia treatment from Ontario Case Costing Initiative as the cost of chemotherapy-related death. The cost of tamoxifen was applied to all patients for a total of 5 years or until development of distant recurrence or death if events occurred before 5 years.
The costs of recurrence-free follow-up care, treatment for breast cancer recurrence, and terminal care were obtained from the literature [24].
Outcomes
The model generated estimates of the health care costs in Canadian dollars, the life year, and QALYs associated with each strategy, and also the incremental cost per life year gained and per QALY gained (incremental cost-effectiveness ratio, ICER) with the RS strategy. ICER was estimated as the difference in cumulative costs between RS- and AOL-guided treatments, divided by the difference in QALYs between the two strategies.
Sensitivity Analysis
One-way sensitivity analysis was performed on all variables to assess the robustness of the model. In particular, we evaluated the impact of age, cost of different chemotherapeutic regimens, cost of 21-gene assay, and discount rate on ICER. Two-way sensitivity analysis was performed by varying the patient age and the cost of 21-gene assay. The ranges for the variables were derived from a review of available literature. In the cases of paucity of data, ranges used were derived from 20% above and 20% below baseline values. The cost of 21-gene assay ranging between $2000 and $5000 was used.
Results
Base-Case Analysis
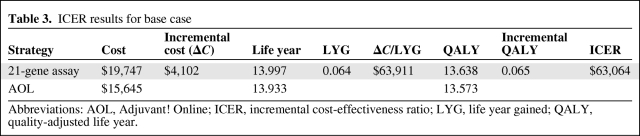
The cost of RS-guided treatment in lymph node–negative HR-positive HER-2–negative breast cancer in a 50-year-old patient was estimated to be $19,747, compared with AOL-guided treatment of $15,645, for an incremental cost of $4,102 (Table 3). RS-guided treatment was associated with a gain of 0.066 life years. With utility taken into account, a difference of 0.064 QALY was observed. ICER was $63,064 per QALY.
Table 3.
ICER results for base case
Abbreviations: AOL, Adjuvant! Online; ICER, incremental cost-effectiveness ratio; LYG, life year gained; QALY, quality-adjusted life year.
Sensitivity Analysis
One-way sensitivity analysis was performed on all variables in the model (Table 2). The model was most sensitive to changes in probabilities relating to risk groups and recurrence rates. All variables for probabilities crossed the threshold when the willingness to pay threshold was set at $50,000 per QALY, making AOL-guided treatment more favorable. The ICER remained stable with the variation of utility ranging between $59,000 and $80,000 per QALY. Varying all costs also did not change the ICER significantly ($61,000–$71,000 per QALY) with the exception of the cost of 21-gene assay. A 50% reduction in the cost of the test ($2,202) would reduce the ICER to $29,207 per QALY. Varying the discount rate from 0% to 5% significantly changed the ICER from $15,888 to $63,064 per QALY. ICER was sensitive to patient's age. ICER for a 30-year-old patient was $40,573 per QALY compared with $200,273 per QALY for a 70-year-old patient. If the willingness to pay threshold of $50,000 per QALY was applied, RS-guided treatment would be cost-effective in patients under 43 years old. The cost-effectiveness would become marginal in women older than 60 if a threshold of $100,000 was used.
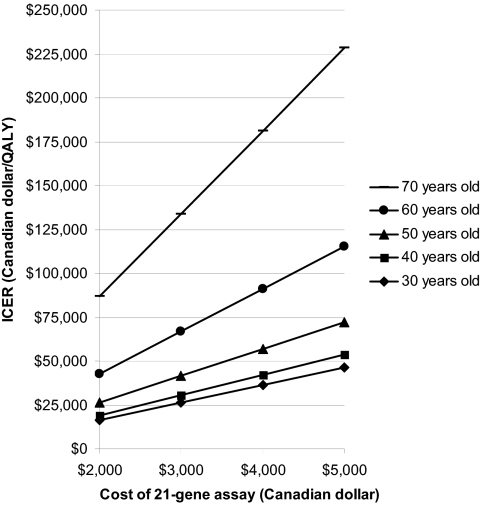
Two-way sensitivity analysis was performed by varying the patient age and the cost of 21-gene assay (Figure 3). It showed that ICER was positively associated with an increased cost of 21-gene assay and an increasing age of patients. If the willingness to pay threshold was increased, or if the cost of 21-gene assay dropped, RS-guided treatment would become cost-effective to the older population in this cohort of patients.
Figure 3.
Two-way sensitivity analysis. Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life years.
Discussion
This study demonstrated that treatment of lymph node–negative HR-positive HER-2–negative breast cancer guided by RS using 21-gene assay was more effective than AOL-guided treatment with regard to life year gained (0.064 years) and QALY gained (0.065 years), at an additional cost of $4,102 per person over a lifetime. Incremental cost-effectiveness ratio for the base case was $63,064 per QALY.
Sensitivity analyses showed that the cost-effectiveness estimates were favorable in younger women. This was due to higher QALY gained with RS strategy in younger patients than in older patients (0.099 QALY in 30-year-olds vs. 0.021 QALY in 70-year-olds) whereas incremental cost with an RS-guided strategy remained similar ($4,036 in 30-year-olds vs. $4,239 in 70-year-olds).
The model was sensitive to changes in the probabilities relating to risk groups and recurrence rates. Varying these values in effect resulted in alteration of test performance of these two strategies. The presence of threshold values within the plausible ranges suggests that the sensitivity and specificity for these two strategies are likely to be comparable. There are little data available in the literature on test properties of a 21-gene assay and AOL. We adapted the results from Bryant et al., the only data available in the literature in which these two methods were compared and a 10-year recurrence rate was assessed in each risk group [12]. We used an arbitrary range of base value ± 20% for sensitivity analysis, which might have led to an overestimation. The robustness of the model could be more vigorously assessed when this information becomes available.
The ICER was largely driven by the cost of 21-gene assay as it accounted for 22% of total cost in the RS-guided strategy in the base case. Because this was an upfront cost, it was not affected by discounting, yet the effect measured in QALY is heavily discounted as it occurred many years from original diagnosis. The disproportionate change in cost and benefit with time explains the sensitivity of the model to discounting. The utility of various health states and the cost of chemotherapeutic agents had no significant impact on the ICER.
Three studies examining the cost-effectiveness of RS-guided treatment all demonstrated its superiority over conventional methods of risk classification. Hornberger et al. compared RS-guided treatment to treatment guided by National Comprehensive Cancer Network (NCCN) guideline, whereas Kondo et al. compared RS-guided strategy with two other strategies—NCCN guideline or St Gallen recommendation—in the context of Japan's health care system [25, 26]. Both studies showed increased QALY and acceptable ICER. Lyman et al. showed a gain in life expectancy of 2.2 years with RS-guided treatment compared with treating all patients with tamoxifen alone; and a net cost savings of $2,256 per patient compared with the cost of treating all patients with chemotherapy and tamoxifen. QALY was not evaluated in their study [27].
It is generally agreed that consensus guidelines are crude tools in risk classification. Risks are determined by one or more clinicopathological features. AOL, however, is a validated tool based on a number of clinicopathological features that gives estimates for outcome at 10 years according to therapy used [16]. It is seen as a more sophisticated alternative with stronger predictive power and is now widely used to assist in making decisions regarding adjuvant treatment. We therefore chose to use AOL to compare with 21-gene assay.
Our study has limitations. First, there are little data regarding the test performances of 21-gene assay and AOL. We used the data from the only study available and had to estimate the plausible ranges for sensitivity analysis, which might have influenced the robustness of our model. Second, the data we adapted from Bryant et al. grouped the intermediate-risk patients together with the high-risk patients in the RS category. There are currently no data as to whether chemotherapy is beneficial in the intermediate-risk group, and this is the subject of an ongoing phase III trial (TAILORx). In addition, AOL does not provide risk categories. Bryant ranked order output from AOL so that a similar proportion of cases would be categorized as low risk (50%) when compared with 21-gene assay. This method is arbitrary and may not be optimal in identifying patients who are likely to benefit from chemotherapy. Third, we did not consider local recurrence as a health state, and hence might have missed out accounting for its associated cost and utility loss. None of the models in previous studies on 21-gene assay included this state because of the lack of data in validation studies. Fourth, this model did not consider HER-2–positive disease, which represents up to 25% of early breast cancer. It is assumed that patients with HER-2–positive disease are generally advised to receive combined chemotherapy-trastuzumab treatment.
Our study modeled based on the Canadian health system. It would be most interesting to see such analysis performed in the U.S. setting. Because of the likely differences in the practice patterns including patient follow-up, treatment for metastatic disease, and the difference in health care structure, it is anticipated that the estimates of the incremental cost-effectiveness ratio would be even more favorable.
Conclusion
RS-guided treatment using 21-gene assay for lymph node–negative HR-positive HER-2–negative breast cancer is both more effective and more costly compared with AOL-guided treatment with a relatively acceptable ICER of $61,800 per QALY, from a health care perspective in the context of a willingness to pay thresholds of $50,000–$100,000 per QALY. Nevertheless, further research is required to validate 21-gene assay and AOL and to identify subgroups of patients who may derive more benefit from the RS-guided treatment strategy.
Acknowledgments
We acknowledge Dr. Ahmed Bayoumi for his helpful advice on modeling and his review of the manuscript, and Dr. Harindra Wijeysundera for review of the Markov model.
Author Contributions
Conception/Design: Daphne T. Tsoi, Miho Inoue, Catherine M. Kelly, Sunil Verma, Kathleen I. Pritchard
Provision of study material or patients: Daphne T. Tsoi, Miho Inoue
Collection and/or assembly of data: Daphne T. Tsoi, Miho Inoue
Data analysis and interpretation: Daphne T. Tsoi, Miho Inoue, Sunil Verma, Kathleen I. Pritchard
Manuscript writing: Daphne T. Tsoi, Miho Inoue, Catherine M. Kelly, Sunil Verma, Kathleen I. Pritchard
Final approval of manuscript: Daphne T. Tsoi, Miho Inoue, Catherine M. Kelly, Sunil Verma, Kathleen I. Pritchard
References
- 1.Canadian Cancer Society. Canadian Cancer Statistics, 2009. [accessed August 5, 2009]. Available at www.cancer.ca.
- 2.Jemal A, Siegal R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Invasive breast cancer, 2008. [accessed December 10, 2008]. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 4.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Redmond C. Systemic therapy in node-negative patients: updated findings from NSABP clinical trials. National Surgical Adjuvant Breast and Bowel Project. J Natl Cancer Inst Monogr. 1992;(11):105–116. [PubMed] [Google Scholar]
- 6.Goldhirsch A, Wood WC, Gelber RD, et al. Meeting highlights: Updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol. 2003;21:3357–3365. doi: 10.1200/JCO.2003.04.576. [DOI] [PubMed] [Google Scholar]
- 7.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 8.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 9.BlueCross BlueShield Association. Gene expression profiling of breast cancer to select women for adjuvant chemotherapy, 2008. [accessed December 10, 2008]. Available at www.bcbs.com/tec. [PubMed]
- 10.Marchionni L, Wilson RF, Wolff AC, et al. Systematic review: Gene expression profiling assays in early-stage breast cancer. Ann Intern Med. 2008;148:358–369. doi: 10.7326/0003-4819-148-5-200803040-00208. [DOI] [PubMed] [Google Scholar]
- 11.Canadian Agency for Drugs and Technologies in Health. 3rd ed. Ottawa: CADTH; 2006. [accessed December 10, 2008]. Guidelines for the economic evaluation of health technologies: Canada. Available at http://www.acmts.ca/media/pdf/186_EconomicGuidelines_e.pdf. [Google Scholar]
- 12.Bryant J. Marchionni L, et al. Toward a more rational selection of tailored adjuvant therapy: Data from the National Surgical Adjuvant Breast and Bowel Project. Ann Intern Med; paper presented at St Gallen Breast Cancer Symposium, 2005; 2008. 0:0000605-200803040-00208-E-208. [Google Scholar]
- 13.Hillner BE, Smith TJ. Efficacy and cost effectiveness of adjuvant chemotherapy in women with node-negative breast cancer. A decision-analysis model. N Engl J Med. 1991;324:160–168. doi: 10.1056/NEJM199101173240305. [DOI] [PubMed] [Google Scholar]
- 14.Partridge AH, Burstein HJ, Winer EP. Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Natl Cancer Inst Monogr. 2001;(30):135–142. doi: 10.1093/oxfordjournals.jncimonographs.a003451. [DOI] [PubMed] [Google Scholar]
- 15.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 16.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 17.Skedgel C, Rayson D, Dewar R, et al. Cost-utility of adjuvant hormone therapies for breast cancer in post-menopausal women: Sequential tamoxifen-exemestane and upfront anastrozole. Breast Cancer Res Treat. 2007;101:325–333. doi: 10.1007/s10549-006-9299-4. [DOI] [PubMed] [Google Scholar]
- 18.Younis T, Rayson D, Dewar R, et al. Modeling for cost-effective-adjuvant aromatase inhibitor strategies for postmenopausal women with breast cancer. Ann Oncol. 2007;18:293–298. doi: 10.1093/annonc/mdl410. [DOI] [PubMed] [Google Scholar]
- 19.Younis T, Rayson D, Sellon M, et al. Adjuvant chemotherapy for breast cancer: a cost-utility analysis of FEC-D vs. FEC 100. Breast Cancer Res Treat. 2008;111:261–267. doi: 10.1007/s10549-007-9770-x. [DOI] [PubMed] [Google Scholar]
- 20.Statistics Canada. Life Tables, Canada, Provinces and Territories, 2000–2002. [accessed December 10, 2008]. Available at http://www.statcan.gc.ca.
- 21.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38:583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Earle CC, Chapman RH, Baker CS, et al. Systematic overview of cost-utility assessments in oncology. J Clin Oncol. 2000;18:3302–3317. doi: 10.1200/JCO.2000.18.18.3302. [DOI] [PubMed] [Google Scholar]
- 23.Statistics Canada. Consumer Price Index, Health and Personal Care, by Province, 2008. [accessed December 10, 2008]. Available at http://www.statcan.gc.ca.
- 24.Will BP, Berthelot JM, Le Petit C, et al. Estimates of the lifetime costs of breast cancer treatment in Canada. Eur J Cancer. 2000;36:724–735. doi: 10.1016/s0959-8049(99)00340-8. [DOI] [PubMed] [Google Scholar]
- 25.Hornberger J, Cosler LE, Lyman GH. Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care. 2005;11:313–324. [PubMed] [Google Scholar]
- 26.Kondo M, Hoshi SL, Ishiguro H, et al. Economic evaluation of 21-gene reverse transcriptase-polymerase chain reaction assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer in Japan. Breast Cancer Res Treat. 2008;112:175–187. doi: 10.1007/s10549-007-9842-y. [DOI] [PubMed] [Google Scholar]
- 27.Lyman GH, Cosler LE, Kuderer NM, et al. Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: an economic analysis based on prognostic and predictive validation studies. Cancer. 2007;109:1011–1018. doi: 10.1002/cncr.22506. [DOI] [PubMed] [Google Scholar]
- 28.Oncotype DX, Genomic Health. www.genomichealth.com/OncotypeDX/Index.aspx.
- 29.Odette Cancer Centre pharmacy. 2008 Personal communication; information not publicly available. [Google Scholar]
- 30.Ontario Case Costing Initiative. Cost for treatment of sepsis, July 2008. [accessed December 10, 2008]. Available at http://www.occp.com.