This review discusses the distinct phenotypes of inherited renal cancer syndromes and advances in diagnosis and management. Recommendations for screening in families are discussed.
Keywords: Renal cancer, Familial, MET, Papillary, Clear cell
Learning Objectives
After completing this couse, the reader will be able to:
Apply presymptomatic gene testing to family members with familial renal cancer in order to facilitate earlier diagnosis and treatment for this population.
Use genetic testing for timely detection of familial renal cancer in carriers to enable earlier use and increased efficacy of VEGF and mTOR pathway inhibiting drugs.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Abstract
We discuss recent advances in the diagnosis and management of renal cell cancer (RCC) given the enhanced molecular genetics knowledge in this area. A number of hereditary renal cancer syndromes have been described, including von Hippel-Lindau disease, Birt-Hogg-Dubé syndrome, hereditary leiomyomatosis/RCC syndrome, and hereditary papillary renal cancer. Early molecular diagnosis now facilitates the management and prevention of RCC in families. Recommendations for screening in families are discussed.
Introduction
Renal cell cancer (RCC) affects around 1 in 100 people. It is the seventh most common cancer in men and the ninth in women [1]. The etiological factors have been well described and are not discussed further in this review. However, around 3%–4% of renal cancers have a familial basis, and several (mainly autosomal dominant) syndromes have been defined. This review discusses the distinct phenotypes of these inherited cancer syndromes and advances in diagnosis and management. Increased effort is being devoted to cancer prevention and early detection of RCC in individuals with an inherited susceptibility to these tumors.
Hereditary Renal Cancer
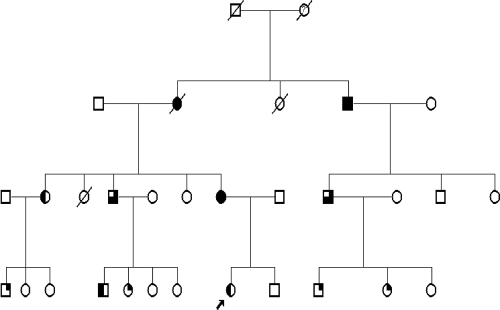
Around 3%–4% of RCC cases result from familial susceptibility. Hereditary RCC cases, as in other hereditary cancers, tend to present earlier than sporadic cancers and may be bilateral at the time of diagnosis. The main RCC disorders are shown in Table 1. The majority are autosomal dominant (Fig. 1), and a typical family history of RCC in multiple generations can often be elucidated. Careful questioning can also help to identify other extrarenal features, including skin and central nervous system manifestations.
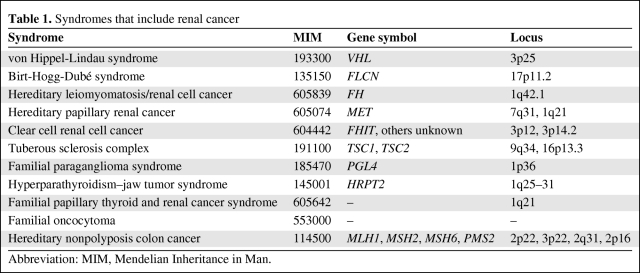
Table 1.
Syndromes that include renal cancer
Abbreviation: MIM, Mendelian Inheritance in Man.
Figure 1.
Family tree showing a pedigree with Birt-Hogg-Dubé syndrome displaying clear autosomal dominant inheritance. Proband indicated with an arrow. Symbols: full shaded, renal cancer and skin lesions; 75% shaded, renal cancer, pneumothorax, and skin lesions; 50% shaded, skin lesions and pneumothorax; 25% shaded, skin lesions only.
Pathology
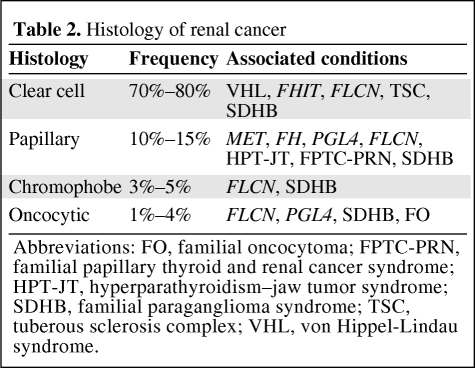
The histological type of renal cancer varies with the inherited disorder, and is shown in Table 2. At least 75% of cases have clear cell histology, but knowing the histological type and then taking a careful family history and examining patients for extrarenal clinical signs help in identifying the particular genetic disorder and subsequently allow tailored prevention and screening programs. Papillary RCCs are usually divided into a milder type I (basophilic) and a more aggressive type II (eosinophilic). Chromophobe, medullary, collecting duct, and unclassified histology are rare subtypes. Angiomyolipomas and oncocytomas are generally benign and are considered here because of the characteristic findings in particular syndromes, including tuberous sclerosis and familial oncocytoma. Generally, the age at onset of hereditary renal cancer is one or two decades earlier than the age at onset of sporadic RCC cases. Sporadic cancers usually occur in the sixth or seventh decades of life, with a mean age at diagnosis of 60 years.
Table 2.
Histology of renal cancer
Abbreviations: FO, familial oncocytoma; FPTC-PRN, familial papillary thyroid and renal cancer syndrome; HPT-JT, hyperparathyroidism–jaw tumor syndrome; SDHB, familial paraganglioma syndrome; TSC, tuberous sclerosis complex; VHL, von Hippel-Lindau syndrome.
von Hippel-Lindau Disease
von Hippel-Lindau (VHL) disease is an autosomal dominant disorder characterized by clear cell RCC (CCRCC), retinal hemangiomata, cerebellar and spinal hemangioblastomas, pheochromocytomas, and endocrine pancreatic tumors [2]. The mean age at renal cancer onset is 39 years, and around 30% of patients develop cancer, primarily of clear cell histology [3]. The prevalence of VHL disease is around one in 50,000 cases. The VHL gene is a tumor suppressor gene on chromosome 3p25 and forms a ubiquitin ligase complex in the hypoxia induced factor (HIF)-1α pathway. The mutated VHL protein promotes overexpression of HIF-activated genes, leading to increased angiogenic activity resulting in a hypervascularized state in the majority of VHL tumors. VHL can be associated with erythrocytosis secondary to constitutive activation of erythropoietin caused by impaired degradation of HIF [4]. Renal cysts are also commonly present but transition from a cyst to a solid lesion is rare [5], although there is debate as to whether carcinoma in situ arises from the walls of complex cysts. Cysts therefore require careful follow-up, and surgical management may be appropriate if there is a change suggestive of cancer on further imaging. Because cases in patients as young as 16 years old have been reported, screening by ultrasound needs to start in adolescence [1].
Birt-Hogg-Dubé Syndrome
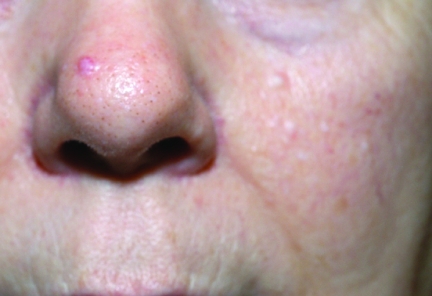
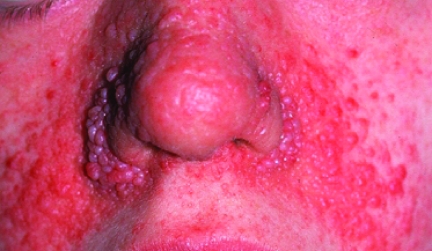
Birt-Hogg-Dubé (BHD) syndrome is caused by abnormalities in the folliculin (FLCN) gene, an autosomal dominant tumor suppressor gene [6, 7]. The incidence of BHD syndrome is unknown [8]. BHD has characteristic skin manifestations, including multiple fibrofolliculomas, trichodiscomas, and achrocordons (Fig. 2), which typically appear over the scalp, midface, and upper trunk and breast. The associated RCCs have primarily an oncocytic or chromophobe histological type, although papillary RCC (PRCC) and CCRCC have also been described [9]. The mean age at onset is around 38 years, with an age range of 20–75 years. Associated features include colon cancer, renal cysts, and lung cysts, with the lung abnormalities often predisposing to multiple pneumothoraces, which may be a presenting feature in isolation [10]. Around 25% of BHD patients eventually develop renal cancer [8]. The onset of renal cysts should alert clinicians to provide more frequent and detailed surveillance because they may precede overt renal cancer. FLCN functions within the mammalian target of rapamycin (mTOR) pathway, with downregulation of FLCN leading to mTOR inhibition [11].
Figure 2.
Characteristic multiple dome-shaped papules on the nose and cheek of a carrier of a folliculin gene mutation.
Hereditary Leiomyomas and Renal Cancer
Hereditary leiomyomas may occur with PRCC (HLRCC). Families have been described with multiple uterine leiomyomata and occasionally leiomyosarcoma. The gene fumarate hydratase (FH) is an autosomal dominant tumor suppressor gene. The finding of cutaneous leiomyomas or uterine fibroids should prompt further consideration of this disorder in patients, with appropriate screening for renal cancers [12, 13]. Type II papillary renal cancer has been associated with HLRCC, with an onset often at 30–50 years of age, but cases as young as 11 years have been described; hence, screening may need to start in late childhood even if there is later onset in other family members [14].
Hereditary PRCC
PRCC is divided into type I (basophilic) and type II (eosinophilic). Hereditary PRCC, caused by mutations in the c-MET proto-oncogene, is inherited in an autosomal dominant manner and is associated with type I PRCC. Breast, pancreas, and stomach cancers have been associated in some families [15]. By age 50, around 30% of MET carriers develop renal cancer [16].
CCRCC with Chromosome 3 Translocation
Some families have been described with various chromosome 3 rearrangements, including one with a balanced translocation interrupting the FHIT gene on 3p14, and may be involved in somatic loss of VHL leading to RCC. Other families have had 3q translocations, and these families have had other cancers including thyroid, gastric, pancreatic, and bladder cancers [17].
CCRCC Without Chromosome 3 Translocation
Some autosomal dominant families without obvious rearrangements or linkage to chromosome 3 have been described, characterized by earlier onset and often bilateral tumors, compared with sporadic cases [18, 19].
Tuberous Sclerosis Complex
Tuberous sclerosis complex (TSC) is an autosomal dominant disorder characterized by facial angiofibromas (Fig. 3), periungal fibromas, hypopigmented macules, and shagreen patches. The prevalence is around one in 25,000 RCC cases. Learning problems and epilepsy are associated. Renal manifestations include angiomyolipomata, and cases of multifocal CCRCC have also been noted in up to 3% of cases [20]. Two tumor suppressor genes, TSC1 and TSC2, are causal. They are inherited in an autosomal dominant fashion on chromosomes 9q34 and 16p13.3, respectively. They are involved in regulation of the mTOR pathway. Downregulation of TSC1 and TSC2 leads to mTOR activation, in contrast to the role of FLCN [21]. Interestingly, both TSC and BHD (see earlier) cause facial rash, which is often in the same nasolabial distribution with a similar appearance, and a propensity for lung cysts. Renal ultrasound screening usually starts in early childhood, with cases diagnosed as early as 5 years of age. Larger angiomyolipomas may need surgical removal or detailed imaging to ensure that they do not mask renal carcinoma [22].
Figure 3.
Characteristic facial angiofibromas in the nasolabial folds of the face of a carrier of a tuberous sclerosis complex 2 gene mutation.
Familial Paraganglioma Syndrome
The prevalence of hereditary paraganglioma disorders is currently unknown, but clear cell, chromophobe, type II papillary, and mixed RCCs, and oncocytomas have all been described in families with succinate dehydrogenase B (SDHB) gene mutations. The age at onset of renal cancer is variable, with cases as young as 16 years of age being described with papillary cancer [23], but the age range is broad, with cases typically aged 20–73 years [24]. SDHB mutations cause hereditary paraganglioma in association with extra-adrenal pheochromocytoma and (very rarely) thyroid cancer. No significant associations with renal cancer have been described as yet with SDHC or SDHD mutations [25, 26].
Familial Papillary Thyroid and Renal Cancer Syndrome
A distinct, but rare, inherited tumor syndrome with familial papillary thyroid cancer (PTC), nodular thyroid disease, and papillary renal neoplasia has been described in a large three-generation PTC kindred with apparent autosomal dominant inheritance [27].
Familial Renal Hamartomas and Hyperparathyroidism–Jaw Tumor Syndrome
This rare disorder presents predominantly with renal hamartomas and ossifying fibromas of the jaw in association with parathyroid adenoma or carcinoma. Occasionally renal carcinomas with PRCC histology are noted in later life. The gene is an autosomal dominant tumor suppressor gene [28].
Autosomal Recessive Renal Cancer
Analysis of Swedish and Icelandic cancer registry data suggests a possible association between renal cancer and presumed autosomal recessive inheritance. Such inheritance should be considered when there are affected siblings with no obvious mutations in the relevant genes discussed earlier, with normal parents at an older age, apparently free from renal cancer and no prior history. No genes or specific causes have yet been identified, and the majority of hereditary renal cancers still appear to have an autosomal dominant pattern of inheritance [29]. Those with possible autosomal recessive inheritance have an age at onset <50 years [29].
Familial Oncocytoma
Oncocytoma of the kidney is usually a benign tumor with eosinophilia, different from CCRCC. The familial inheritance pattern is typically autosomal dominant and most affected individuals have FLCN mutations. Those without may have another gene, or an unidentified FLCN mutation. One family has been described with a reciprocal translocation (8;9)(q24.1;q34.3), which may or may not be coincidental. Further examination of families with renal oncocytoma for skin and other physical signs, and FLCN analysis, is needed [30]. Renal oncocytomas in patients as young as 25 years old and as old as 65 years of age have been described [25].
Hereditary Nonpolyposis Colon Cancer
Hereditary nonpolyposis colon cancer is an autosomal dominant disease characterized by colon, stomach, and endometrial tumors. Around 5% of colon cancer cases may be hereditary and part of this condition. Four main genes involved in the colon mismatch repair pathway—MLH1, MSH2, MSH6, and PMS2—are present in ∼80% of families fitting the Amsterdam criteria (three cases of gastrointestinal or endometrial cancer in two generations, one patient affected at age <50). Immunohistochemistry of the tumor is helpful in identifying a mismatch repair gene abnormality. Cancers of the renal and ureteric tracts, including bladder, are occasionally associated; they are usually transitional cell cancers and occur in 2% of cases. Screening in families with renal cancer in a relative should start from ∼40 years of age [31]. It is debated whether prostate cancer is also a feature [32].
Screening of At Risk Family Members
Depending on the histological and syndromic features, genetic testing for a particular mutation can be initiated in an affected member. In most cases, this provides presymptomatic diagnosis, or confirms the diagnosis, especially in cases in which a family mutation is already known. Mutation negative cases can be reassured that there is no significantly higher risk for renal cancer in them or their children. Mutation carriers can be entered into a screening program.
For BHD syndrome, this includes renal screening with at least ultrasound from 20–25 years of age, because cancers mainly occur at ages 25–80 years. Regular computed tomography (CT) scanning would provide too high a radiation dose over a patient's lifetime, and increasingly magnetic resonance (MR) imaging is used. Ultrasound is useful in distinguishing a simple benign cyst from a more complex cyst or a solid tumor mass. Screening should be every 1 to 2 years and can be tailored appropriately for family members with advice from clinical genetics and nephrology or uro-oncology specialists dealing with the disorder. More complex renal cysts may be evaluated by the Bosniak classification [33], which is based on the morphologic and enhancement characteristics determined by CT scanning with contrast. Nephron-sparing surgery is generally recommended for indeterminate cystic masses with thickened, irregular, or smooth walls when there is measurable enhancement on a contrast CT scan. If cysts contain contrast-enhancing soft tissue, then again surgical removal is recommended.
Regular colonoscopy is also probably indicated for patients with BHD syndrome, but the exact starting age and repeat interval are still to be determined. Our own practice is to offer an initial colonoscopy screen at 25–30 years and thereafter to repeat it every 3 years, but this may be insufficiently frequent and will need to be revised as evidence accumulates.
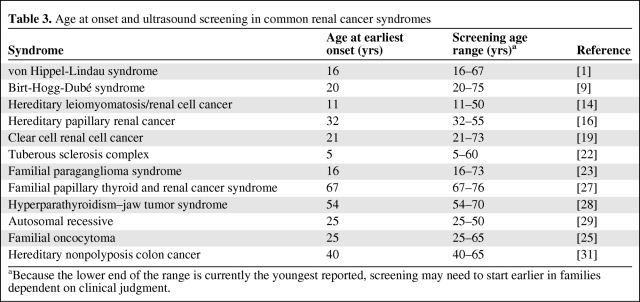
For renal-specific syndromes, a similar annual or every-2-years ultrasound or MR screening from the age of 25 is appropriate, continuing throughout life. For complications in other organs, specific recommendations have been published. For VHL, additional screening for pheochromocytoma and ocular and cerebellar complications is needed, with renal screening starting around 18 years of age, or earlier if indicated from the age at onset in family members; and for TSC, screening should include regular CT brain imaging. SDHB mutation carriers should be offered screening for paraganglioma and pheochromocytoma [24], although it has to be recognized that there is insufficient experience as yet. Table 3 shows the earliest recorded renal cancer in the literature for the more common renal cancer syndromes, and the range of age at onset. Screening with ultrasound can therefore be tailored for families depending on the hereditary disorder.
Table 3.
Age at onset and ultrasound screening in common renal cancer syndromes
aBecause the lower end of the range is currently the youngest reported, screening may need to start earlier in families dependent on clinical judgment.
Targeted Treatment
Surgical and pharmaceutical treatment options have been detailed elsewhere, and aim to spare renal function, reduce metastatic disease, and maximize quality of life. Targeted therapies have great potential given characterization of the mTOR and vascular endothelial growth factor (VEGF) pathways, and a series of recently developed VEGF and mTOR inhibiting drugs is improving treatment in this area [35]. VEGF-inhibiting drugs include sunitinib, sorafenib, and bevacizumab. The latter is a monoclonal antibody that binds and neutralizes circulating VEGF protein. mTOR-inhibiting drugs include temsirolimus and everolimus, both rapamycin analogs.
Conclusions
Advances in the molecular genetics of RCC syndromes have allowed targeted genetic testing and surgical and therapeutic approaches in the 3%–4% of renal cancers that have a familial basis. Early screening is allowing rapid detection and better treatment of known gene mutation carriers. Improvements in our understanding of the molecular basis of these disorders will continue to refine and redefine the classification of this group of disorders and can result in earlier diagnosis, better treatment, and longer term management of this challenging group of patients. Genetic testing and early detection of renal cancer in carriers of these disorders will allow VEGF and mTOR pathway inhibiting drugs to be used early, thus raising the possibility of greater efficacy of treatment. Over the next decade, it will be important to assess if the prognosis can be improved significantly using carefully targeted screening and therapy.
Author Contributions
Conception/Design: Patrick J. Morrison
Provision of study material or patients: Patrick J. Morrison
Collection and/or assembly of data: Patrick J. Morrison, Deirdre E. Donnelly, A. Brew Atkinson, Alexander P. Maxwell
Data analysis and interpretation: Patrick J. Morrison, Deirdre E. Donnelly, A. Brew Atkinson, Alexander P. Maxwell
Manuscript writing: Patrick J. Morrison, Deirdre E. Donnelly, A. Brew Atkinson, Alexander P. Maxwell
Final approval of manuscript: Patrick J. Morrison, Deirdre E. Donnelly, A. Brew Atkinson, Alexander P. Maxwell
References
- 1.American Cancer Society. What Are the Key Statistics for Kidney Cancer? [accessed January 20, 2010]. Available at http://www.cancer.org/docroot/cri/content/cri_2_4_1x_what_are_the_key_statistics_for_kidney_cancer_22.asp?sitearea=&level=
- 2.Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 3.Maher ER, Yates JR, Harries R, et al. Clinical features and natural history of von Hippel-Lindau disease. Q J Med. 1990;77:1151–1163. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- 4.Wiesener MS, Seyfarth M, Warnecke C, et al. Paraneoplastic erythrocytosis associated with an inactivating point mutation of the von Hippel-Lindau gene in a renal cell carcinoma. Blood. 2002;99:3562–3565. doi: 10.1182/blood.v99.10.3562. [DOI] [PubMed] [Google Scholar]
- 5.Choyke PL, Glenn GM, Walther MM, et al. The natural history of renal lesions in von Hippel-Lindau disease: A serial CT study in 28 patients. AJR Am J Roentgenol. 1992;159:1229–1234. doi: 10.2214/ajr.159.6.1442389. [DOI] [PubMed] [Google Scholar]
- 6.Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell. 2002;2:157–164. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 7.Toro JR, Wei MH, Glenn GM, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dubé syndrome: A new series of 50 families and a review of published reports. J Med Genet. 2008;45:321–331. doi: 10.1136/jmg.2007.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlovich CP, Grubb RL, 3rd, Hurley K, et al. Evaluation and management of renal tumors in the Birt-Hogg-Dubé syndrome. J Urol. 2005;173:1482–1486. doi: 10.1097/01.ju.0000154629.45832.30. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt LS, Nickerson ML, Warren MB, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dubé syndrome. Am J Hum Genet. 2005;76:1023–1033. doi: 10.1086/430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunji Y, Akiyoshi T, Sato T, et al. Mutations of the Birt-Hogg-Dubé gene in patients with multiple lung cysts and recurrent pneumothorax. J Med Genet. 2007;44:588–593. doi: 10.1136/jmg.2007.049874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menko FH, van Steensel MA, Giraud S, et al. Birt-Hogg-Dubé syndrome: Diagnosis and management. Lancet Oncol. 2009;10:1199–1206. doi: 10.1016/S1470-2045(09)70188-3. [DOI] [PubMed] [Google Scholar]
- 12.Alam NA, Rowan AJ, Wortham N, et al. Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet. 2003;12:1241–1252. doi: 10.1093/hmg/ddg148. [DOI] [PubMed] [Google Scholar]
- 13.Wei MH, Toure O, Pithukpakorn M, et al. Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet. 2006;43:18–27. doi: 10.1136/jmg.2005.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alrashdi I, Levine S, Paterson J, et al. Hereditary leiomyomatosis and renal cell carcinoma: Very early diagnosis of renal cancer in a paediatric patient. Fam Cancer. 2010;9:239–244. doi: 10.1007/s10689-009-9306-0. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt L, Junker K, Weirich G, et al. Two North American families with hereditary papillary renal carcinoma and identical novel mutations in the MET proto-oncogene. Cancer Res. 1998;58:1719–1722. [PubMed] [Google Scholar]
- 16.Choyke PL, Walther MM, Glenn GM, et al. Imaging features of hereditary papillary renal cancers. J Comput Assist Tomogr. 1997;21:737–741. doi: 10.1097/00004728-199709000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt L, Li F, Brown RS, et al. Mechanism of tumorigenesis of renal carcinomas associated with the constitutional chromosome 3;8 translocation. Cancer J Sci Am. 1995;1:191–195. [PubMed] [Google Scholar]
- 18.Woodward ER, Ricketts C, Killick P, et al. Familial non-VHL clear cell (conventional) renal cell carcinoma: Clinical features, segregation analysis, and mutation analysis of FLCN. Clin Cancer Res. 2008;14:5925–5930. doi: 10.1158/1078-0432.CCR-08-0608. [DOI] [PubMed] [Google Scholar]
- 19.Newmann HP, Pawlu C, Peczkowska M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292:943–951. doi: 10.1001/jama.292.8.943. [DOI] [PubMed] [Google Scholar]
- 20.Cook JA, Oliver K, Mueller RF, et al. A cross sectional study of renal involvement in tuberous sclerosis. J Med Genet. 1996;33:480–484. doi: 10.1136/jmg.33.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison PJ. Tuberous sclerosis: Epidemiology, genetics and progress towards treatment. Neuroepidemiology. 2009;33:342–343. doi: 10.1159/000254570. [DOI] [PubMed] [Google Scholar]
- 22.Robertson FM, Cendron M, Klauber GT, et al. Renal cell carcinoma in association with tuberous sclerosis in children. J Pediatr Surg. 1996;31:729–730. doi: 10.1016/s0022-3468(96)90689-2. [DOI] [PubMed] [Google Scholar]
- 23.Srirangalingam U, Walker L, Khoo B, et al. Clinical manifestations of familial paraganglioma and phaeochromoctyomas in succinate dehydrogenase B (SDH-B) gene mutation carriers. Clin Endocrinol (Oxf) 2008;69:587–596. doi: 10.1111/j.1365-2265.2008.03274.x. [DOI] [PubMed] [Google Scholar]
- 24.Ricketts CJ, Forman JR, Rattenbury E, et al. Tumour risks and genotype-phenotype-proteotype analysis in 358 patients with germline mutations in SDHB and SDHD. Hum Mutat. 2010;31:41–51. doi: 10.1002/humu.21136. [DOI] [PubMed] [Google Scholar]
- 25.Henderson A, Douglas F, Perros P, et al. SDHB-associated renal oncocytoma suggests a broadening of the renal phenotype in hereditary paragangliomatosis. Fam Cancer. 2009;8:257–260. doi: 10.1007/s10689-009-9234-z. [DOI] [PubMed] [Google Scholar]
- 26.Morrison PJ, Atkinson AB. Genetic aspects of familial thyroid cancer. The Oncologist. 2009;14:571–577. doi: 10.1634/theoncologist.2009-0046. [DOI] [PubMed] [Google Scholar]
- 27.Malchoff CD, Sarfarazi M, Tendler B, et al. Papillary thyroid carcinoma associated with papillary renal neoplasia: Genetic linkage analysis of a distinct heritable tumor syndrome. J Clin Endocr Metab. 2000;85:1758–1764. doi: 10.1210/jcem.85.5.6557. [DOI] [PubMed] [Google Scholar]
- 28.Carpten JD, Robbins CM, Villablanca A, et al. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet. 2002;32:676–680. doi: 10.1038/ng1048. [DOI] [PubMed] [Google Scholar]
- 29.Hemminki K, Li X. Familial renal cell cancer appears to have a recessive component. J Med Genet. 2004;41:e58. doi: 10.1136/jmg.2003.014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teh BT, Blennow E, Giraud S, et al. Bilateral multiple renal oncocytomas and cysts associated with a constitutional translocation (8;9)(q24.1;q34.3) and a rare constitutional VHL missense substitution. Genes Chromosomes Cancer. 1998;21:260–264. doi: 10.1002/(sici)1098-2264(199803)21:3<260::aid-gcc12>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 31.Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis colon cancer) J Med Genet. 2007;44:353–362. doi: 10.1136/jmg.2007.048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devlin LA, Graham CA, Price JH, et al. Germline MSH6 mutations are more prevalent in endometrial cancer patient cohorts than hereditary non polyposis colorectal cancer cohorts. Ulster Med J. 2008;77:25–30. [PMC free article] [PubMed] [Google Scholar]
- 33.Israel GM, Bosniak MA. An update of the Bosniak renal cyst classification system. Urology. 2005;66:484–488. doi: 10.1016/j.urology.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]