This study explored the impact of gender on cancer patients' needs and preferences, and found that, of all the patient- and disease-related factors, gender was the most important independent predictor of patient preferences.
Keywords: Patient-centered care, Patient characteristics, Gender, Patient preferences, Quantitative research, Questionnaire
Learning Objectives
After completing this course, the reader will be able to:
Enumerate reasons for a patient-centered model of care and plan changes in your practice/facility consistent with patient-centered care.
Differentiate between the general preferences of male and female cancer patients and tailor care of individual patients accordingly.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Aim.
Improving quality of care for cancer patients requires insight into their specific wishes, needs, and preferences concerning cancer care. The aim of this study was to explore the impact of gender on cancer patients' needs and preferences.
Patients and Methods.
Data were obtained from 386 questionnaires assessing cancer patients' preferences for health care. Multivariate regression analyses were performed with data obtained from medical oncology patients treated in seven Dutch hospitals, using the scales of the questionnaire as dependent variables.
Results.
Patients rated safety, expertise, performance, and attitude of physicians and nurses highest on their list of preferences. There were significant differences between male and female patients concerning preferences in health care in 15 of the 21 scales and in two of the eight single items. Without exception, women found the care aspects mentioned in these scales and items more important than men. Multivariate regression analysis showed that, of all the patient- and disease-related factors, gender was the most important independent predictor of patient preferences.
Conclusion.
Gender impacts cancer patients' needs and preferences and should be taken into account for optimal cancer care. Cancer care might be tailored toward gender, for example, with regard to the means and extent of communication, manner and extent of support, counseling and rehabilitation, consultation length, and physician assignment. The results of this study may guide health care professionals and organizations to develop a gender-specific health care approach to further improve cancer patient–centered care.
Introduction
In a report from the Institute of Medicine (Washington) from 2001, “patient centered care” was defined as “care that is respectful of and responsive to individual patient preferences, needs and values, ensuring that patient values guide all clinical decisions” [1]. Today, health care organizations put effort into making their care and treatment based as much as possible on the wishes of patients. An important argument for health care organizations to increase patient satisfaction is the belief that satisfied patients are more likely to cooperate with their treatment, continue their use of medical services, and maintain a good relationship with their physicians. Greater patient satisfaction is associated with better clinical outcomes [2–7].
At the same time, health care organizations need to save costs in an increasingly competitive environment that compels them to deliver demonstrably efficient, effective, and high-quality care. As a result of these developments, the emphasis on providing health care is shifting from a service-centered and fragmented care organization to integrated patient-centered care. To further improve health care, organizations have therefore focused on assessments of specific needs and wishes of patients.
Patients with cancer are a specific subgroup of patients. They encounter severe physical, existential, and emotional problems. Evaluation of the best possible health care for cancer patients concerns not only aspects that are medical but also aspects that are directly linked to the patients' quality of life, their personal aspirations, needs, and values, and the quality of their relations. Therefore, it seems likely that cancer patients have different needs and expectations with regard to their care than other patients [2, 3, 8, 9]. The severe impact of cancer on the patient and his/her family results in the desire for information and a more critical appraisal of the care received. Consequently, there is an increasing demand from patients to play an active role in improving the quality of care they receive [10]. To reach this goal, it is important to gain insight into cancer patients' views on health care and their specific wishes, needs, and preferences [11].
To obtain insight into the specific preferences of cancer patients, we developed a patient health care preference questionnaire, based on the patients' unrestricted input [12]. It should be noted that this questionnaire is not a satisfaction questionnaire, but a questionnaire that evaluates the importance of care aspects.
Over the last decade, there has been increasing attention on differences between men and women concerning health care. Therefore, we wondered whether the gender of cancer patients would influence their preferences as assessed by our questionnaire. Generally, men and women differ with regard to thinking, solving problems, memory, and sensitivity to danger or threat [13, 14]. The literature suggests significant differences concerning health care between men and women with respect to communication styles [15], confiding in crisis [16], coping with illness-related distress [17, 18], the use of psychosocial support [16–21], and their involvement in medical decision making [22, 23].
The aim of this analysis was to determine the impact of gender on cancer patients' preferences for health care. We compared the influence of gender with that of other patient- and disease-related variables that might influence patient preferences (including age, educational level, type of cancer, presence or absence of metastatic disease, years since diagnosis, and days of hospitalization) [24–28].
Patients and Methods
The research protocol was approved by the Medical Ethics Commission of the University Medical Center Utrecht.
Questionnaire
The development of the Cancer Patients' Health Care Preference Questionnaire is described elsewhere [12]. Briefly, items were generated during 10 focus group interviews between June 2004 and December 2005 with a total of 51 cancer patients. During the focus group interviews, participants were stimulated to have a free flow of ideas without any interruption from the interviewer.
Based on the focus group interviews, a questionnaire containing 136 items was generated. Each item evaluates the level of importance on a four-point scale, ranging from not important (1) to somewhat important (2), important (3), and extremely important (4). All scores of scales and single items are transformed to a score of 0–100, with high values indicating a high level of importance.
After a pretest, the questionnaire was distributed among patients in care of medical oncologists from six community hospitals and one university medical center. Doctors and nurses of these departments handed out the questionnaires to an unselected sample of consecutive cancer patients. The questionnaires were encoded by the hospital. A cover letter informed patients about the aim of the study and the importance of their input. Respondents were assured that their answers would be kept confidential and that the data would be processed anonymously. A phone number and e-mail address to contact the project manager were provided. Respondents could complete the questionnaire at home and send it back anonymously in a self-addressed prestamped envelope. A reminder was sent to each patient after 4 weeks.
Patients did not sign a consent form for the study.
An explorative factor analysis was performed, resulting in 21 scales containing 115 items and eight single items. The process of deleting and including items into scales is described elsewhere [12]. The internal consistency of the 21 scales was sufficient for most of the scales. Six scales (Mistakes by Professionals, Consultation and Transfer, Patient File Confidentiality, Accessibility of Services, Appointments, and Fellow-Patient Interaction) had a Cronbach's α value < 0.70, probably because of the low number of items (two to four) in these scales. Because the mean interitem correlation coefficient was sufficient, we decided to retain these scales in the questionnaire [12].
Statistical Analyses
Data were analyzed using the Statistical Package for the Social Sciences, version 15.0 (SPSS Inc., Chicago, IL).
Differences between male and female patients with regard to the means of the scales and single items of the questionnaire were studied using Mann-Whitney tests and by calculating effect sizes for statistically significant differences. According to Cohen's thresholds [29], an effect size (ES) <0.20 indicates a trivial effect, an ES ≥0.20 to <0.50 indicates a small effect, an ES ≥0.50 to <0.80 indicates a moderate effect, and an ES ≥0.80 indicates a large effect. An ES ≥0.20 reflects a relevant difference between groups [30].
Next, each of the 21 scales was analyzed separately. First, a simple regression analysis was performed, analyzing which of the following patient and disease characteristics (independent variables) had a significant influence on the scales of the questionnaire (dependent variables): gender (male or female), age (<50 years, 50–65 years, or >65 years), educational level (high or low), presence or absence of metastases (as indicated by the patients), type of cancer (breast, gastrointestinal, urogenital, or other), years since diagnosis (<1 year, 1–5 years, or >5 years), days of previous hospitalization (<1 week, >1 week), and hospital (academic or affiliated).
Variables with a p-value < .2 in the simple regression analysis were included in a multivariate regression analysis, using a forward stepwise method. To avoid an inflated type I error resulting from multiple testing, we applied a Bonferroni-type correction procedure, considering independent variables to be significant in the multiple regression model only if they had a p-value < .0024 (p = .05/21 variables).
Results
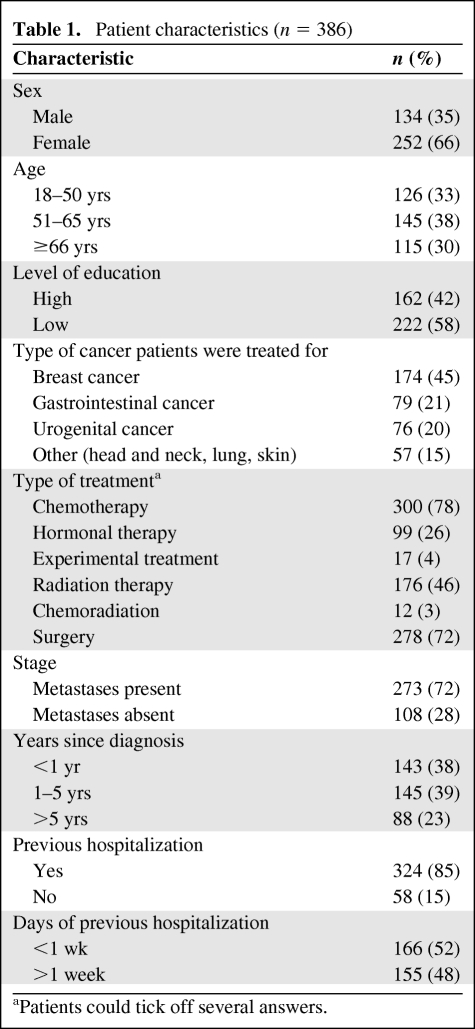
Between October 2006 and March 2007, 681 questionnaires were handed out to patients. In total, 396 questionnaires were returned. Ten questionnaires were received after the cutoff date and were not included in the analysis. The data are based on responses from 386 patients, translating into a 57% response rate. The characteristics (self-reported) of these patients are summarized in Table 1.
Table 1.
Patient characteristics (n = 386)
aPatients could tick off several answers.
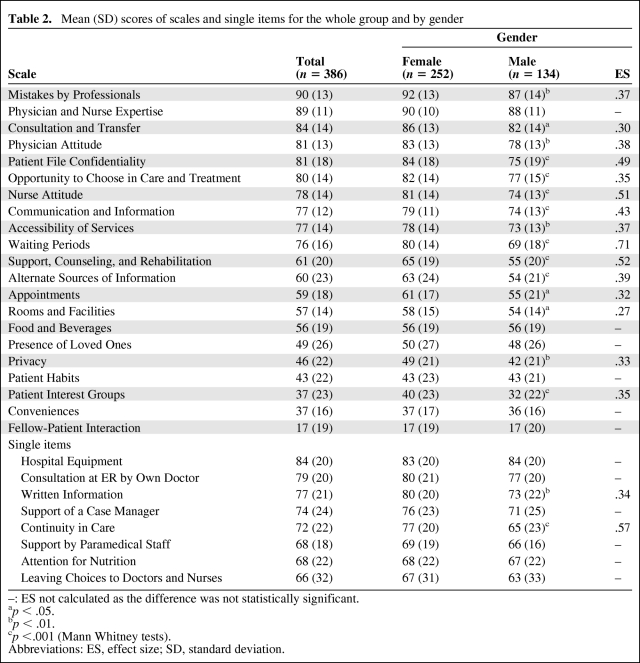
The mean scores of scales and single items for the whole group and by gender are shown in Table 2.
Table 2.
Mean (SD) scores of scales and single items for the whole group and by gender
–: ES not calculated as the difference was not statistically significant.
ap < .05.
bp < .01.
cp <.001 (Mann Whitney tests).
Abbreviations: ES, effect size; SD, standard deviation.
Patients set the highest value on treatment in a safe environment by skilled doctors and nurses, able to communicate well. Of relatively less importance were the organizational and environmental factors.
There were significant differences between male and female patients concerning preferences in health care for 15 of the 21 scales (71%) and for two of the eight single items (25%). For all these scales and single items, without exception, women found the care aspects mentioned in these scales and the single items more important than did their male counterparts. A moderate to large effect was found for the scales Waiting Periods, Nurse Attitude, and Support, Counseling, and Rehabilitation, and for the single item Continuity in Care.
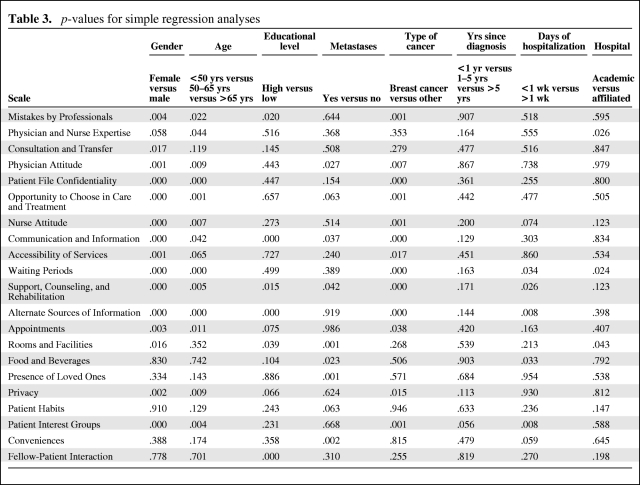
The p-values of the simple regression analysis are shown in Table 3. Of the variables examined, gender, age, and type of cancer showed the lowest p-values. With regard to age, there were significant differences (p-value < .05) among age groups for 13 scales. In all these 13 scales, patients aged >65 years showed the lowest mean scores; the scores for the age groups <50 years and 50–65 years were generally comparable. In other words, older patients attached the lowest value to care aspects mentioned in 62% of the scales (data not shown).
Table 3.
p-values for simple regression analyses
Because there was a clear pattern of differences in mean scores of scales and single items between breast cancer on the one hand (invariably showing higher scores) and gastrointestinal, urogenital, and other tumors on the other hand (data not shown), we dichotomized type of cancer (breast cancer versus other) for the multivariate regression analysis.
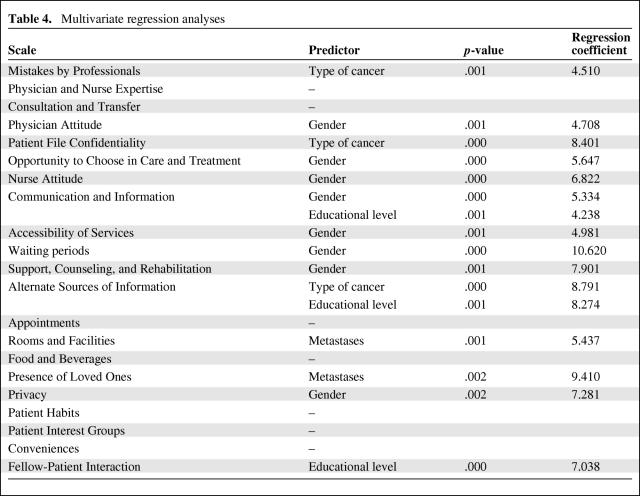
The multivariate analysis (Table 4) showed that gender had the strongest impact on patient preference. It was an independent predictor for eight of the 21 scales (38%): Physician Attitude, Opportunity to Choose in Care and Treatment, Nurse Attitude, Communication and Information, Accessibility of Services, Waiting Periods, Support, Counseling, and Rehabilitation, and Privacy.
Table 4.
Multivariate regression analyses
Furthermore, type of cancer (three scales), educational level (three scales), and presence/absence of metastases (two scales) independently influenced the degree to which patients found care aspects important.
In all scales for which type of cancer influenced the degree to which patients found care aspects important, breast cancer patients scored the highest. Concerning educational level, patients with a lower educational level found communication and information and fellow-patient interaction more important than did higher educated patients. Higher educated patients found aspects related to alternate sources of information more important. The presence or absence of metastases was a predictor for rooms and facilities and for the presence of loved ones. In these cases, patients with metastases found the mentioned care aspects in these scales more important than did patients without metastases.
Age, years since diagnoses, days of hospitalization, and hospital had no influence on the scales. Seven scales were not influenced by any independent variable, namely, Physician and Nurse Expertise, Consultation and Transfer, Appointments, Food and Beverages, Patient Habits, Patient Interest Groups, and Conveniences.
Discussion
Providing optimal care for patients with cancer requires insight into the true preferences and wishes of this vulnerable patient group. The aim of this study was to determine the impact of gender on cancer patient preferences for health care. Although several studies have been published on the relationship between patient characteristics and patient satisfaction [25–27, 31, 32], there is limited information about the impact of gender on cancer patient preferences.
Previous studies concerning gender and satisfaction with care have reached inconsistent conclusions. Some studies found a clear relation between patient gender and satisfaction [25, 26, 31], whereas others did not [24, 33, 34]. In the studies in which a relation was found between gender and satisfaction, men tended to be more satisfied with several aspects of care than women [25, 26, 31]. Larsson et al. [35] found that female patients receiving medical and surgical care attached significantly more value to the quality of care.
Our study showed that there are significant differences between female and male cancer patients with regard to health care preferences. Generally, men regarded most care aspects as less important than women did.
Multivariate analyses revealed that gender had much more impact on patient preferences than other patient- and disease-related factors. Women particularly attached higher value to aspects as measured by the scales Nurse Attitude, Support Counseling and Rehabilitation, and Continuity of Care. These scales and single item are related to attitude and support issues. That women attach more value to psychosocial support is consistent with other research [18, 21, 36]. Compared with men, women may access support services more readily [16, 17, 19–21], and they value the opportunity to share their feelings and concerns with more confidantes [20, 21]. Men tend to seek out psychosocial support from different sources than women (i.e., often from their wives) [16, 17, 20, 21]. Female patients report higher levels of unmet support needs [36] and feel less satisfied even if emotional support is available [21]. The importance of nurse attitude in this study is probably related to the important role nurses play with regard to psychosocial support.
For some types of cancer (e.g., breast, ovarian, gynecological, or prostate cancer), gender and type of cancer are obviously interrelated. If type of cancer is found to be associated with health care preferences, this may be a result of the influence of gender. As our multiple regression analysis showed, gender was more important than type of cancer for most preference variables. Having breast cancer was the strongest independent predictor for only three scales.
Contrary to our expectations, age was not an independent predictor for cancer care needs and preferences. Age was only significant in the simple regression analyses and not in any one of the final models. Satisfaction studies generally show a tendency for older patients to be more satisfied than young and middle-aged patients [25, 26, 32]. Younger patients prefer a more active role in decision making and participation in health care [22, 23]. It is important to realize that our study had a relatively low number of young people with cancer (patients aged 18–35 years comprised only 5% of the study population). This reflects the low incidence of cancer at this young age, but it may lead to an underestimation of the specific needs of young patients. The younger generation today is more educated and trained to find information when needed, is more critical toward authorities, and demands dialog, respect, and good service [28]. During the focus group interviews, young people expressed specific needs and preferences concerning care and treatment, including continued support to reintegrate into their previous daily routine (home, work, school, etc.), clustering patients of roughly the same age during their hospital stays, access to leisure activities, and being able to maintain their own individual daily rhythm. Additional studies of this younger patient group are required.
A possible limitation to our study is patient selection. Because our patient population was recruited through medical oncologists, our findings may only reflect the need of this patient group and not that of other cancer patients. This aspect warrants further study.
Furthermore, the impact of gender may be nationally or culturally determined and not be valid in other countries or cultures.
The results of our study may be used to make health care more patient centered. Health care organizations have recognized that patient-centered care not only provides a benefit for the patient but also saves costs. True patient-centered care should ensure that each patient receives the best possible care. For example, the optimal care for a highly educated woman with metastatic breast cancer will be different from the optimal care for a lower-educated man with a nonmetastasized form of cancer. With regard to gender, care should be tailored to certain aspects of care, for example, the extent and manner of communication, extent and manner of support, counseling and rehabilitation, length of consultation, assignment of physician, choices in treatment and care, and offering privacy.
Conclusion
Male and female cancer patients differ in their preferences concerning health care. While gender is but one of the aspects influencing patients' health care preferences, in our study population it is apparently the most important. These results should encourage health care professionals to become more aware of gender differences and help them to better recognize, understand, and address the specific needs and wishes of patients. Therefore, in striving for providing optimal patient-centered cancer care, gender should be taken into account. Based on our findings, future research should focus on the impact of gender on health care preferences in a prospective setting.
Acknowledgments
We gratefully thank the cancer patients for filling out our questionnaire and the medical oncologists and nurses for distributing questionnaires to the patients. We also wish to thank Annelies Hetharia, M.Sc. (UMC Utrecht), Else Mulder, M.C.C. (UMC Utrecht), Rachel Giles, Ph.D. (UMC Utrecht), Mirjam Majoor, M.Sc. (NFK), and Margriet van der Heiden, M.Sc. (IKMN) for their valuable contributions and support.
This study was supported by grants from the Health Insurers Innovation Foundation (dossier 894) and the Comprehensive Cancer Center Middle Netherlands (dossier 2005/01).
Author Contributions
Conception/Design: Hester Wessels, Alexander de Graeff, Klaske Wynia, Emile E. Voest
Provision of study material or patients: Hester Wessels, Miriam de Heus, Alexander de Graeff, Saskia C.C.M. Teunissen, Emile E. Voest
Collection and/or assembly of data: Hester Wessels, Miriam de Heus
Data analysis and interpretation: Hester Wessels, Miriam de Heus, Cas L.J.J. Kruitwagen, Alexander de Graeff, Gerda T.G.J. Woltjer, Klaske Wynia, Emile E. Voest
Manuscript writing: Hester Wessels, Cas L.J.J. Kruitwagen, Alexander de Graeff, Gerda T.G.J. Woltjer, Saskia C.C.M. Teunissen, Klaske Wynia, Emile E. Voest
Final approval of manuscript: Hester Wessels, Miriam de Heus, Cas L.J.J. Kruitwagen, Alexander de Graeff, Gerda T.G.J. Woltjer, Saskia C.C.M. Teunissen, Klaske Wynia, Emile E. Voest
References
- 1.Institute of Medicine Committee on Quality of Health Care in America. Washington, DC: National Academy Press; 2001. Crossing the Quality Chasm: A New Health System for the 21st Century; pp. 1–337. [Google Scholar]
- 2.Skarstein J, Dahl AA, Laading J, et al. 'Patient satisfaction' in hospitalized cancer patients. Acta Oncol. 2002;41:639–645. doi: 10.1080/028418602321028256. [DOI] [PubMed] [Google Scholar]
- 3.Wiggers JH, Donovan KO, Redman S, et al. Cancer patient satisfaction with care. Cancer. 1990;66:610–616. doi: 10.1002/1097-0142(19900801)66:3<610::aid-cncr2820660335>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 4.Sandoval GA, Brown AD, Sullivan T, et al. Factors that influence cancer patients' overall perceptions of the quality of care. Int J Quality Health Care. 2006;18:266–274. doi: 10.1093/intqhc/mzl014. [DOI] [PubMed] [Google Scholar]
- 5.Sandoval GA, Levinton C, Blackstien-Hirsch P, et al. Selecting predictors of cancer patients' overall perceptions of the quality of care received. Ann Oncol. 2006;17:151–156. doi: 10.1093/annonc/mdj020. [DOI] [PubMed] [Google Scholar]
- 6.Gesell SB, Gregory N. Identifying priority actions for improving patient satisfaction with outpatient cancer care. J Nurs Care Qual. 2004;19:226–233. doi: 10.1097/00001786-200407000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Brédart A, Razavi D, Delvaux N, et al. A comprehensive assessment of satisfaction with care for cancer patients. Support Care Cancer. 1998;6:518–523. doi: 10.1007/s005200050207. [DOI] [PubMed] [Google Scholar]
- 8.Tamburini M, Gangeri L, Brunelli C, et al. Cancer patients' needs during hospitalisation: A quantitative and qualitative study. BMC Cancer. 2003:312. doi: 10.1186/1471-2407-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamburini M, Gangeri L, Brunelli C, et al. Assessment of hospitalised cancer patients' needs by the Needs Evaluation Questionnaire. Ann Oncol. 2000;11:31–37. doi: 10.1023/a:1008396930832. [DOI] [PubMed] [Google Scholar]
- 10.Crawford MJ, Rutter D, Manley C, et al. Systematic review of involving patients in the planning and development of health care. BMJ. 2002;325:1263. doi: 10.1136/bmj.325.7375.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Campen CC, Sixma H, Friele RD, et al. Quality of care and patient satisfaction: A review of measuring instruments. Med Care Res Rev. 1995;52:109–133. doi: 10.1177/107755879505200107. [DOI] [PubMed] [Google Scholar]
- 12.Wessels H, de Graeff A, Wynia K, et al. Medical oncology patients' preferences with regard to health care: Development of a patient-driven questionnaire. Ann Oncol. 2009;20:1708–1713. doi: 10.1093/annonc/mdp044. [DOI] [PubMed] [Google Scholar]
- 13.Conner MG. Understanding the Difference Between Men and Women. [accessed May 6, 2010]. Available at http://www.oregoncounseling.org/ArticlesPapers/Documents/DifferencesMenWomen.htm.
- 14.Witzemann TM, Pardue ML. Washington, DC: Institute of Medicine, National Academy Press; 2001. Exploring the Biological Contributions to Human Health, Does Sex Matter? pp. 1–288. [PubMed] [Google Scholar]
- 15.Street RL., Jr Gender differences in health care provider-patient communication: Are they due to style, stereotypes, or accommodation? Patient Educ Couns. 2002;48:201–206. doi: 10.1016/s0738-3991(02)00171-4. [DOI] [PubMed] [Google Scholar]
- 16.Harrison J, Maguire P, Pitceathly C. Confiding in crisis: Gender differences in pattern of confiding among cancer patients. Soc Sci Med. 1995;41:1255–1260. doi: 10.1016/0277-9536(94)00411-l. [DOI] [PubMed] [Google Scholar]
- 17.Keller M, Henrich G. Illness-related distress: Does it mean the same for men and women? Gender aspects in cancer patients' distress and adjustment. Acta Oncol. 1999;38:747–755. doi: 10.1080/028418699432905. [DOI] [PubMed] [Google Scholar]
- 18.Volkers N. In Coping With Cancer, Gender Matters. J. Natl Cancer Inst. 1999. [accessed May 6, 2010]. Available at http://jnci.oxfordjournals.org/cgi/content/full/91/20/1712. [DOI] [PubMed]
- 19.Kiss A, Meryn S. Effect of sex and gender on psychosocial aspects of prostate and breast cancer. BMJ. 2001;323:1055–1058. doi: 10.1136/bmj.323.7320.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kandrack MA, Grant KR, Segall A. Gender differences in health related behaviour: Some unanswered questions. Soc Sci Med. 1991;32:579–590. doi: 10.1016/0277-9536(91)90293-l. [DOI] [PubMed] [Google Scholar]
- 21.Clarke SA, Booth L, Velikova G, et al. Social support: Gender differences in cancer patients in the United Kingdom. Cancer Nurs. 2006;29:66–72. doi: 10.1097/00002820-200601000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Say R, Murtagh M, Thomson R. Patients' preference for involvement in medical decision making: A narrative review. Patient Educ Couns. 2006;60:102–114. doi: 10.1016/j.pec.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Hamann J, Neuner B, Kasper J, et al. Participation preferences of patients with acute and chronic conditions. Health Expect. 2007;10:358–363. doi: 10.1111/j.1369-7625.2007.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sitzia J, Wood N. Patient satisfaction: A review of issues and concepts. Soc Sci Med. 1997;45:1829–1843. doi: 10.1016/s0277-9536(97)00128-7. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen Thi PL, Briançon S, Empereur F, et al. Factors determining inpatient satisfaction with care. Soc Sci Med. 2002;54:493–504. doi: 10.1016/s0277-9536(01)00045-4. [DOI] [PubMed] [Google Scholar]
- 26.Quintana JM, González N, Bilbao A, et al. Predictors of patient satisfaction with hospital health care. BMC Health Serv Res. 2006;6:102. doi: 10.1186/1472-6963-6-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hargraves JL, Wilson IB, Zaslavsky A, et al. Adjusting for patient characteristics when analyzing reports from patients about hospital care. Med Care. 2001;39:635–641. doi: 10.1097/00005650-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Rosén P, Anell A, Hjortsberg C. Patient views on choice and participation in primary health care. Health Policy. 2001;55:121–128. doi: 10.1016/s0168-8510(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Second Edition. New York: Academic Press; 1988. pp. 1–567. [Google Scholar]
- 30.Middel B, Stewart R, Bouma J, et al. How to validate clinically important change in health-related functional status. Is the magnitude of the effect size consistently related to magnitude of change as indicated by a global question rating? J Eval Clin Pract. 2001;7:399–410. doi: 10.1046/j.1365-2753.2001.00298.x. [DOI] [PubMed] [Google Scholar]
- 31.Foss C. Gender bias in nursing care? Gender-related differences in patient satisfaction with the quality of nursing care. Scand J Caring Sci. 2002:19–26. doi: 10.1046/j.1471-6712.2002.00045.x. [DOI] [PubMed] [Google Scholar]
- 32.Rahmqvist M. Patient satisfaction in relation to age, health status and other background factors: A model for comparisons of care units. Int J Qual Health Care. 2001;13:385–390. doi: 10.1093/intqhc/13.5.385. [DOI] [PubMed] [Google Scholar]
- 33.Hall JA, Dornan MC. Patient sociodemographic characteristics as predictors of satisfaction with medical care: A meta-analysis. Soc Sci Med. 1990;30:811–818. doi: 10.1016/0277-9536(90)90205-7. [DOI] [PubMed] [Google Scholar]
- 34.Weisman CS, Henderson JT, Schifrin E, et al. Gender and patient satisfaction in managed care plans: Analysis of the 1999 HEDIS/CAHPS 2.0H Adult Survey. Womens Health Issues. 2001;11:401–415. doi: 10.1016/s1049-3867(01)00093-7. [DOI] [PubMed] [Google Scholar]
- 35.Larsson BW, Larsson G, Starrin B. Patients' views on quality of care: A comparison of men and women. J Nurs Manag. 1999;7:133–139. doi: 10.1046/j.1365-2834.1999.00121.x. [DOI] [PubMed] [Google Scholar]
- 36.Sanson-Fisher R, Girgis A, Boyes A, et al. The unmet supportive care needs of patients with cancer. Paper presented at the 25th Annual Scientific Meeting of the Clinical Oncology Society of Australia: Sydney; November 25–26, 1999; Australia. [Google Scholar]