The article examines the importance of managing weight to reduce risk for developing cancer and for survival among cancer patients and presents a set of strategies that can be useful to guide clinical advice to patients for whom weight control is an important adjunct to risk management or to improve quality of life and disease-free survival after diagnosis.
Keywords: Obesity, Prevention, Survival, Mechanisms, Cancer
Abstract
Weight, weight gain, and obesity account for approximately 20% of all cancer cases. Evidence on the relation of each to cancer is summarized, including esophageal, thyroid, colon, renal, liver, melanoma, multiple myeloma, rectum, gallbladder, leukemia, lymphoma, and prostate in men; and postmenopausal breast and endometrium in women. Different mechanisms drive etiologic pathways for these cancers. Weight loss, particularly among postmenopausal women, reduces risk for breast cancer. Among cancer patients, data are less robust, but we note a long history of poor outcomes after breast cancer among obese women. While evidence on obesity and outcomes for other cancers is mixed, growing evidence points to benefits of physical activity for breast and colon cancers. Dosing of chemotherapy and radiation therapy among obese patients is discussed and the impact on therapy-related toxicity is noted. Guidelines for counseling patients for weight loss and increased physical activity are presented and supported by strong evidence that increased physical activity leads to improved quality of life among cancer survivors. The “Five A's” model guides clinicians through a counseling session: assess, advise, agree, assist, arrange. The burden of obesity on society continues to increase and warrants closer attention by clinicians for both cancer prevention and improved outcomes after diagnosis.
Introduction
Because weight, weight gain, and strategies to manage weight are important both for the risk for developing cancer and for survival among cancer patients, we divided this review into two sections relating to the patient population. We then present a set of strategies that can be useful to guide clinical advice to patients for whom weight control is an important adjunct to risk management or to improve quality of life and disease-free survival after diagnosis.
Weight, Weight Gain, and Risk for Cancer
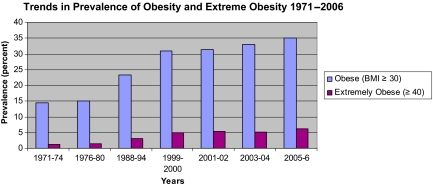
Although evidence shows that adult overweight and obesity are related to risk for many cancers, the growing epidemic of obesity provides a challenge to clinical practice and the implementation of guidelines for the management of weight. Historical data from the past 25 years point to obesity as a cause of approximately 14% of cancer deaths in men and up to 20% of cancer deaths in women [1]. These may be conservative estimates because the population has gained substantial weight over this time period and the prevalence of overweight and obesity has increased from 15% in 1980 to 35% in 2005 [2] (Fig. 1). Some now estimate that the total health burden of overweight and obesity may exceed that for cigarette smoking [3].
Figure 1.
Trends in obesity, U.S.
Abbreviation: BMI, body mass index.
Source: NCHS 2008. Available at http://www.cdc.gov/nchs/data/hestat/overweight/overweight_adult.htm.
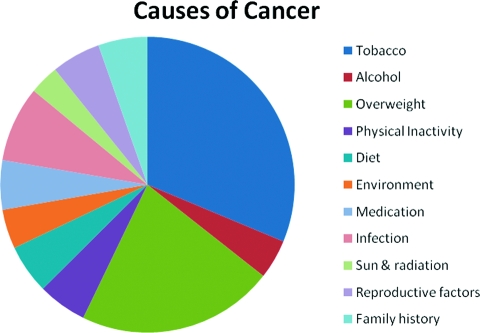
A major review of weight, physical activity, and cancer incidence by the International Agency for Research on Cancer (IARC) used obesity prevalence data from Europe and relative risks from a meta-analysis of published studies and concluded, in 2002, that obesity was a cause of 11% of colon cancer cases, 9% of postmenopausal breast cancer cases, 39% of endometrial cancer cases, 25% of kidney cancer cases, and 37% of esophageal cancer cases [4]. In addition, data from the American Cancer Society suggested that overweight and obesity were related to mortality from liver cancer, pancreatic cancer, non-Hodgkin's lymphoma, and myeloma [1]. This effect on mortality reflects both the excess incidence and excess mortality among those with cancer. Since the 2002 IARC report, substantial new evidence has supported a cause-and-effect relation between overweight and obesity and the onset of these cancers, further increasing the burden of cancer resulting from obesity [5]. The American Institute for Cancer Research and World Cancer Research Fund reported that there is convincing evidence for a relation between obesity and esophageal, pancreatic, colorectal, postmenopausal breast, endometrial, and kidney cancers, with probable evidence for gallbladder cancer. In addition, they found probable evidence that abdominal fatness, in particular, increases the risk for pancreatic, endometrial, and postmenopausal breast cancer. Finally, emerging evidence suggests that obesity increases risk for aggressive prostate cancer [6]. Overall, we estimate that overweight and obesity cause approximately 20% of all cancer cases. Previously Doll and Peto [7] included overnutrition (overweight) with diet causing a combined 35% of all cancer cases. In Figure 2, we break out overweight and obesity from diet and provide updated estimates for the causes of cancer.
Figure 2.
Estimated proportion of cancer in the U.S. that could have been avoided by changes in each category of nongenetic cancer causes.
To conclude that a cause-and-effect relation exists between obesity and cancer at each site, one often pursues studies of mechanisms that confirm the underlying biology of this relation and provide insights into prevention strategies. Take, for example, postmenopausal breast cancer. Among postmenopausal women, obesity is directly related to circulating estradiol levels [8], which themselves are directly related to breast cancer risk [9, 10]. When the action of estrogens is interrupted by estrogen receptor modulators in randomized controlled trials, breast cancer incidence is approximately 50% lower [11, 12].
Just as smoking cessation leads to a reduction in the risk for lung cancer, adding to the evidence of a cause-and-effect relation, a documented 50% reduction in the risk for breast cancer among women who lost ≥10 kg after menopause and kept it off [13] adds to our understanding of this causal relation. In addition, focusing on weight loss after menopause, a time in life when obesity clearly increases the risk for breast cancer, provides important evidence on the time frame for change in cause (weight) and subsequent change in cancer incidence. This lower incidence of postmenopausal breast cancer follows the decline in circulating estrogen after weight loss.
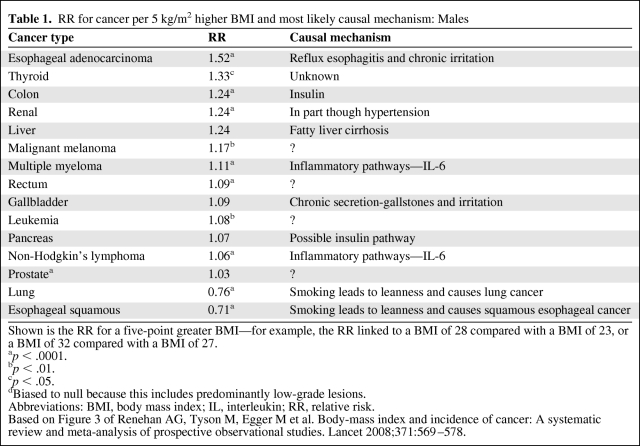
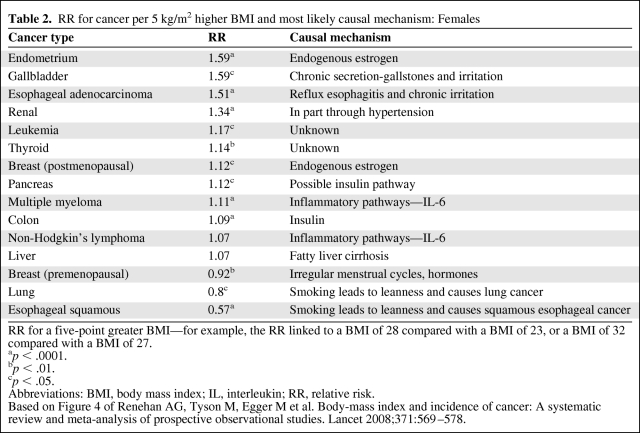
For colon cancer, growing evidence points to insulin pathways mediating the effect of body mass index BMI and risk [14]. Studies of blood glucose levels and colon cancer show a direct relation between higher glucose and subsequent risk [14]. Providing further biologic rationale, c-peptide [15], a marker of insulin production, also shows this positive relation, and animal models using insulin injection versus saline show a significantly higher incidence of colon cancer among those injected with insulin [16]. Finally, preclinical data provide additional support for the insulin–insulin-like growth factor (IGF) hypothesis of cancer risk, as outlined in several excellently detailed recent reviews [17, 18]. The phosphoinositide 3-kinase/Akt pathway likely compromises the downstream target of insulin, and is one of the pathways most commonly altered in epithelial tumors [18, 19]. In sum, strong evidence points to hyperinsulinemia as the direct pathway from adiposity to colon cancer. For each cancer site, we present summary estimates of relative risk from the rigorous meta-analysis by Renehan et al. [5] and the likely pathway or mechanism for a causal relation between obesity and cancer (Tables 1 and 2).
Table 1.
RR for cancer per 5 kg/m2 higher BMI and most likely causal mechanism: Males
Shown is the RR for a five-point greater BMI—for example, the RR linked to a BMI of 28 compared with a BMI of 23, or a BMI of 32 compared with a BMI of 27.
ap < .0001.
bp < .01.
cp < .05.
dBiased to null because this includes predominantly low-grade lesions.
Abbreviations: BMI, body mass index; IL, interleukin; RR, relative risk.
Based on Figure 3 of Renehan AG, Tyson M, Egger M et al. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–578.
Table 2.
RR for cancer per 5 kg/m2 higher BMI and most likely causal mechanism: Females
RR for a five-point greater BMI—for example, the RR linked to a BMI of 28 compared with a BMI of 23, or a BMI of 32 compared with a BMI of 27.
ap < .0001.
bp < .01.
cp < .05.
Abbreviations: BMI, body mass index; IL, interleukin; RR, relative risk.
Based on Figure 4 of Renehan AG, Tyson M, Egger M et al. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–578.
Of particular importance is the rapid rise in adenocarcinoma of the esophagus over the past 20 years. In parallel with the global epidemic of obesity, the morphology of esophageal cancer has shifted from squamous to adenocarcinoma, and a growing body of research points to the role of obesity in esophageal reflux, a pathway for this malignancy [20]. Increasing BMI in the Nurses' Health Study was associated with a significantly higher risk for reflux esophagitis [21]. Weight gain was associated with a higher risk for developing frequent reflux, and a weight loss of 3.5 kg was associated with a significantly lower risk for frequent symptoms of gastroesophageal reflux disease [21]. The role of weight loss and the time course of the lower risk for esophageal cancer remain to be documented.
Mechanisms Differ
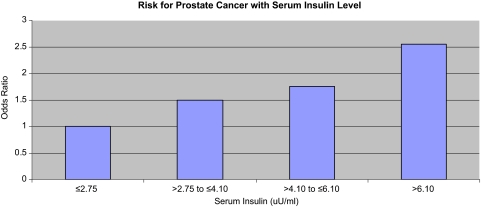
One concern raised by some is that obesity cannot cause cancer through so many different mechanisms. This opinion seems misplaced because the etiology of cancers is known to differ for different organ sites. Female hormones cause breast and endometrial cancer but have much less impact on other cancers, for example. Insulin may drive colon and prostate cancer [22] (Fig. 3), whereas inflammation may drive other malignancies. All these mechanisms can have a role in mediating the relation between obesity and cancer risk. As noted by Roberts et al. [17], in their review of the biologic mechanisms linking obesity and cancer risk, the pathophysiology of obesity is complex and multisystemic, and thus, it is unlikely that “one size fits all.”
Figure 3.
Serum insulin and risk for prostate cancer. p (trend) = .02.
Based on Table 3 of Albanes D, Weinstein SJ, Wright ME et al. Serum insulin, glucose, indices of insulin resistance, and risk of prostate cancer. J Natl Cancer Inst 2009;101:1272–1279.
Weight Loss and Cancer Risk Reduction
Despite extensive evidence showing a deleterious effect of overweight and obesity on cancer, relatively few data exist on the effects of weight gain or weight loss on altering the risk for cancer. The lack of data on weight loss is likely a function of the small number of individuals able to achieve a sustained weight loss.
The IARC evaluated data through 2000 and found limited evidence for an association between weight change and the risk for colorectal cancer [4]. However, subsequent studies have added evidence to support this adverse effect. Cohort studies examined changes in weight from early adulthood to later in life and found modestly higher relative risks (1.4–1.6). Case–control studies provided additional support. More recent evidence confirms that weight gain in adulthood appears to increase the risk for colon cancer. In a case–control study in Canada, men who gained ≥21 kg after the age of 20 had a 60% higher risk for colorectal cancer than men who had gained 1–5 kg [23]. The association was stronger when rectal tumors were excluded, suggesting that studies that examine the association between weight gain and colorectal cancer may underestimate the association for colon cancer. No association between weight gain and colorectal cancer risk was observed among women, for whom higher estrogen levels may counter the adverse effect of obesity through insulin pathways. Another study of men and women found that, compared with those whose BMI had remained stable, those whose BMI had increased from age 30 or 50 to diagnosis had a 25%–35% higher risk for colorectal cancer [24]. Finally, a study of Austrian adults found evidence for a direct association between weight loss and a reduction in colon cancer risk among men [25].
Perhaps the best evidence that weight loss can reduce the risk for cancer comes from recent studies in bariatric surgery patients. Emerging evidence from two large cohort studies suggests that large weight loss from bariatric surgery reduces the risk for cancer death [26, 27]. The mean weight loss was in the range of 14%–27% 15 years after surgery in the Swedish patient population [27]. In the U.S. patient sample, cancer death rates, excluding prevalent cancers, were 38% lower (hazard ratio [HR], 0.62; 95% confidence interval [CI], 0.61–0.74) in patients undergoing Roux-en-Y gastric bypass than in BMI-matched controls, with some indication that the reduction in the death rate was stronger in men than in women [26]. The cancer death rate reduction was larger when including prevalent cases of cancer at baseline (HR, 0.40; 95% CI, 0.25–0.65). The limited sample sizes in both studies precluded examination of cancer-specific rates, though Sjöström and colleagues noted that the results included both deaths from obesity-related cancers and deaths from cancers unrelated to obesity [27]. More recently, both studies demonstrated a lower cancer incidence in surgical patients. The Swedish study demonstrated a lower risk for cancer in women undergoing bariatric surgery than matched obese controls (HR, 0.58; 95% CI, 0.44–0.77), though no such effect was observed in men (HR, 0.97; 95% CI, 0.62–1.52) [28]. The amount of weight loss was not associated with cancer risk. Similarly, the U.S.-based study found a significant cancer risk reduction in bariatric surgery patients (HR, 0.76; 95% CI, 0.65–0.89). That study found that this risk reduction was largely concentrated in obesity-related cancers (i.e., esophageal adenocarcinoma, colorectal cancer, pancreatic cancer, postmenopausal breast cancer, corpus and uterus cancers, kidney cancer, non-Hodgkin's lymphoma, leukemia, multiple myeloma, liver cancer, and gallbladder cancer). Subsequent studies have reported similar cancer risk reductions [29, 30].
Ample observational data support a detrimental effect of obesity on the risk for several cancers, including breast and colon cancer, two of the most common cancers in North America and Europe. Examination of possible mechanisms provides further evidence that the observed associations have biologic rationale. Finally, growing research indicates that a change in weight is associated with subsequent changes in the risk for several cancers. Weight loss after menopause significantly reduces the risk for breast cancer [13]. Taken together, this indicates an important role for obesity and weight change in cancer risk.
Impact of Obesity Among Cancer Patients
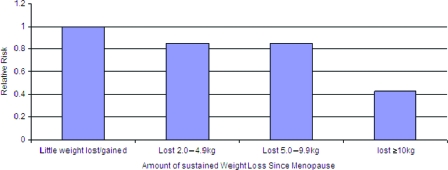
Far fewer studies address weight or weight change and survival following a cancer diagnosis. A long-standing association between obesity and poor outcomes for women with breast cancer [31, 32] has received increasing attention [33]. The majority of evidence points to weight at diagnosis as the major lifestyle risk for poor breast cancer outcomes (and poorer quality of life) [34], with growing evidence that weight gain after diagnosis exacerbates risk [33]—a result seen most clearly among women who were lean at diagnosis or nonsmokers. Nonsmoking women who gained >2 kg/m2 after a breast cancer diagnosis had a relative risk for death during 9 years of follow-up that was 1.64 (95% CI, 1.07–2.51), compared with stable weight women (Fig. 4). Insulin pathways have been suggested as one mechanism for this effect [35]. Weight gain following diagnosis may be particularly problematic because research suggests that it is largely an increase in fat mass and not muscle mass [36]. Furthermore, evidence that physical activity after diagnosis reduces the risk for breast cancer recurrence [37] and intervention trials of diet and physical activity that showed longer disease-free survival times among the intervention group, the members of which lost substantially more weight than the control group [38], all point to the importance of energy balance after breast cancer.
Figure 4.
Sustained weight loss and breast cancer risk in postmenopausal women who never used postmenopausal hormones.
Based on Table 3 of Eliassen AH, Colditz G, Rosner B et al. Adult weight change and risk of postmenopausal breast cancer. JAMA 2006;296:193–201.
Although obesity is not associated with prostate cancer incidence overall, it is associated with fatal and aggressive disease [6]. There is also emerging evidence that prostate cancer is more difficult to detect in obese men [6]. Obese men have a higher risk for recurrence following prostatectomy [6, 39, 40], but there is no evidence for higher rates of recurrence among obese men undergoing brachytherapy [6, 39, 41]. Obese men also have higher rates of death from prostate cancer [6]. However, some reports suggest that obese patients with metastatic prostate cancer may have better postdiagnosis outcomes, perhaps because obesity acts as a cachexia preventive [42].
Evidence for an effect of obesity on colon cancer survival is mixed. Meyerhardt et al. [43] found no association with disease-free survival time or overall mortality (patients all received weight-based treatment, so this could not be attributed to chemotherapy underdosing). Subsequent reports have indicated that those who are very obese (class 2 obesity) have greater overall mortality and shorter disease-free survival intervals [44–46]. However, the one study to look at the effects of weight change found no association with survival outcomes [44]. In addition, in one study [45], chemotherapy dose was capped for the very obese, which may confound the findings. The implications of worse outcomes for the morbidly obese are particularly worrisome because this is a rapidly increasing segment of the population.
In addition to the impact of obesity on disease incidence and progression, concern has been raised regarding the potential for dosing of therapy to be poorly matched to weight in heavier cancer patients. The narrow therapeutic index associated with many chemotherapeutic drugs prompts a rational concern on the part of the medical oncologist that the high doses of chemotherapy required by the very obese will result in excess toxicity. Research in this area has suggested that obese patients are frequently treated at lower chemotherapy dose intensities than the nonobese. Paradoxically, studies have not demonstrated greater chemotherapy-related toxicity in obese patients treated at full dose intensities [47]. This refutes the notion that obese patients should uniformly receive dose-reduced therapy. For obese patients being treated with curative intent, dose reductions should be approached with particular caution.
A similar problem with regard to chemotherapy in obese patients surrounds the common use of body surface area (BSA) for dose calculation. Historically, cytotoxic chemotherapeutic agents have been dosed this way based on the assumption that metabolism is proportional to BSA, a theory first described by Rubner in 1883. One of the more commonly used formulae is based on measurements performed on a small number of individuals, reported nearly 100 years ago [48]. Although the BSA approach may be helpful when extrapolating doses between mice and humans during the translational period from preclinical to early-phase clinical studies, some have questioned whether this approach is appropriate in clinical practice [49]. For example, a man who is 180-cm tall with an ideal body weight of 71 kg has a BSA of 1.9, according to the Dubois formula, whereas another man who is 180-cm tall and who weighs twice as much (142 kg) has a BSA of 2.55. The BSA in the latter man is 34% greater than the BSA in the man with ideal body weight, whereas the weight of the latter man is 100% greater. Particularly for drugs with any degree of fat solubility (higher volume of distribution), BSA dosing is likely to result in systematic underdosing, compared with weight-based dosing. With increasing attention being paid to chemotherapy dose intensity as a predictor of treatment response in numerous tumor types, careful reconsideration of the optimal dosing in obese patients may be appropriate for some drugs [47, 50].
Obesity may also interfere with the ability to deliver other forms of treatment. Wong et al. [51] found that there was a shift in the delivery of external-beam radiation in obese patients, resulting in the target location not receiving the full dose. In addition, research has suggested that, among men undergoing prostatectomy, surgical margins may not be as clean in obese men and they may have fewer nerve bundles preserved [52].
Quality of life among cancer patients and those free from cancer is reduced by higher BMI. Limited data suggest that weight loss is associated with improved quality of life. More substantial data point to an increase in physical activity among cancer survivors leading to significant increases in quality of life [53].
Mechanisms
Among cancer survivors, the likely pathways from obesity leading to recurrence and death again may vary by tumor site. Insulin receptors are increasingly being studied as a mechanism for obesity to have adverse effects among breast cancer survivors. The insulin receptor is overexpressed and may bind both insulin and IGF-II [54]. Among colon cancer patients, the insulin pathway has again been identified as potentially mediating adverse outcomes, and recent research addressing obesity and STMN1 (stathmin or oncoprotein-18), which destabilizes microtubules and reorganizes cytoskeleton and functions in cell progression and cell migration, indicates that the adverse effect of BMI among colon cancer patients may be limited to those who are STMN1+ [55].
Metformin is associated with a lower cancer incidence among diabetics [56] and with lower cancer mortality among patients with type 2 diabetes [57]. Among mechanisms for such a benefit are the inhibition of cancer cell growth, suppression of ErbB-2 oncoprotein overexpression, and inhibition of mammalian target of rapamycin [58–60]. This rapidly expanding area of laboratory and human evidence points to a role for metformin among diabetic patients, with clinical trials now proposed [61].
Inflammation is also a source of great interest. Adipocytes release inflammatory markers, which are increasingly being found to be associated with a worse postdiagnosis prognosis. In the Healthy Eating, Activity, and Living (HEAL) study, serum amyloid a and c-reactive protein levels were associated with shorter overall survival in breast cancer patients [62]. As with the hypothesized mechanisms linking obesity to cancer risk, ample preclinical data detail the linkages in these pathways [17, 18].
Weight Loss and Intermediate Endpoints
Many randomized controlled trials of weight loss and measures of insulin, glucose, and blood pressure show a strong response to an increase in activity and a reduction in adiposity [63]. Lack of energy expenditure in the form of physical activity is a leading determinant of higher body mass. For those who are already overweight, physical activity, in combination with dietary changes, is important for achieving, and particularly for maintaining, weight loss [63]. There is increasing evidence [64] that attaining >9,000 daily steps as an indicator of physical activity is associated with a lower likelihood of being obese, including among lower income and racial/ethnic minority populations. TV viewing is a highly prevalent sedentary activity; Americans watch at least 4 hours of TV each day and TV viewing is the most prevalent activity after work and sleep. TV viewing is positively associated with excess body weight among children and adults [65, 66]. TV viewing may also promote obesity through the promotion of consumption of calorically dense foods through advertising and other media content [67]. The strong and consistent association between TV viewing and obesity suggests the importance of including reduction in TV hours as a target in behavioral weight reduction efforts.
Benefits of Weight Loss and Increased Physical Activity Among Cancer Survivors
Strong evidence supports the idea that an increase in physical activity leads to improved quality of life among cancer survivors [53]. Among men after prostate surgery, being lean and physically active is associated with superior symptoms of incontinence, compared with obese and inactive men, suggesting a role for weight loss after prostate resection to improve quality of life [68].
The National Heart, Lung and Blood Institute evidence review on weight loss [63] evaluated 86 randomized, controlled trials and concluded that “low calorie diets are recommended for weight loss in overweight and obese persons” (evidence category A) and that “Physical activity is recommended as part of a comprehensive weight loss therapy and weight control program because it modestly contributes to weight loss” (evidence category A) [63]. Specific recommended intake levels vary based on a number of factors, including current weight, activity levels, and weight loss goals. There are many other behavioral factors that influence weight loss and may be more effectively intervened upon over an extended intervention period to achieve sustained weight loss. We outline several of these in Figure 5. Rather than focus on specific calorie calculations, we recommend behavioral targets that should lead to an energy deficit as well as increase and maintain patient motivation.
Figure 5.
Physical activity, diet, and behavioral goals for sustained weight loss.
Counseling for Weight Loss
Providers play a central role in guiding their patients to follow strategies to reduce or maintain their weight and thus improve their quality of life and reduce their risk for developing a primary malignancy, or improve survival among those diagnosed with cancer. Given the prevalence of overweight and obesity, weight loss is a sound strategy for reducing the risk for cancer. Here, we briefly outline strategies that a provider can use to counsel patients to maintain weight and achieve a sustained weight loss.
For effective weight change interventions, one would like to have on hand strategies to address individual behaviors, social factors that might reinforce individual behavior change, and environmental strategies (such as safe sidewalks and effective transportation systems, etc.) [69] that promote or discourage a given behavior.
The Diabetes Prevention Trial demonstrated the efficacy of an intensive health care provider–based intervention to reduce weight and increase physical activity over a 24-month period and thereby prevent diabetes [70]. Translating this efficacy study into effective approaches that work in the broader clinical setting remains to be demonstrated. One approach, derived from the methodology originally developed by the National Cancer Institute to guide physicians in counseling their patients to quit smoking, the “Five As” model guides clinicians through a counseling session, with each “A” corresponding to a brief behavioral intervention—assess, advise, agree, assist, arrange — which together have been shown to be effective. Clinicians may use the Five As approach to deliver behavior change strategy messages (such as those in Fig. 5).
Interactive technologies can also address a wide variety of health behavior domains simultaneously. Thus, interactive technology can provide a streamlined, consistent method for conducting many aspects of evidence-based behavior change counseling, including assessing current health behaviors, identifying barriers to change, allowing the patient to set goals and select relevant activities, and arranging follow-up support.
Evidence from randomized trials demonstrates that the use of interactive technologies supports lifestyle behavior change [71], although most of this research was conducted using a single-disease and/or single behavior change focus. Practice-based research indicates that clinicians who have limited financial, space, and personnel resources favor tools that address comorbid conditions and health behaviors simultaneously. In addition, tools that can be accessed via patients' homes or from community settings are useful and effective and appeal to clinicians who face barriers to providing self-management care tools [71].
Thus, there is great interest in the ability of interactive and Web-based tools to deliver and reinforce behavior change strategies in a cost-effective manner. Increasingly, Web-based tools for supporting positive lifestyle changes are making their way into the clinical setting. However, it is unclear whether such interventions are effective. An increasing number of studies are beginning to assess their potential to reduce weight, both in the health care setting and beyond.
Summary of Provider Role
Because the factors that influence energy balance range from the individual to the environment, efforts to shift the population distribution of physical activity upward and diet toward healthier choices will require emphasis on all the intervention points: health care settings, communities, and environment. The challenge for providers is to engage the patient in understanding the importance of weight control and increased physical activity to begin on the path to behavior change.
Safety Issues
Although the benefits of weight maintenance and greater physical activity are well established for many chronic diseases, the risks must also be considered. These include injuries, alteration in glucose control for diabetics, and sustained increases in joint pain. In general, these risks are outweighed by the vast benefits of weight loss and increased physical activity and can generally be reduced with simple steps. To date, drug-based strategies to aid with weight loss have had side effects that reduce adherence.
Conclusion
Obesity causes a substantial proportion of all cancers, and emerging evidence suggests that adult weight loss reduces cancer risk. Increasing physical activity and avoiding weight gain after cancer, as in adult life, have substantial benefits.
Acknowledgment
G.A.C. is supported by an American Cancer Society Clinical Research Professorship.
Author Contributions
Conception/design: Graham A. Colditz, Kathleen Y. Wolin, Kenneth Carson
Collection and/or assembly of data: Graham A. Colditz, Kathleen Y. Wolin, Kenneth Carson
Data analysis and interpretation: Graham A. Colditz, Kathleen Y. Wolin, Kenneth Carson
Manuscript writing: Graham A. Colditz, Kathleen Y. Wolin, Kenneth Carson
Final approval of manuscript: Graham A. Colditz, Kathleen Y. Wolin, Kenneth Carson
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 3.Stewart ST, Cutler DM, Rosen AB. Forecasting the effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2009;361:2252–2260. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer. Weight Control and Physical Activity. Volume 6. Lyon: International Agency for Research on Cancer; 2002. pp. 1–315. [Google Scholar]
- 5.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 6.Freedland SJ, Platz EA. Obesity and prostate cancer: Making sense out of apparently conflicting data. Epidemiol Rev. 2007;29:88–97. doi: 10.1093/epirev/mxm006. [DOI] [PubMed] [Google Scholar]
- 7.Doll R, Peto R. New York: Oxford University Press; 1981. The Causes of Cancer: Quantitative Estimates of Avoidable Risks of Cancer in the United States Today; pp. 1–115. [PubMed] [Google Scholar]
- 8.Hankinson SE, Colditz GA, Hunter DJ, et al. Reproductive factors and family history of breast cancer in relation to plasma estrogen and prolactin levels in postmenopausal women in the Nurses' Health Study (United States) Cancer Causes Control. 1995;6:217–224. doi: 10.1007/BF00051793. [DOI] [PubMed] [Google Scholar]
- 9.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 10.Missmer SA, Eliassen AH, Barbieri RL, et al. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96:1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 11.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 12.Martino S, Cauley JA, Barrett-Connor E, et al. Continuing outcomes relevant to Evista: Breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 13.Eliassen AH, Colditz GA, Rosner B, et al. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: A review. Am J Clin Nutr. 2007;86:s836–s842. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 15.Wei EK, Ma J, Pollak MN, et al. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2005;14:850–855. doi: 10.1158/1055-9965.EPI-04-0661. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6:164–179. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 17.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: New perspectives. Annu Rev Med. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 18.Hursting SD, Lashinger LM, Wheatley KW, et al. Reducing the weight of cancer: Mechanistic targets for breaking the obesity-carcinogenesis link. Best Pract Res Clin Endocrinol Metab. 2008;22:659–669. doi: 10.1016/j.beem.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Carling D. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson BC, Somers SC, Fuchs CS, et al. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340–2348. doi: 10.1056/NEJMoa054391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albanes D, Weinstein SJ, Wright ME, et al. Serum insulin, glucose, indices of insulin resistance, and risk of prostate cancer. J Natl Cancer Inst. 2009;101:1272–1279. doi: 10.1093/jnci/djp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell PT, Cotterchio M, Dicks E, et al. Excess body weight and colorectal cancer risk in Canada: Associations in subgroups of clinically defined familial risk of cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1735–1744. doi: 10.1158/1055-9965.EPI-06-1059. [DOI] [PubMed] [Google Scholar]
- 24.Russo A, Franceschi S, La Vecchia C, et al. Body size and colorectal-cancer risk. Int J Cancer. 1998;78:161–165. doi: 10.1002/(sici)1097-0215(19981005)78:2<161::aid-ijc7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 25.Rapp K, Klenk J, Ulmer H, et al. Weight change and cancer risk in a cohort of more than 65,000 adults in Austria. Ann Oncol. 2008;19:641–648. doi: 10.1093/annonc/mdm549. [DOI] [PubMed] [Google Scholar]
- 26.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 27.Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 28.Sjöström L, Gummesson A, Sjöström CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): A prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–662. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 29.McCawley GM, Ferriss JS, Geffel D, et al. Cancer in obese women: Potential protective impact of bariatric surgery. J Am Coll Surg. 2009;208:1093–1098. doi: 10.1016/j.jamcollsurg.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 30.Christou NV, Lieberman M, Sampalis F, et al. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis. 2008;4:691–695. doi: 10.1016/j.soard.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 31.Coates RJ, Clark WS, Eley JW, et al. Race, nutritional status, and survival from breast cancer. J Natl Cancer Inst. 1990;82:1684–1692. doi: 10.1093/jnci/82.21.1684. [DOI] [PubMed] [Google Scholar]
- 32.Goodwin PJ, Boyd NF. Body size and breast cancer prognosis: A critical review of the evidence. Breast Cancer Res Treat. 1990;16:205–214. doi: 10.1007/BF01806329. [DOI] [PubMed] [Google Scholar]
- 33.Kroenke CH, Chen WY, Rosner B, et al. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 34.Fine JT, Colditz GA, Coakley EH, et al. A prospective study of weight change and health-related quality of life in women. JAMA. 1999;282:2136–2142. doi: 10.1001/jama.282.22.2136. [DOI] [PubMed] [Google Scholar]
- 35.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 36.Demark-Wahnefried W, Peterson BL, Winer EP, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19:2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 37.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 38.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 39.Stroup SP, Cullen J, Auge BK, et al. Effect of obesity on prostate-specific antigen recurrence after radiation therapy for localized prostate cancer as measured by the 2006 Radiation Therapy Oncology Group-American Society for Therapeutic Radiation and Oncology (RTOG-ASTRO) Phoenix consensus definition. Cancer. 2007;110:1003–1009. doi: 10.1002/cncr.22873. [DOI] [PubMed] [Google Scholar]
- 40.Jayachandran J, Schroeck F, Sun L, et al. The Shared Equal Access Regional Cancer Hospital (SEARCH) nomogram for risk stratification in intermediate risk group of men with prostate cancer: Validation in the Duke Prostate Center database. BJU Int. 2010;105:180–184. doi: 10.1111/j.1464-410X.2009.08728.x. [DOI] [PubMed] [Google Scholar]
- 41.Merrick GS, Butler WM, Wallner KE, et al. Influence of body mass index on biochemical outcome after permanent prostate brachytherapy. Urology. 2005;65:95–100. doi: 10.1016/j.urology.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 42.Halabi S, Ou SS, Vogelzang NJ, et al. Inverse correlation between body mass index and clinical outcomes in men with advanced castration-recurrent prostate cancer. Cancer. 2007;110:1478–1484. doi: 10.1002/cncr.22932. [DOI] [PubMed] [Google Scholar]
- 43.Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–495. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 44.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: Findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26:4109–4115. doi: 10.1200/JCO.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98:1647–1654. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 46.Sinicrope FA, Foster NR, Sargent DJ, et al. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010;16:1884–1893. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005;165:1267–1273. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 48.Dubois D, Dubois EF. Title? Arch of Int Med. 1916;17:863–871. [Google Scholar]
- 49.Mathijssen RH, de Jong FA, Loos WJ, et al. Flat-fixed dosing versus body surface area based dosing of anticancer drugs in adults: Does it make a difference? The Oncologist. 2007;12:913–923. doi: 10.1634/theoncologist.12-8-913. [DOI] [PubMed] [Google Scholar]
- 50.Pettengell R, Schwenkglenks M, Bosly A. Association of reduced relative dose intensity and survival in lymphoma patients receiving CHOP-21 chemotherapy. Ann Hematol. 2008;87:429–430. doi: 10.1007/s00277-008-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong JR, Gao Z, Merrick S, et al. Potential for higher treatment failure in obese patients: Correlation of elevated body mass index and increased daily prostate deviations from the radiation beam isocenters in an analysis of 1,465 computed tomographic images. Int J Radiat Oncol Biol Phys. 2009;75:49–55. doi: 10.1016/j.ijrobp.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 52.Freedland SJ, Isaacs WB, Platz EA, et al. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: A search database study. J Clin Oncol. 2005;23:7546–7554. doi: 10.1200/JCO.2005.05.525. [DOI] [PubMed] [Google Scholar]
- 53.Speed-Andrews AE, Courneya KS. Effects of exercise on quality of life and prognosis in cancer survivors. Curr Sports Med Rep. 2009;8:176–181. doi: 10.1249/JSR.0b013e3181ae98f3. [DOI] [PubMed] [Google Scholar]
- 54.Frasca F, Pandini G, Sciacca L, et al. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008;114:23–37. doi: 10.1080/13813450801969715. [DOI] [PubMed] [Google Scholar]
- 55.Ogino S, Nosho K, Baba Y, et al. A cohort study of STMN1 expression in colorectal cancer: Body mass index and prognosis. Am J Gastroenterol. 2009;104:2047–2056. doi: 10.1038/ajg.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer: A cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landman GW, Kleefstra N, van Hateren KJ, et al. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33:322–326. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–915. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 59.Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle. 2009;8:88–96. doi: 10.4161/cc.8.1.7499. [DOI] [PubMed] [Google Scholar]
- 60.Vázquez-Martin A, Oliveras-Ferraros C, del Barco S, et al. mTOR inhibitors and the anti-diabetic biguanide metformin: New insights into the molecular management of breast cancer resistance to the HER2 tyrosine kinase inhibitor lapatinib (Tykerb) Clin Transl Oncol. 2009;11:455–459. doi: 10.1007/s12094-009-0384-0. [DOI] [PubMed] [Google Scholar]
- 61.Goodwin PJ, Ligibel JA, Stambolic V. Metformin in breast cancer: Time for action. J Clin Oncol. 2009;27:3271–3273. doi: 10.1200/JCO.2009.22.1630. [DOI] [PubMed] [Google Scholar]
- 62.Pierce BL, Ballard-Barbash R, Bernstein L, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.National Institutes of Health. Obesity Initiative Expert Panel. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6(suppl 2):51s–209s. [PubMed] [Google Scholar]
- 64.Bennett GG, Wolin KY, Puleo E, et al. Pedometer-determined physical activity among multiethnic low-income housing residents. Med Sci Sports Exerc. 2006;38:768–773. doi: 10.1249/01.mss.0000210200.87328.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berkey CS, Rockett HR, Gillman MW, et al. One-year changes in activity and in inactivity among 10- to 15-year-old boys and girls: Relationship to change in body mass index. Pediatrics. 2003;111:836–843. doi: 10.1542/peds.111.4.836. [DOI] [PubMed] [Google Scholar]
- 66.Hu FB, Li TY, Colditz GA, et al. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289:1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 67.Bennett GG, Wolin KY, Viswanath K, et al. Television viewing and pedometer-determined physical activity among multiethnic residents of low-income housing. Am J Public Health. 2006;96:1681–1685. doi: 10.2105/AJPH.2005.080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolin KY, Luly J, Sutcliffe S, et al. Risk of urinary incontinence following prostatectomy: The role of physical activity and obesity. J Urol. 2010;183:629–633. doi: 10.1016/j.juro.2009.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sallis J, Owen N. Physical Activity and Behavioral Medicine. Volume 3. Thousand Oaks, CA: SAGE Publications Ltd; 1999. pp. 1–210. [Google Scholar]
- 70.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glasgow RE, Bull SS, Piette JD, et al. Interactive behavior change technology. A partial solution to the competing demands of primary care. Am J Prev Med. 2004;27(2 suppl):80–87. doi: 10.1016/j.amepre.2004.04.026. [DOI] [PubMed] [Google Scholar]