Abstract
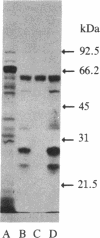
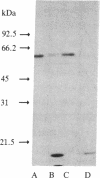
Specific monoclonal antibody coupled to Affi-Gel 10 was used to purify a major membrane glycoprotein of Leishmania mexicana amazonensis, one of a group of parasitic protozoa that specifically infect mammalian macrophages. Immobilized antigen was eluted at a 34% efficiency with buffers at either pH 2.5 or 11 or with MgCl2, but only the antigen eluted under basic conditions could be readsorbed to the immunobeads. Sephacryl S-300 gel filtration of the purified antigen gave a single peak of protein estimated to have a molecular mass of 400 kDa. However, NaDodSO4/polyacrylamide gel electrophoresis showed a single band of this protein with an apparent molecular mass of 63 kDa. The antigen is an N-linked glycoprotein, as indicated by its increase in electrophoretic mobility after treatment with endoglycosidase H and by its binding to lentil lectin-Sepharose, elutable with methyl alpha-D-mannoside and methyl alpha-D-glucoside. Purified antigen inhibits the binding of leishmania cells to macrophages by 50%, suggesting that it may play a role in the process of infection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell J. M., Ezekowitz R. A., Roberts M. B., Channon J. Y., Sim R. B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985 Jul 1;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P. Cellular and molecular mechanisms of intracellular symbiosis in leishmaniasis. Int Rev Cytol Suppl. 1983;14:267–305. [PubMed] [Google Scholar]
- Chang K. P. Human cutaneous lieshmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science. 1980 Sep 12;209(4462):1240–1242. doi: 10.1126/science.7403880. [DOI] [PubMed] [Google Scholar]
- Chang K. P. Leishmania donovani-macrophage binding mediated by surface glycoproteins/antigens: characterization in vitro by a radioisotopic assay. Mol Biochem Parasitol. 1981 Nov;4(1-2):67–76. doi: 10.1016/0166-6851(81)90030-x. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M., Gottlieb M. The surface membrane chemistry of Leishmania: its possible role in parasite sequestration and survival. J Cell Biochem. 1983;23(1-4):35–45. doi: 10.1002/jcb.240230105. [DOI] [PubMed] [Google Scholar]
- Fong D., Chang K. P. Surface antigenic change during differentiation of a parasitic protozoan, Leishmania mexicana: Identification by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7366–7370. doi: 10.1073/pnas.79.23.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Goding J. W. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985 Feb;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T., Toyoshima S., Osawa T. Lectin-like molecules on the murine macrophage cell surface. Biochim Biophys Acta. 1984 Nov 13;805(3):235–244. doi: 10.1016/0167-4889(84)90078-8. [DOI] [PubMed] [Google Scholar]
- Jaffe C. L., Bennett E., Grimaldi G., Jr, McMahon-Pratt D. Production and characterization of species-specific monoclonal antibodies against Leishmania donovani for immunodiagnosis. J Immunol. 1984 Jul;133(1):440–447. [PubMed] [Google Scholar]
- Kasper L. H., Crabb J. H., Pfefferkorn E. R. Purification of a major membrane protein of Toxoplasma gondii by immunoabsorption with a monoclonal antibody. J Immunol. 1983 May;130(5):2407–2412. [PubMed] [Google Scholar]
- Nakagawa H., Kotani T., Ohtaki S., Nakamura M., Yamazaki I. Purification of thyroid peroxidase by monoclonal antibody-assisted immunoaffinity chromatography. Biochem Biophys Res Commun. 1985 Feb 28;127(1):8–14. doi: 10.1016/s0006-291x(85)80118-2. [DOI] [PubMed] [Google Scholar]
- Remaley A. T., Das S., Campbell P. I., LaRocca G. M., Pope M. T., Glew R. H. Characterization of Leishmania donovani acid phosphatases. J Biol Chem. 1985 Jan 25;260(2):880–886. [PubMed] [Google Scholar]
- Schwarz R. T., Datema R. The lipid pathway of protein glycosylation and its inhibitors: the biological significance of protein-bound carbohydrates. Adv Carbohydr Chem Biochem. 1982;40:287–379. doi: 10.1016/s0065-2318(08)60111-0. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Friesen H. G. Solubilization and purification of a prolactin receptor from the rabbit mammary gland. J Biol Chem. 1974 Dec 25;249(24):7902–7911. [PubMed] [Google Scholar]
- Staehelin T., Hobbs D. S., Kung H., Lai C. Y., Pestka S. Purification and characterization of recombinant human leukocyte interferon (IFLrA) with monoclonal antibodies. J Biol Chem. 1981 Sep 25;256(18):9750–9754. [PubMed] [Google Scholar]
- Stahl P., Gordon S. Expression of a mannosyl-fucosyl receptor for endocytosis on cultured primary macrophages and their hybrids. J Cell Biol. 1982 Apr;93(1):49–56. doi: 10.1083/jcb.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. S., Nelson R. S., Silverstein S. C. Yeast mannans inhibit binding and phagocytosis of zymosan by mouse peritoneal macrophages. J Cell Biol. 1983 Jan;96(1):160–166. doi: 10.1083/jcb.96.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Harari I., Schlessinger J. Purification of an active EGF receptor kinase with monoclonal antireceptor antibodies. J Biol Chem. 1985 Jan 10;260(1):315–319. [PubMed] [Google Scholar]