The present study assessed subsequent cancer risks in type 2 diabetes patients first hospitalized for this disease at age >39 years. Twenty-four cancer types showed an elevated risk when follow-up was started after the last hospitalization for type 2 diabetes. No additional risk was found in familial diabetics.
Keywords: Diabetes, Cancer, Risk factors, Liver cancer, Prostate cancer
Abstract
Objectives.
Cancer and type 2 diabetes (T2D) are two common diseases that may share risk factors. We aimed at determining subsequent cancer risks in patients hospitalized for T2D in Sweden.
Methods.
T2D patients were obtained from the nationwide Hospital Discharge Register; cancers were recorded from the Swedish Cancer Registry. Standardized incidence ratios (SIRs) were calculated for cancer following last hospitalization for T2D. The comparison group was the general Swedish population.
Results.
The number of hospitalized T2D patients from 1964 to 2007 was 125,126, of whom 26,641 had an affected family member. Altogether 24 cancers showed an elevated risk when follow-up was started after the last hospitalization. The highest SIRs were for pancreatic (6.08) and liver (4.25) cancers. The incidences of these cancers were even elevated when follow-up was started 5 years after the last hospitalization for T2D, with primary liver cancer showing the highest SIR of 4.66. Also increased were the incidences of upper aerodigestive tract, esophageal, colon, rectal, pancreatic, lung, cervical, endometrial, ovarian, and kidney cancers. Prostate cancer showed a lower risk. Familial T2D patients showed no exceptional elevated cancer risks but their prostate cancer and melanoma risks were lower.
Conclusions.
This study, covering approximately one half of Swedish T2D patients, showed an elevated risk for several cancers after hospitalization for T2D, probably indicating the profound metabolic disturbances of the underlying disease. The highest risks were found for liver and pancreatic cancers. No excess cancer risks were observed in familial diabetics. The lower risk for prostate cancer remains intriguing.
Introduction
Type 2 diabetes (T2D) is considered to be one of the major public health challenges. The prevalence of T2D is in the range of 2%–10% in industrialized countries, with an increasing trend [1–3]. T2D is characterized by a loss of metabolic fuel homeostasis caused by insulin resistance, that is, the inability of tissues to respond to insulin [4]. As the normal functions of insulin, including the control of glucose uptake into peripheral tissues and suppression of the release of stored lipids from adipose tissue, become defective, hyperglycemia and dyslipidemia follow. The chronic increase in circulating glucose and lipid levels can further impair insulin secretion and lead to progressive β-cell failure. T2D is often associated with and preceded by obesity and metabolic syndrome [5]. The disease is thought to be caused by environmental and inherited factors in about equal proportions [6]. Many environmental risks factors are known, and they include obesity, sedentary lifestyle, low or high birth weight, stress, nutritional factors, and toxins [1, 2]. Tobacco smoking has been found to be a risk factor with a relative risk of about 1.6 for smokers of one pack or more per day [7]. The data on alcohol consumption are controversial, but most data suggest a U-shaped relationship between consumption and T2D [8]. Family history is an important risk factor that has been shown in twins and singleton siblings [1, 6, 9–13]. Recent genetic studies have revealed some 20 genes/loci to be associated with T2D [6, 12]. T2D is also manifested in rare Mendelian forms that account for <2%–5% of all cases and that are of early onset [6]. Some 10% of T2D patients could be diagnosed with latent autoimmune diabetes of the adult [14, 15].
T2D shares risk factors with cancer, such as obesity and sedentary lifestyle. It is also conceivable that the chronic metabolic and hormonal disturbances that are characteristics of T2D predispose to cancer; these include aberrations in the insulin-like growth factor pathway and steroid hormone metabolism [16–18]. Consequently, there is evidence that T2D is associated with elevated risks for and mortality from liver, pancreatic, breast, endometrial, kidney, bladder, and colorectal cancers and non-Hodgkin's lymphoma [16–22].
In the present study, we assessed subsequent cancer risks in T2D patients first hospitalized for T2D at age >39 years. The age limit and some other inclusion criteria were applied because “diabetes mellitus” has been distinguished as T2D only since 1997. The present nationwide study on 125,126 T2D patients is the largest yet published and has statistical power to address cancer risk at sites for which data are lacking or inconclusive. As a novel approach, a separate analysis of patients with a family history of T2D was carried out in order to test for shared genetic and environmental risk factors for T2D and cancer.
Materials and Methods
All the databases used in this study were nationwide, covering the whole population of Sweden over a defined period of time (9.0 million in 2005). The research database used for this study was a subset of the national MigMed 2 datasets at the Center for Primary Health Care Research, Malmö, Lund University. The MigMed database was compiled using data from several national Swedish registers provided by Statistics Sweden, including the Multigeneration Register, in which persons (second generation) born in Sweden in 1932 and thereafter are registered shortly after birth and are linked to their parents (first generation). Sibships could be defined for the second generation. National Census Data (1960–1990) and the Swedish population register (1990–2001) were incorporated into the database to obtain information on individuals' socioeconomic status. Dates of hospitalization for T2D were obtained from the Swedish Hospital Discharge Register for the years 1964–2007. Patients registered for hospitalization stayed at least one night in the hospital, usually in wards with specialists; the Register does not include outpatients in hospitals or health care centers. Diagnoses were reported according to the different versions of the International Classification of Diseases (ICD), and only the primary diagnosis was considered. The codes for T2D and type 1 diabetes were first separated in ICD-10 (≥1997) and we thus included only patients aged >39 years at first hospitalization; this age limit is the same one used by the National Swedish Diabetes Registry [23]. Further, in order to minimize the number of patients with type 1 diabetes, from 1997 onward, only the diagnosis of T2D was accepted and anyone who was diagnosed with any non-T2D diagnosis in ICD-10 was removed from the analysis. All linkages were performed using the national 10-digit civic identification number that is assigned to each person in Sweden for his or her lifetime. This number was replaced by a serial number for each person in order to provide anonymity and to check that each individual was entered only once, for his or her first hospitalization for T2D. Mistakes in the civic identification number are rare because it contains a control code. For cancer registration, the accuracy is very high because of established routines [24]. Quality checks on the Hospital Discharge Register have shown large differences based on, for example, diagnosis, hospital, and time, with coding errors given at 6%–8%; no representative data are available for T2D. However, the very high familial risks that we have reported for T2D in families of multiple affected individuals would not be possible without a reasonable diagnostic accuracy [13] (see also Discussion). Over 11.8 million individuals in >3.5 million families were included in this database; 8.9 million individuals belonged to the second generation, which had reached the age of 75 years at the end of the follow-up, which spanned 1964–2007 [25]. Family history was defined through hospitalization for T2D in a first-degree relative [13].
Cancer cases were obtained from the nationwide Swedish Cancer Registry. Person-years of follow-up were calculated from the start of follow-up on January 1, 1964 until hospitalization for T2D, death, emigration, or the closing date, December 31, 2007. The median follow-up time was 15 years from the last hospitalization, 13 years when the follow-up was started 1 year later, and 9 years when it was started 5 years later. Last hospitalization and the various follow-up times were used in order to reduce the possibility for biased surveillance in patients who underwent treatment. Standardized incidence ratios (SIRs) were calculated as the ratio of observed to the expected number of cases. Expected numbers were calculated for anyone not hospitalized for T2D, that is, for the whole Swedish population >39 years old and not hospitalized for T2D. The expected numbers were calculated as age (5-year groups), sex, period (5-year groups), region, and socioeconomic status–specific standard incidence rates. An additional adjustment was made for hospitalization for obesity using codes ICD-7 = 287.00, 287.09; ICD-8 = 277.99; ICD-9 = 278A; and ICD-10 = E65-E68 [26]. In total, 30,020 individuals were hospitalized for obesity and 1,171 of those were T2D patients. Ninety-five percent confidence intervals (95% CI) were calculated assuming a Poisson distribution, and they were adjusted for dependence between sibling pairs [27].
The study was approved by the regional ethical review board at Lund University.
Results
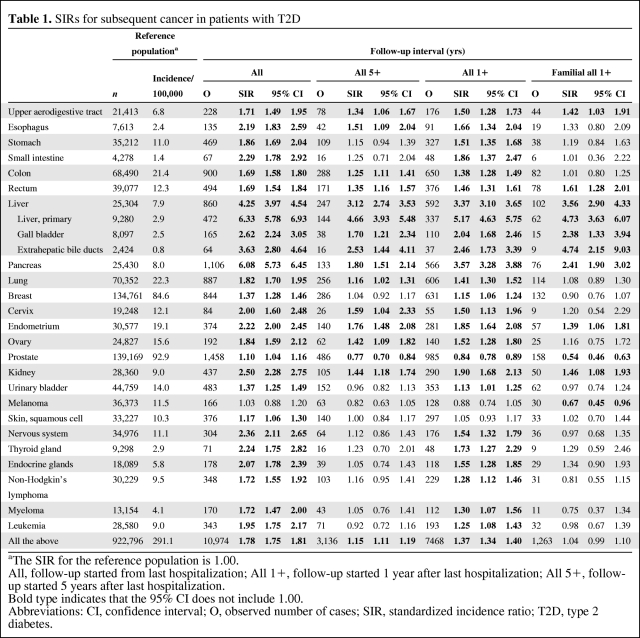
The number of T2D patients aged >39 years at first hospitalization and fulfilling the other entrance criteria was 125,126, of whom 51,468 were first hospitalized at age >69 years; 26,641 patients had an affected family member. In Table 1, the first columns show the number of cases and incidences in the reference population, those not hospitalized for T2D. The SIR for the reference population was 1.00. Follow-up for cancer was started after last hospitalization for T2D. Cancer risks for T2D patients are shown in Table 1 separately for the whole follow-up period (All) and for the follow-up that started 1 year (All 1+) or 5 years (All 5+) after hospitalization for T2D, because the SIRs were higher for All than for the other follow-up periods, probably because of a concomitant diagnosis of T2D and cancer. This was seen in the decreasing SIRs for all cancers, 1.78 for All, 1.37 for All 1+, and 1.15 for All 5+. Only sites with ≥50 cases or significant SIRs for the All period are shown. For the All period, the highest SIRs were for pancreatic (6.08) and liver (4.25) cancers, and liver cancer showed the highest SIR of 3.12, even in the All 5+ period. Notably, the risk for primary liver cancer was higher than those for gall bladder and extrahepatic bile duct tumors; the SIR was 4.66 in the All 5+ period. In that follow-up period, even the risks for upper aerodigestive tract, esophageal, colon, rectal, pancreatic, lung, cervical, endometrial, ovarian, and kidney cancers were significant.
Table 1.
SIRs for subsequent cancer in patients with T2D
aThe SIR for the reference population is 1.00.
All, follow-up started from last hospitalization; All 1+, follow-up started 1 year after last hospitalization; All 5+, follow-up started 5 years after last hospitalization.
Bold type indicates that the 95% CI does not include 1.00.
Abbreviations: CI, confidence interval; O, observed number of cases; SIR, standardized incidence ratio; T2D, type 2 diabetes.
Familial T2D patients (only data for the All 1+ period are shown in Table 1) had no difference in risk for all cancers (1.04) than all patients (1.37), which was influenced in part by the significantly lower risk for prostate cancer (0.54) in the familial patients than in all patients (0.84). In familial T2D patients, the risk for prostate cancer decreased in a uniform fashion according to the number of hospitalizations, reaching a SIR of 0.43 (n = 13; 95% CI, 0.23–0.74) for three to five hospitalizations and 0.10 for more than five hospitalizations (n = 1; 95% CI, 0.00–0.58). The risk for pancreatic cancer was significantly lower in familial than in all patients (2.41 and 3.57, respectively). Some cancers that had higher risks in all patients were not higher in familial patients because of the lower number of cases. However, the risk for melanoma was lower only in familial patients (0.67).
The SIRs for all sites combined were about 0.2 decimal units higher for women than for men, which was mainly explained by the low SIR for prostate cancer (data not shown). Compared with the All 5+ period in Table 1, only the male SIR of 4.09 for liver cancer was significantly greater (n = 161; 95% CI, 3.48–4.77). For females for the All 5+ period, the SIRs were higher for gastric cancer (1.51; n = 51; 95% CI, 1.12–1.98) and for nervous system tumors (1.43; n = 38; 95% CI, 1.01–1.96). For melanoma, the female SIR was lower at 0.56 (n = 17; 95% CI, 0.33–0.90).
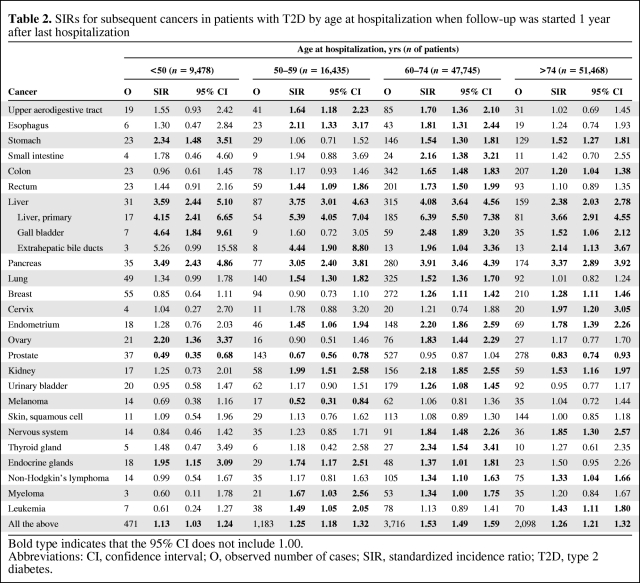
Table 2 shows cancer risks according to age at last hospitalization for T2D when follow-up started 1 year later (All 1+). For some high-risk cancers, such as liver and pancreatic cancers, the age at hospitalization appeared not to be critical. For stomach, ovarian, and endocrine tumors, age at first hospitalization was associated with risk. For prostate cancer, the lower SIR was stronger when hospitalization took place at a young age, which was also true of the lower risk for melanoma.
Table 2.
SIRs for subsequent cancers in patients with T2D by age at hospitalization when follow-up was started 1 year after last hospitalization
Bold type indicates that the 95% CI does not include 1.00.
Abbreviations: CI, confidence interval; O, observed number of cases; SIR, standardized incidence ratio; T2D, type 2 diabetes.
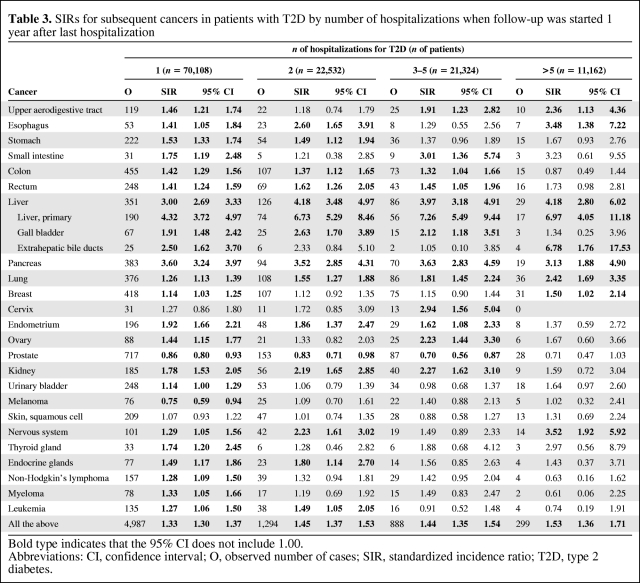
The number of hospitalizations for T2D may be an indication of the severity of the disease. The effect of number of hospitalizations was analyzed and results are presented in Table 3. For all cancers, there were no large effects and all the SIRs were around 1.3–1.5. Cancers at some sites, including the upper aerodigestive tract, esophagus, liver (primary), lung, and nervous system, were associated with the number of hospitalizations. For many other tumors, such as colon and endometrial cancers, non-Hodgkin's lymphoma, and leukemia, the significant SIRs were usually observed for patients with a lower number of hospitalizations. The lower risk for prostate cancer was largest for patients with multiple hospitalizations; the lower risk for melanoma was only observed in patients hospitalized once.
Table 3.
SIRs for subsequent cancers in patients with T2D by number of hospitalizations when follow-up was started 1 year after last hospitalization
Bold type indicates that the 95% CI does not include 1.00.
Abbreviations: CI, confidence interval; O, observed number of cases; SIR, standardized incidence ratio; T2D, type 2 diabetes.
We also analyzed the frequencies of other diseases for which T2D patients were hospitalized after their last hospitalization for T2D in order to find relationships with cancer risk factors. However, the most common hospitalizations were conditions related to T2D, such as coronary heart disease, stroke, atherosclerosis, cardiac fibrillation, abdominal and chest pain, and femur fracture.
Discussion
The use of hospital discharge data has great advantages, such as access to a nationwide patient pool and a reasonably high diagnostic accuracy, because the discharge diagnoses are often delivered by specialists during extended examinations in the clinic. In Sweden, hospitalization for T2D may be a secondary or tertiary referral step; T2D is diagnosed in primary care centers that refer patients to hospital outpatient clinics or directly to inpatient clinics. Hospital clinics are directed by specialists in internal medicine or endocrinology/diabetology [23]. With poor diagnostic accuracy, any effects would be expected to regress toward null. One limitation is that not all T2D patients are hospitalized and there is probably a selection toward severe disease presentation and complications. However, even among the present T2D cancer patients, 56% had been hospitalized only once (Table 3), and among those patients the number of cancers with an elevated risk was similar to that for all T2D patients (22 cancers had higher risks for patients hospitalized once, compared with 23 cancers in the All 1+ period shown in Table 1; cervical cancer was the only difference). By extrapolation from regional rates, it has been estimated that there would have been some 350,000 T2D patients in Sweden in the year 2004 [28]. On the other hand, at the same time, the National Diabetes Register in Sweden only included 57,000 patients with an age at onset >39 years [14, 23]. The Register figure is an underestimate because not all primary care centers or diabetes clinics participate. Thus, the present overall figure of 125,126 patients hospitalized at age >39 years and defined as having T2D by the ICD-10 appears to lie between the National Diabetes Register number, corrected for full coverage, and the extrapolated figure for T2D patients. Even if some type 1 diabetes patients could not be excluded, they would not essentially contribute to the present results because of the unclear association with any risk for cancer [18]. Regarding the present patients series, we discussed diagnostic accuracy and coverage in more detail in a recent article [13]. We had no data on many risk factors of or treatment for T2D, which might have influenced the results. The data were adjusted for the risk factors for which data were available, including obesity, based on hospital records on patients ever discharged with this condition. This adjustment, however, had hardly any effect on the risks, in agreement with a U.S. study [19].
Another issue relating to the interpretation of the results is the change in SIR depending on the definition of the follow-up time (All, All 1+, and All 5+). For all cancers, the highest risks were observed during the year of hospitalization for T2D, which is likely to be a result of lead time bias. The Cancer Registry records all new cases of cancer, and close to 100% of the cases are histologically or cytologically confirmed [24]. Thus, lead time bias only shifts the diagnoses earlier, and because diagnostic accuracy is not compromised, even early cases are true cancers. Thus, while the All follow-up period overestimates, the All 1+ and All 5+ periods are likely to underestimate the true risk. The avoidance of lead time bias was also the reason for our selection of starting follow-up from the last hospitalization. It is likely that cancers would be diagnosed earlier in persons who are hospitalized, even for conditions not related to cancer, than in nonhospitalized persons. However, the effects are expected to be small [29–33].
The literature on T2D and cancer is far too extensive to be covered here, and we refer to the five reviews published since 2007 [16–18, 21–22]. One of them concluded that an association with pancreatic cancer has been established, and that an association with colorectal, liver, endometrial, and bladder cancers is supported by some data, similar to the inverse association with prostate cancer [18]. In the present study, we confirmed all these conditional associations in reporting increased risks for 25 cancers in the All period and increased risks for 23 cancers in the All 1+ period. The risks were highest for liver cancer through all follow-up times, except for the All period, in which the risk for pancreatic cancer was highest. Five years after the last hospitalization for T2D (the All 5+ period), 11 cancers had excess risks, with liver cancer showing the highest risk of 3.12 (primary liver cancer, 4.66), followed by pancreatic (1.80), endometrial (1.76), cervical (1.59), and esophageal (1.51) cancers. The higher risks for cervical, upper aerodigestive tract, and esophageal cancers may suggest impairment of defenses against viral transformation. Esophageal and upper aerodigestive tract cancers are related to smoking, but because the SIR for lung cancer was only 1.16 (for the All 5+ period), smoking alone cannot explain the elevated risks [7]. However, excessive consumption of alcohol in combination with smoking may contribute to a greater risk for these cancers [8]. T2D has been assumed to interfere with estrogen metabolism, and previous studies have shown evidence for higher risks for endometrial and postmenopausal breast cancers [16, 18]. Most breast cancer patients in the present population, aged >39 years at the start of the follow-up period, would be postmenopausal and the higher breast cancer risk was only seen for follow-up periods that started shortly after hospitalization for T2D. In contrast, the higher risks for endometrial and ovarian cancers were clear. Obesity is another shared risk factor between T2D and cancers of the endometrium, kidney, colon, and (postmenopausal) breast; however, the applied adjustment for obesity did not change the present results [18, 34]. We lacked data on the treatment for T2D and cannot distinguish between the effects caused by the disease and those caused by the treatment received [21]. However, one can assume that treatment-related effects were related to the age at hospitalization and the number of hospitalizations, parameters that did not correlate with risk for most cancers.
To our knowledge, no previous study has followed cancer in familial T2D patients. There were a few differences between familial T2D patients and all T2D patients. Notably, the risk for prostate cancer was lower in familial patients and it reached an SIR of 0.42 in patients with three to five hospitalizations and 0.10 in patients with more than five hospitalizations. The risks for pancreatic cancer and melanoma were also lower in familial patients than in all patients. The lower risk for prostate cancer has been observed in other studies, which is consistent with a lower level of serum prostate-specific antigen in diabetics [35]. The effects have been ascribed to low androgen levels, but the relationships remain to be established [17, 36]. The data on cancer risk for familial T2D patients suggest that the genetic and environmental mechanisms contributing to familial T2D appear not to be risk factors for cancer. These data indicate that obesity is not likely to be a large confounder of the present associations because high heritability of obesity should lead to higher risks for cancer in familial T2D patients [37]. Similarly, smoking clusters in families [38]; thus, the lack of excess familial risk at smoking-related sites (esophagus, kidney) suggests that smoking was not a confounder. The markedly lower risk for prostate cancer appeared not to be a chance finding. Unraveling of the underlying mechanisms may give important clues about the shared pathways of T2D and prostate cancer.
In summary, this large study on Swedish T2D patients found an elevated risk for 23 cancers when follow-up started 1 year after hospitalization for T2D. The large number of novel cancer sites may be explained by the high statistical power of the study and, probably, by patient selection toward those with severe disease leading to hospitalization. The highest risks were found for liver and pancreatic cancers. The increased risks at these and many other sites cannot be explained by the risk factors for familial T2D because no excess risks were observed in familial diabetics. Rather, the effects on many cancers may be related to the multitude of metabolic disturbances of T2D. The decreased risks for prostate cancer and melanoma remain intriguing and challenging for the future.
Acknowledgments
Supported by the Swedish Council for Working Life and Social Research, the Swedish Cancer Society, and Deutsche Krebshilfe. The database used was created by linking registers maintained at Statistics Sweden and the Swedish Cancer Registry.
Author Contributions
Conception/Design: Xinjun Li, Kari Hemminki, Jan Sundquist, Kristina Sundquist
Provision of study material or patients: Xinjun Li, Kari Hemminki, Jan Sundquist, Kristina Sundquist
Collection and/or assembly of data: Xinjun Li, Jan Sundquist, Kristina Sundquist
Data analysis and interpretation: Xinjun Li
Manuscript writing: Xinjun Li, Kari Hemminki
Final approval of manuscript: Xinjun Li, Kari Hemminki, Jan Sundquist, Kristina Sundquist
References
- 1.Adeghate E, Schattner P, Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci. 2006;1084:1–29. doi: 10.1196/annals.1372.029. [DOI] [PubMed] [Google Scholar]
- 2.Hussain A, Claussen B, Ramachandran A, et al. Prevention of type 2 diabetes: A review. Diabetes Res Clin Pract. 2007;76:317–326. doi: 10.1016/j.diabres.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Ringborg A, Lindgren P, Martinell M, et al. Prevalence and incidence of type 2 diabetes and its complications 1996–2003—estimates from a Swedish population-based study. Diabet Med. 2008;25:1178–1186. doi: 10.1111/j.1464-5491.2008.02541.x. [DOI] [PubMed] [Google Scholar]
- 4.Muoio DM, Newgard CB. Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 5.Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doria A, Patti ME, Kahn CR. The emerging genetic architecture of type 2 diabetes. Cell Metab. 2008;8:186–200. doi: 10.1016/j.cmet.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willi C, Bodenmann P, Ghali WA, et al. Active smoking and the risk of type 2 diabetes: A systematic review and meta-analysis. JAMA. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 8.Carlsson S, Hammar N, Grill V. Alcohol consumption and type 2 diabetes meta-analysis of epidemiological studies indicates a U-shaped relationship. Diabetologia. 2005;48:1051–1054. doi: 10.1007/s00125-005-1768-5. [DOI] [PubMed] [Google Scholar]
- 9.Kaprio J, Tuomilehto J, Koskenvuo M, et al. Concordance for type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia. 1992;35:1060–1067. doi: 10.1007/BF02221682. [DOI] [PubMed] [Google Scholar]
- 10.Grill V, Persson G, Carlsson S, et al. Family history of diabetes in middle-aged Swedish men is a gender unrelated factor which associates with insulinopenia in newly diagnosed diabetic subjects. Diabetologia. 1999;42:15–23. doi: 10.1007/s001250051106. [DOI] [PubMed] [Google Scholar]
- 11.Weires MB, Tausch B, Haug PJ, et al. Familiality of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2007;115:634–640. doi: 10.1055/s-2007-984443. [DOI] [PubMed] [Google Scholar]
- 12.Ridderstråle M, Groop L. Genetic dissection of type 2 diabetes. Mol Cell Endocrinol. 2009;297:10–17. doi: 10.1016/j.mce.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Hemminki K, Li X, Sundquist K, et al. Familial risks for type 2 diabetes in Sweden. Diabetes Care. 2010;33:293–297. doi: 10.2337/dc09-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eliasson B, Cederholm J, Nilsson P, et al. The gap between guidelines and reality: Type 2 diabetes in a National Diabetes Register 1996–2003. Diabet Med. 2005;22:1420–1426. doi: 10.1111/j.1464-5491.2005.01648.x. [DOI] [PubMed] [Google Scholar]
- 15.Carlsson S, Midthjell K, Grill V. Influence of family history of diabetes on incidence and prevalence of latent autoimmune diabetes of the adult: Results from the Nord-Trn̸delag Health Study. Diabetes Care. 2007;30:3040–3045. doi: 10.2337/dc07-0718. [DOI] [PubMed] [Google Scholar]
- 16.Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: A review of the current evidence. Am J Clin Nutr. 2007;86:s823–s835. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 18.Hjartåker A, Langseth H, Weiderpass E. Obesity and diabetes epidemics: Cancer repercussions. Adv Exp Med Biol. 2008;630:72–93. doi: 10.1007/978-0-387-78818-0_6. [DOI] [PubMed] [Google Scholar]
- 19.Coughlin SS, Calle EE, Teras LR, et al. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 20.Rousseau MC, Parent ME, Pollak MN, et al. Diabetes mellitus and cancer risk in a population-based case-control study among men from Montreal, Canada. Int J Cancer. 2006;118:2105–2109. doi: 10.1002/ijc.21600. [DOI] [PubMed] [Google Scholar]
- 21.Smith U, Gale EA. Does diabetes therapy influence the risk of cancer? Diabetologia. 2009;52:1699–1708. doi: 10.1007/s00125-009-1441-5. [DOI] [PubMed] [Google Scholar]
- 22.Vigneri P, Frasca F, Sciacca L, et al. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 23.Gudbjörnsdottir S, Cederholm J, Nilsson PM, et al. The National Diabetes Register in Sweden: An implementation of the St. Vincent Declaration for Quality Improvement in Diabetes Care. Diabetes Care. 2003;26:1270–1276. doi: 10.2337/diacare.26.4.1270. [DOI] [PubMed] [Google Scholar]
- 24.Centre for Epidemiology. Cancer Incidence in Sweden 2005. Stockholm: The National Board of Health and Welfare; 2007. pp. 1–112. [Google Scholar]
- 25.Hemminki K, Granström C, Sundquist J, et al. The updated Swedish family-cancer database used to assess familial risks of prostate cancer during rapidly increasing incidence. Heredit Cancer Clin Pract. 2006;4:186–192. doi: 10.1186/1897-4287-4-4-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolk A, Gridley G, Svensson M, et al. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12:13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 27.Hemminki K, Vaittinen P, Dong C, et al. Sibling risks in cancer: Clues to recessive or X-linked genes? Br J Cancer. 2001;84:388–391. doi: 10.1054/bjoc.2000.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ringborg A, Martinell M, Stålhammar J, et al. Resource use and costs of type 2 diabetes in Sweden—estimates from population-based register data. Int J Clin Pract. 2008;62:708–716. doi: 10.1111/j.1742-1241.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 29.Hemminki K, Li X, Sundquist J, et al. Cancer risks in ulcerative colitis patients. Int J Cancer. 2008;123:1417–1421. doi: 10.1002/ijc.23666. [DOI] [PubMed] [Google Scholar]
- 30.Hemminki K, Li X, Sundquist J, et al. Cancer risks in Crohn disease patients. Ann Oncol. 2009;20:574–580. doi: 10.1093/annonc/mdn595. [DOI] [PubMed] [Google Scholar]
- 31.Ji J, Shu X, Li X, et al. Cancer risk in hospitalised asthma patients. Br J Cancer. 2009;100:829–833. doi: 10.1038/sj.bjc.6604890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji J, Shu X, Li X, et al. Cancer risk in hospitalized sarcoidosis patients: A follow-up study in Sweden. Ann Oncol. 2009;20:1121–1126. doi: 10.1093/annonc/mdn767. [DOI] [PubMed] [Google Scholar]
- 33.Ji J, Shu X, Sundquist K, et al. Cancer risk in hospitalised psoriasis patients: A follow-up study in Sweden. Br J Cancer. 2009;100:1499–1502. doi: 10.1038/sj.bjc.6605027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calle EE, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 35.Werny DM, Saraiya M, Gregg EW. Prostate-specific antigen values in diabetic and nondiabetic US men, 2001–2002. Am J Epidemiol. 2006;164:978–983. doi: 10.1093/aje/kwj311. [DOI] [PubMed] [Google Scholar]
- 36.Wigle DT, Turner MC, Gomes J, et al. Role of hormonal and other factors in human prostate cancer. J Toxicol Environ Health B Crit Rev. 2008;11:242–259. doi: 10.1080/10937400701873548. [DOI] [PubMed] [Google Scholar]
- 37.Bell CG, Walley AJ, Froguel P. The genetics of human obesity. Nat Rev Genet. 2005;6:221–234. doi: 10.1038/nrg1556. [DOI] [PubMed] [Google Scholar]
- 38.Lorenzo Bermejo J, Hemminki K. Familial lung cancer and aggregation of smoking habits: A simulation of the effect of shared environmental factors on the familial risk of cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1738–1740. doi: 10.1158/1055-9965.EPI-05-0201. [DOI] [PubMed] [Google Scholar]