The study investigated the feasibility of longitudinal assessment of functional performance measures in newly diagnosed postsurgical malignant glioma patients and concluded that longitudinal quantitative functional assessments are safe and feasible among select patients undergoing chemoradiation for primary malignant glioma.
Keywords: High-grade glioma, Cardiorespiratory fitness, Longitudinal, Functional status, Quantitative evaluation
Abstract
Purpose.
To investigate the feasibility of longitudinal assessment of functional performance measures in newly diagnosed postsurgical malignant glioma patients.
Methods.
Patients with histologically confirmed, clinically stable, postsurgical, and previously untreated high-grade glioma (HGG) or low-grade glioma (LGG) were studied. Using a prospective design, all participants performed a cardiopulmonary exercise test with expired gas analysis to assess cardiorespiratory function (VO2peak) immediately following surgical resection (mean, 10 days). Additional functional outcomes were skeletal muscle cross-sectional area (CSA) via magnetic resonance imaging, isokinetic muscle strength (isokinetic dynamometry), and body composition (air displacement plethysmography). Quality of life (QOL) was assessed by the Functional Assessment of Cancer Therapy–Brain scale. All study assessments were repeated at 6 and 24 weeks following surgery.
Results.
Thirty-five patients (HGG, n = 25; LGG, n = 10) completed baseline assessments. Of these, 20 HGG (80%) and nine LGG (90%) and 15 HGG (60%) and nine LGG (90%) patients completed study assessments at 6 weeks and 24 weeks, respectively. Intention-to-treat analyses indicated several significant time-by-group interactions, with favorable improvements in functional and QOL endpoints from baseline to 24 weeks in the LGG cohort and unfavorable changes in the HGG cohort. Per-protocol analyses including participants assessed at all three study timepoints indicated significant improvements in VO2peak and fatigue from baseline to 24 weeks in the HGG cohort; peak workload, body composition, and muscle strength improved from baseline to 6 weeks (all p-values < .05).
Conclusions.
Longitudinal quantitative functional assessments are safe and feasible among select patients undergoing chemoradiation for primary malignant glioma. Large prospective studies investigating the clinical importance of these measures appear warranted.
Introduction
The current “standard of care” for high-grade glioma (HGG) is surgical resection followed by 6 weeks of conventional fractioned radiotherapy and chemotherapy, followed by 12 months of adjuvant chemotherapy [1], although use of off-label antiangiogenic agents and other targeted therapies is not uncommon [2]. An additional treatment mainstay is the use of high-dose corticosteroids to control intracranial edema. The use of such aggressive combination therapy together with tumor-related impairments can simultaneously directly (i.e., direct cytotoxic injury) or indirectly (i.e., effects secondary to therapy such as physical inactivity) deleteriously impact the organ components (i.e., the pulmonary-cardiac-muscle axis) that govern exercise tolerance [3]. Poor exercise tolerance leads to a vicious downward cycle characterized by deconditioning (physical inactivity), fatigue, and other functional limitations (e.g., muscle atrophy, body composition changes) that result in further impairments in exercise tolerance and patient-reported outcomes (PROs) (e.g., quality of life [QOL], fatigue, depression, etc.). The importance and devastating functional impact of a primary malignant glioma diagnosis and treatment are widely recognized in clinical practice, yet formal investigations have not been conducted. Consequently, the incidence, magnitude, and pathogenesis of functional sequelae in primary glioma remain to be determined, and interventions to prevent and/or mitigate dysfunction are not available.
In current clinical practice, oncologists rely exclusively on the use of subjective performance status (PS) scoring systems (e.g., Karnofsky, Eastern Cooperative Oncology Group) to evaluate functional status in primary glioma patients. PS is a central component of the established recursive partitioning analysis [4, 5] as well as a newly derived prognosis prediction nomogram for newly diagnosed glioblastoma patients [6]. Given the well-documented limitations of PS scoring systems [7, 8], several research groups, including our own, have started to investigate the utility of alternative measurement tools that provide a quantitative evaluation of cancer patients' functional status [7, 9]. The central hypothesis of this research is that objective functional performance measures may allow for more accurate prognostication as well as guide the development of effective interventions in select cancer patients with “good” PS scores and who are experiencing fewer treatment- and disease-related complications. Recent studies reporting that sarcopenia (i.e., loss of muscle mass) and cardiorespiratory function (i.e., maximal volume of oxygen [VO2peak]) are independent predictors of disease outcome in patients with advanced respiratory, gastrointestinal, or breast cancer [10, 11] and non-small cell lung cancer [12], respectively, provide initial support for this contention. The utility of functional performance measures has not been investigated in patients with primary malignant glioma.
As an initial step, our group recently reported that functional performance measures (e.g., cardiopulmonary exercise testing [CPET], isokinetic dynamometery) were safe and acceptable methods to quantitatively assess functional status in patients with newly diagnosed and untreated, postsurgical, primary malignant glioma [13]. Here, we report on the feasibility of longitudinal assessments of functional performance measures during chemoradiation in this patient cohort. Secondary objectives were to explore changes in functional performance measures and whether or not these changes correlated with quality of life (QOL) or exercise behavior. We hypothesized that longitudinal assessment of functional performance measures would be feasible and safe for malignant glioma patients and that chemoradiation would cause significant unfavorable changes in functional performance measures.
Methods
Participants and Setting
Full details regarding the study sample, recruitment, and procedures were reported previously [13]. In brief, patients with histologically confirmed, clinically stable, postsurgical, and previously untreated HGG (World Health Organization [WHO] grade III–IV) were recruited at the Preston Robert Tisch Brain Tumor Center (PRT-BTC) at Duke University Medical Center (DUMC). Additional major inclusion criteria included: (a) treatment with standard radiotherapy plus concomitant and adjuvant temozolomide (patients scheduled to receive bevacizumab were not eligible), (b) a Karnofsky PS (KPS) score ≥70%, (c) an estimated life expectancy ≥6 months, and (d) no contraindications to CPET [14]. Patients with histologically confirmed, clinically stable, postsurgical, and previously untreated low-grade glioma (LGG) (WHO grade I–II) scheduled not to receive any postsurgical adjuvant therapy were also recruited for comparison purposes. The Duke Institutional Review Board approved the study, and written informed consent was obtained from all participants prior to the initiation of any study procedures.
Study Procedures
Using a prospective, observational design, all potential participants were identified and screened for eligibility via medical chart review of patients scheduled for their primary postsurgical treatment consultation at the PRT-BTC. After obtaining written informed consent, all participants completed the following assessments in order of presentation: (a) air-displacement plethysmography, (b) CPET, (c) isokinetic dynamometery, and (d) magnetic resonance imaging (MRI) of the lower extremities. Body composition was performed between 7:00 a.m. and 10:00 a.m. after an 8-hour, overnight, water-only fast; subsequent assessments were performed after each participant had rested for ≥60 minutes and received a light breakfast. All study-related assessments were performed at DUMC within a 2-day period. Approximately 6 (i.e., following the completion of adjuvant radiotherapy) and 24 weeks following surgery, all baseline assessments were repeated. At follow-up visits, study endpoint assessments were conducted by the same personnel, with the same equipment, in the same order of presentation.
Outcome Assessments
Incremental CPET
To determine the VO2peak, an incremental, physician-supervised CPET with 12-lead echocardiogram monitoring (Mac® 5000; GE Healthcare, Chalfont St Giles, U.K.) was performed according to guidelines published by Jones et al. [14] All tests were performed on an electronically braked cycle ergometer (Ergoselect 100; Ergoline, Bitz, Germany) with expired gas analysis (TrueOne® 2400; ParvoMedics, Sandy, UT). Preceding exercise, 3 minutes of resting metabolic data were collected before participants began cycling at 10–20 W. Workloads were then increased 5–20 W/minute until volitional exhaustion, symptom limitation, or a respiratory exchange ratio >1.0 was achieved. Initial workload and workload increments were determined by patient medical history and metabolic responses to exercise during the first minute. During exercise, blood pressure was measured noninvasively by manual auscultatory sphygmomanometery every 2 minutes. The metabolic measurement system was calibrated before and the calibration was checked after each test. Patients continued with usual medications and were asked to abstain from caffeinated beverages on the day of testing. All data were recorded as the highest 30-second value elicited during the CPET. The mean percentage of age- and sex-predicted peak heart rate and VO2peak were calculated from the equation provided by Jones et al. [15] and Fitzgerald et al. [16] (women) and Wilson and Tanaka [17] (men), respectively.
Skeletal Muscle Function
The muscle cross-sectional area (CSA) of the dominant thigh was assessed using MRI with a 3.0-T scanner (ACS II; Phillips, Shelton, CT). Imaging was performed using sequence gradient echo recall scans at the midfemur level. Seven serial slices were obtained with one at the juncture of the middle third of the femur (this point was landmarked to ensure within-patient reproducibility), as previously described [18]. The CSAs of the quadriceps, hamstrings, and total mid-thigh muscle were assessed using semiautomatic generation of the region of interest using console software. The muscle strength of the right quadriceps was measured by isokinetic dynamometery (Biodex Corporation, Shirley, NY). The isokinetic testing protocols consisted of measurement of three sequential voluntary maximal contractions at an angular velocity of 90°/second. Maximal isokinetic strength was defined as the highest peak torque achieved during the three contractions.
Body Composition
Percent body fat, fat mass (FM), and lean body mass (LBM) were evaluated by air-displacement plethysmography (e.g., BOD POD; Life Measurement Incorporated, Concord, CA), as previously described [19]. In brief, each patient wore spandex and a cap provided by the laboratory while body mass was measured to the nearest 100 g followed by the calculation of thoracic gas volume. Body density was calculated as body mass divided by body volume. Percent body fat was estimated from body density based on a two-compartment model. The system was calibrated prior to each test.
QOL
QOL was assessed using the Functional Assessment of Cancer Therapy–Brain (FACT-BR) scale [20]. The FACT-BR contains subscales for physical (seven items), functional (seven items), emotional (six items), and social/family (seven items) well-being. In addition, the FACT-BR contains a 19-item brain cancer subscale (BCS), which assesses symptoms commonly reported by brain cancer patients [20]. Fatigue was assessed by the 13-item Fatigue Scale of the FACT measurement system developed specifically for cancer patients [21].
Clinical Parameters and PS
Medical characteristics were abstracted from medical records. PS was assessed using the KPS scale and was assessed at the time of study enrollment by the attending oncologist. Self-reported exercise behavior was assessed by the Godin Leisure Time Exercise Questionnaire [22]. Specifically, participants were asked to report their level of exercise since their brain tumor diagnosis (at the baseline assessment; ∼1 month) and over the past month at the 6 and 24 week assessment timepoints.
Statistical Analysis
The initial analysis provided descriptive information on the study endpoints at each assessment timepoint (i.e., baseline and 6 weeks and 24 weeks postsurgery). Baseline characteristics were compared between the two groups using independent-samples t-tests for continuous data and Pearson's χ2 tests for categorical data. Under an intention-to-treat (ITT) principle, a series of repeated measures analyses of variance (ANOVAs) were conducted to assess change in study endpoints within and between groups from baseline to 24 weeks, regardless of nonassessment at all study timepoints. Missing data were imputed under different assumptions according to the reason for nonassessment (e.g., death versus medically ineligible versus transfer of care). One-way ANOVAs were also used to conduct per-protocol analyses that included only patients assessed at all three study timepoints. For both analyses, unadjusted and adjusted (covariate) tests of change were conducted. Covariates included gender, age, histological grade, and the baseline value of the variable under consideration. To determine the univariate associations among change in functional performance endpoints, exercise behavior, and QOL from baseline to 24 weeks, we used linear regression analysis. Imputed data for patients nonassessable at 24 weeks because of progressive disease (PD) or death were not included in this analysis. Data are presented as the mean ± standard deviation. Statistical significance was set at p < .05 for all analyses except the exploratory analysis (p < .10).
Results
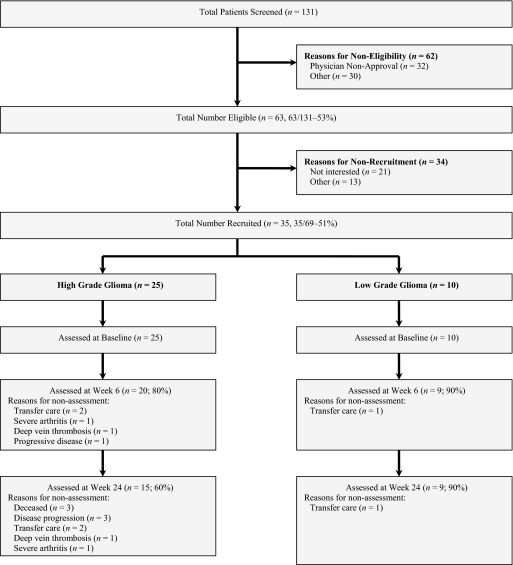
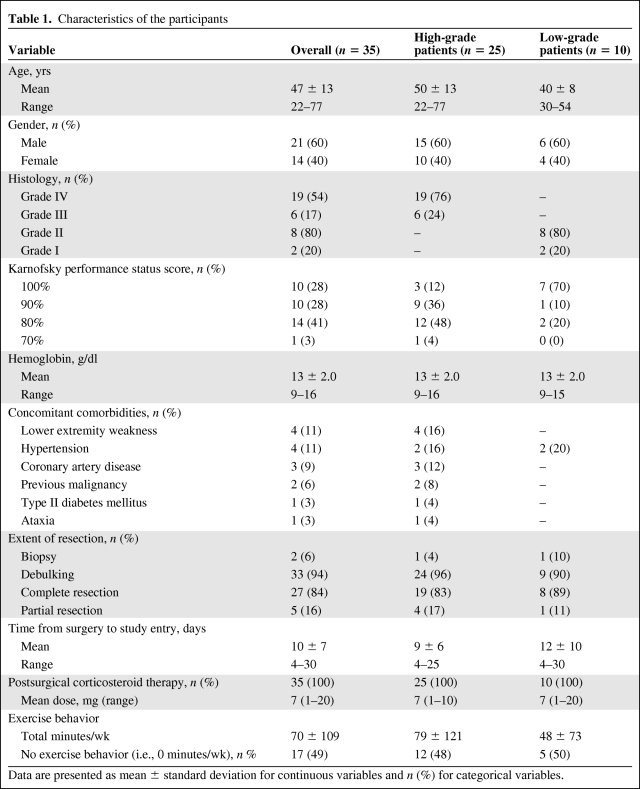
Details regarding study recruitment were reported previously [13]. In brief, in total, 131 patients were screened for study eligibility, 69 (53%) met the inclusion criteria and 35 (51%) (HGG, n = 25; LGG, n = 10) completed baseline assessments. Of these, 20 HGG (80%) and nine LGG (90%) and 15 HGG (60%) and nine LGG (90%) patients completed study assessments at 6 weeks and 24 weeks, respectively (Fig. 1). The baseline characteristics of study participants are reported in Table 1. There were no differences between the HGG and LGG cohorts on any demographic or medical variable. No adverse events were observed during any functional performance assessments at any study timepoint.
Figure 1.
Study flow.
Table 1.
Characteristics of the participants
Data are presented as mean ± standard deviation for continuous variables and n (%) for categorical variables.
Longitudinal Changes in Functional Performance Outcomes and QOL
ITT Analyses
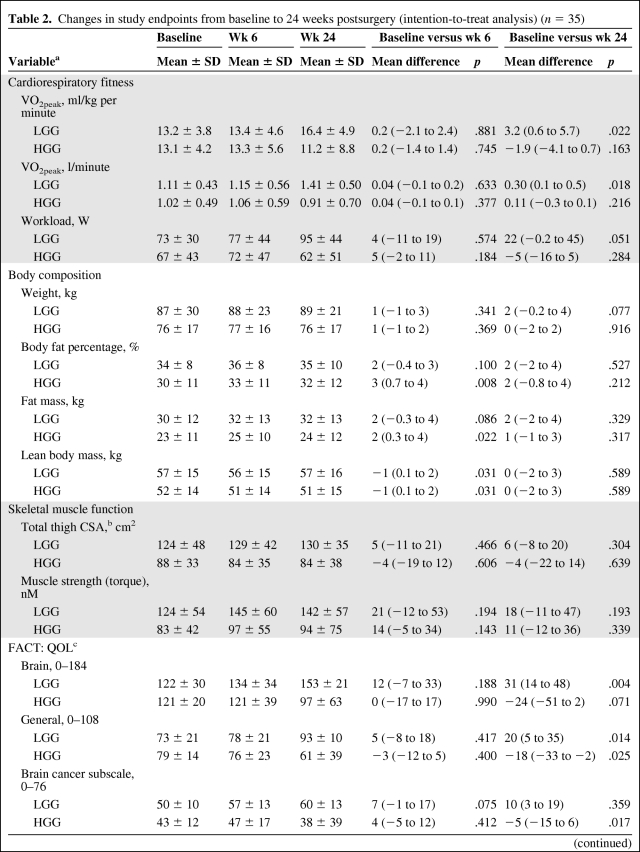
Significant time-by-group interactions were indicated for several study endpoints, including VO2peak (ml/kg per minute), workload, body fat percentage, FM, total CSA, FACT-BR, and Functional Assessment of Cancer Therapy–General (FACT-G) (all p-values < .05). In general, univariate analyses indicated favorable improvements in functional performance endpoints and QOL in the LGG cohort with unfavorable changes in the HGG cohort. Significant main (time) effects were also indicated for VO2peak (l/minute), fatigue, and exercise behavior in the LGG cohort and BCS in the HGG cohort (all p-values < .05) (Table 2). Results were unchanged after adjustment for covariates.
Table 2.
Changes in study endpoints from baseline to 24 weeks postsurgery (intention-to-treat analysis) (n = 35)
Table 2.
(Continued)
Data are presented as mean ± SD.
aHGG, n = 25; LGG, n = 10; for all variables except where indicated.
bHGG, n = 13; LGG, n = 7.
cHGG, n = 20; LGG, n = 10.
Abbreviations: FACT, Functional Assessment of Cancer Therapy; HGG, high-grade glioma; LGG, low-grade glioma; QOL, quality of life; VO2peak, peak oxygen consumption.
Per-Protocol Analyses
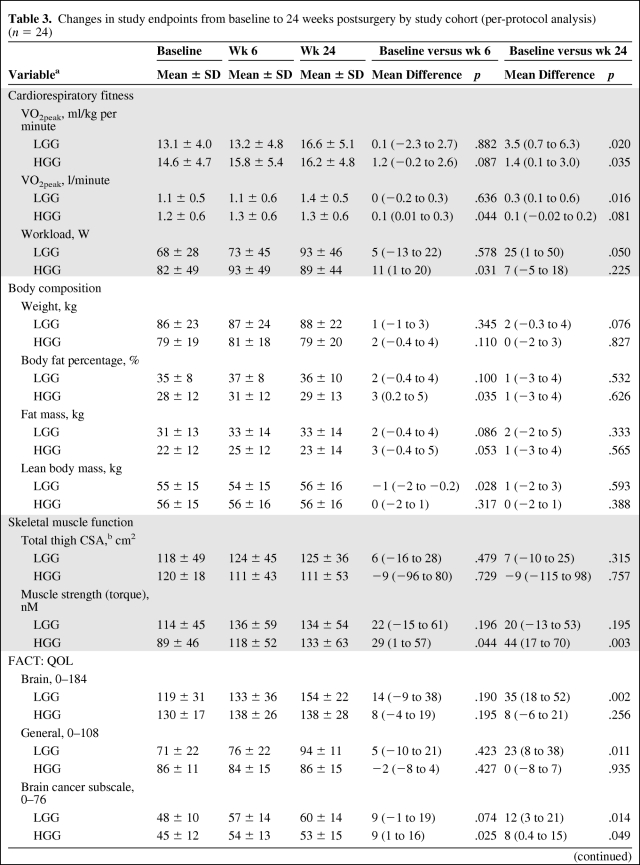
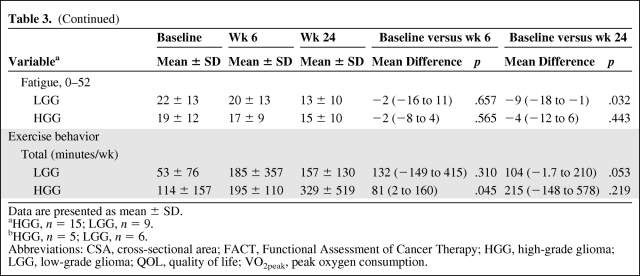
There were no significant time-by-group interactions for any study endpoint. Significant improvements were indicated for VO2peak (ml/kg per minute), fatigue, and BCS in both study cohorts from baseline to 24 weeks. In the HGG cohort, there were significant improvements in workload, body fat percentage, FM, muscle strength, and exercise behavior from baseline to 6 weeks (all p-values < .05) that were no longer significant at 24 weeks (all p-values > .05); only significant improvements in muscle strength were sustained at 24 weeks. In the LGG cohort, there were significant improvements in VO2peak (l/minute), workload, FACT-BR, FACT-G, BCS, fatigue, and exercise behavior from baseline to 24 weeks (all p-values < .05) (Table 3). Results were unchanged after adjustment for covariates.
Table 3.
Changes in study endpoints from baseline to 24 weeks postsurgery by study cohort (per-protocol analysis) (n = 24)
Table 3.
(Continued)
Data are presented as mean ± SD.
aHGG, n = 15; LGG, n = 9.
bHGG, n = 5; LGG, n = 6.
Abbreviations: CSA, cross-sectional area; FACT, Functional Assessment of Cancer Therapy; HGG, high-grade glioma; LGG, low-grade glioma; QOL, quality of life; VO2peak, peak oxygen consumption.
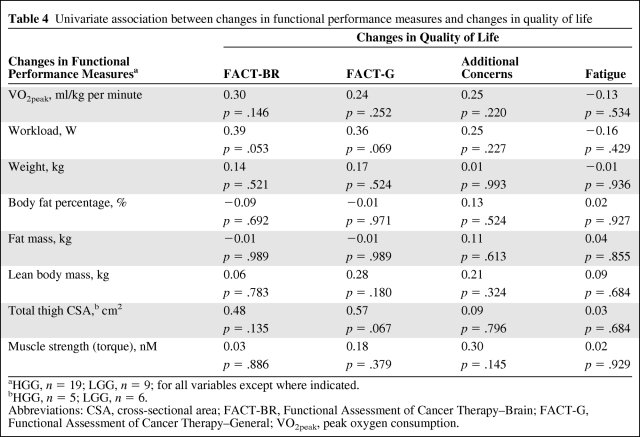
Univariate Associations Among Functional Performance Measures, Exercise Behavior, and QOL
Table 4 presents the univariate associations between changes in functional outcomes and QOL. Greater improvements in the VO2peak, workload, and total CSA were associated with more favorable changes in QOL and fatigue, but these correlations did not reach statistical significance. Similarly, increased self-reported exercise behavior was associated with more favorable changes in cardiopulmonary function, muscle strength, body composition, and QOL, but these correlations did not reach statistical significance (data not reported).
Table 4.
Univariate association between changes in functional performance measures and changes in quality of life
aHGG, n = 19; LGG, n = 9; for all variables except where indicated.
bHGG, n = 5; LGG, n = 6.
Abbreviations: CSA, cross-sectional area; FACT-BR, Functional Assessment of Cancer Therapy–Brain; FACT-G, Functional Assessment of Cancer Therapy–General; VO2peak, peak oxygen consumption.
Discussion
The principal finding of this pilot study was that longitudinal evaluation of quantitative functional performance measures is safe, feasible, and well tolerated among select patients undergoing chemoradiation for primary malignant glioma. Moreover, there was considerable patient variability in changes in functional performance outcomes during chemoradiation, and these changes correlated partially with changes in QOL and fatigue.
Numerous randomized trials have used quantitative functional measures, such as CPET and body composition measurement, to investigate the efficacy of structured exercise training in cancer populations at various stages of treatment and recovery [23–25]. Overall, quantitative functional measures have been found to be safe and feasible tools with minimal risk for an adverse event. The vast majority of these studies, however, have been conducted among cancer populations with good PS score (KPS score ≥70%) and who are experiencing relatively few disease- and/or treatment-related toxicities (e.g., early-stage breast cancer patients) [26, 27]. In contrast, the prognosis for HGG patients is poor; the overall survival rate for glioblastoma multiforme patients is only 17.7% at 1 year. Patients also commonly present with tumor-related neurological impairments that, in turn, impact motor control and functional status. Together with the direct and indirect effects of aggressive combination cytotoxic therapy, HGG patients represent a unique population with distinctive functional impairments and limitations to exercise. Thus, demonstration of the safety, tolerability, and acceptability of functional performance measures during postsurgical cytotoxic adjuvant therapy in this setting is novel and important. Longitudinal functional assessment in HGG patients is, of course, not possible in a significant portion of patients because of physically debilitating PD and/or death; 32% of HGG patients in this study were nonassessable as a result of PD and/or death over the course of this study. Thus, longitudinal functional evaluation studies are limited to those patients with good PS scores, stable disease, and who are experiencing fewer treatment-related complications. Nevertheless, all patients were able to complete functional performance evaluations at baseline (i.e., prior to the initiation of adjuvant cytotoxic therapy), and the majority (68%) were able to complete functional performance measurements up to 24 weeks postsurgery.
Consistent with our primary hypothesis, ITT analyses indicated significant unfavorable reductions in functional performance measures and QOL endpoints in HGG patients, relative to LGG patients, over the study period. However, it is important to state that ITT analyses included a significant portion of imputed data at 24 weeks for patients nonassessable at 24 weeks. Although this is a widely accepted and appropriate statistical approach for our study design, it may not accurately reflect the change in functional endpoints in the cohort of HGG patients surviving >24 weeks. Indeed, per-protocol analyses (including only patients assessable at 24 weeks) generally indicated significant favorable mean improvements in study endpoints in both cohorts. Intriguingly, a closer inspection of the data, however, revealed several distinct patterns of change in study endpoints in the HGG cohort that were not reflected in the group aggregate data. Using VO2peak (ml/kg per minute) as an illustration, VO2peak increased in seven patients (46%) (mean improvement, 3.3 ml/kg per minute) and decreased in one patient (6%) from baseline to 24 weeks. Of importance, in seven patients (46%), the VO2peak increased from baseline to 6 weeks (mean improvement, 3.1 ml/kg per minute) but subsequently decreased from 6 weeks to 24 weeks (mean decrease, 2.7 ml/kg per minute). Intriguingly, in the latter cohort of patients, 60% were receiving decadron, versus only 14% of the cohort of patients experiencing an increase in VO2peak. There were no significant differences in any other clinical or demographic variables at baseline that discriminated these groups (analyses not presented). Similar patterns were observed in several other study endpoints in the HGG cohort. In contrast, progressive improvements in virtually all study endpoints were observed in the LGG cohort. These preliminary data suggest that the impact of chemoradiation on functional performance endpoints may vary considerably even among a relatively homogeneous cohort of HGG patients with stable disease.
Taken together, the present results demonstrate that objective measurements of functional performance are feasible in select patients with primary malignant glioma, and such measures are sensitive to therapy-induced changes in physiology. Although the clinical value is currently unknown, objective measures of functional performance may provide complementary information to current subjective PS scoring systems used to evaluate physical functioning in the oncology setting. Given their subjectivity, current PS scoring systems do not detect more subtle but important therapy-induced physiological changes and, as a result, fail to characterize the degree of physiological injury in malignant glioma and other cancer populations. Further, they do not inform therapeutic intervention. Objective functional assessments provide an unbiased assessment of physical functioning, which may, in turn, provide more accurate prognostication. Recent findings from our group demonstrating that VO2peak is a significant predictor of all-cause mortality beyond PS in non-small cell lung cancer patients provide initial support for this contention [12]. Large prospective studies investigating whether objective functional performance measures are prognostic beyond traditional measures of physical functioning as well as other conventional markers of prognosis in HGG are warranted.
A secondary objective was to examine whether changes in functional measures correlated with changes in QOL endpoints. We previously reported that VO2peak and LBM were inversely associated with fatigue in HGG patients immediately following surgical resection [13]. Here, we found that, in general, improvements in select functional outcomes were associated with favorable changes in QOL and fatigue, but these correlations did not reach statistical significance; the small sample size was likely responsible for the lack of statistical significance. Similarly, a change in self-reported exercise behavior was favorably associated with changes in functional and QOL outcomes, but these correlations were small and did not approach statistical significance. In this regard, an unexpected finding was the high level of exercise behavior in primary malignant glioma patients during chemoradiation. These data support our previous findings [28] and suggest that primary glioma patients are physically capable of exercising during adjuvant therapy.
This is in contrast to several prior cross-sectional investigations that reported positive associations between exercise behavior and PROs in a broad range of cancer populations [29–31]. Differences in the patient populations (advanced versus early-stage disease), study designs (prospective versus cross-sectional), and study endpoints (self-reported versus quantitative) may be partially responsible for these discrepant findings. Larger, prospective studies are required to further investigate the relationship among functional outcomes, PROs, and exercise behavior in HGG patients.
This study has several limitations. The most important limitation is patient selection bias because of the relatively low eligibility rate, the transparent purpose of the investigation, and exclusion of patients with poor KPS scores (<70%). As such, patients with better KPS scores, less advanced disease, and who were experiencing fewer treatment-related complications were probably more likely to participate in this study. Another obvious limitation is the small sample size. Future large-scale studies are required to adequately evaluate the utility and prognostic importance of functional performance measures in primary malignant glioma.
In summary, this study provides the first evidence that longitudinal evaluation of quantitative functional performance measures is safe, feasible, and well tolerated among select patients undergoing chemoradiation for primary malignant glioma. Such measures may provide a more objective method to evaluate PS and improve prognostication in primary malignant glioma.
Acknowledgments
This study was supported by NIH grant CA-126432 and funds from the Preston Robert Tisch Brain Tumor Center, Tug McGraw Foundation, and Boot Camp-to-Beat Cancer awarded to L.W.J. We dedicate this paper to the brave and courageous individuals who made this research possible.
Author Contributions
Conception/Design: Lee W. Jones, David A. Reardon, Allan H. Friedman
Financial support: Lee W. Jones
Provision of study material or patients: David A. Reardon, Allan H. Friedman, Miranda J. West, Stephanie K. Mabe
Collection and/or assembly of data: Lee W. Jones, William E. Kraus
Data analysis and interpretation: Lee W. Jones, Henry S. Friedman, David A. Reardon, Marina Mourtzakis, Katherine B. Peters, William E. Kraus
Manuscript writing: Lee W. Jones, David A. Reardon, Marina Mourtzakis, Katherine B. Peters
Final approval of manuscript: Lee W. Jones, Henry S. Friedman, David A. Reardon, Marina Mourtzakis, Katherine B. Peters, Allan H. Friedman, Miranda J. West, Stephanie K. Mabe, William E. Kraus
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Reardon DA, Desjardins A, Rich JN, et al. The emerging role of anti-angiogenic therapy for malignant glioma. Curr Treat Options Oncol. 2008;9:1–22. doi: 10.1007/s11864-008-0052-6. [DOI] [PubMed] [Google Scholar]
- 3.Jones LW, Eves ND, Haykowsky M, et al. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10:598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- 4.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 5.Scott CB, Scarantino C, Urtasun R, et al. Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: A report using RTOG 90–06. Int J Radiat Oncol Biol Phys. 1998;40:51–55. doi: 10.1016/s0360-3016(97)00485-9. [DOI] [PubMed] [Google Scholar]
- 6.Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: Prognostic factor analysis of EORTC and NCIC trial 26981–22981/CE. 3. Lancet Oncol. 2008;9:29–38. doi: 10.1016/S1470-2045(07)70384-4. [DOI] [PubMed] [Google Scholar]
- 7.Jones LW, Cohen RR, Mabe SK, et al. Assessment of physical functioning in recurrent glioma: Preliminary comparison of performance status to functional capacity testing. J Neurooncol. 2009;94:79–85. doi: 10.1007/s11060-009-9803-x. [DOI] [PubMed] [Google Scholar]
- 8.Abernethy AP, Shelby-James T, Fazekas BS, et al. The Australia-modified Karnofsky performance status (AKPS) scale: A revised scale for contemporary palliative care clinical practice [ISRCTN81117481] BMC Palliat Care. 2005;4:7. doi: 10.1186/1472-684X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones LW, Eves ND, Mackey JR, et al. Safety and feasibility of cardiopulmonary exercise testing in patients with advanced cancer. Lung Cancer. 2007;55:225–232. doi: 10.1016/j.lungcan.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Prado CM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920–2926. doi: 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- 11.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 12.Jones LW, Watson D, Herndon JE, et al. Peak oxygen consumption and long-term all-cause mortality in non-small cell lung cancer. Cancer. 2010 doi: 10.1002/cncr.25396. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones LW, Friedman AH, West MJ, et al. Quantitative assessment of cardiorespiratory fitness, skeletal muscle function, and body composition in adults with primary malignant glioma. Cancer. 2010;116:695–704. doi: 10.1002/cncr.24808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones LW, Eves ND, Haykowsky M, et al. Cardiorespiratory exercise testing in clinical oncology research: Systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- 15.Jones NL, Summers E, Killian KJ. Influence of age and stature on exercise capacity during incremental cycle ergometry in men and women. Am Rev Respir Dis. 1989;140:1373–1380. doi: 10.1164/ajrccm/140.5.1373. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald MD, Tanaka H, Tran ZV, et al. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: A meta-analysis. J Appl Physiol. 1997;83:160–165. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
- 17.Wilson TM, Tanaka H. Meta-analysis of the age-associated decline in maximal aerobic capacity in men: Relation to training status. Am J Physiol Heart Circ Physiol. 2000;278:H829–H834. doi: 10.1152/ajpheart.2000.278.3.H829. [DOI] [PubMed] [Google Scholar]
- 18.Minotti JR, Pillay P, Oka R, et al. Skeletal muscle size: Relationship to muscle function in heart failure. J Appl Physiol. 1993;75:373–381. doi: 10.1152/jappl.1993.75.1.373. [DOI] [PubMed] [Google Scholar]
- 19.Wagner DR, Heyward VH, Gibson AL. Validation of air displacement plethysmography for assessing body composition. Med Sci Sports Exerc. 2000;32:1339–1344. doi: 10.1097/00005768-200007000-00023. [DOI] [PubMed] [Google Scholar]
- 20.Weitzner MA, Meyers CA, Gelke CK, et al. The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75:1151–1161. doi: 10.1002/1097-0142(19950301)75:5<1151::aid-cncr2820750515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 21.Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 22.Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: A concurrent validity study. Can J Public Health. 1986;77:359–362. [PubMed] [Google Scholar]
- 23.Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27:344–351. doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 24.Courneya KS, Jones LW, Peddle CJ, et al. Effects of aerobic exercise training in anemic cancer patients receiving darbepoetin alfa: A randomized controlled trial. The Oncologist. 2008;13:1012–1020. doi: 10.1634/theoncologist.2008-0017. [DOI] [PubMed] [Google Scholar]
- 25.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 26.McNeely ML, Campbell KL, Rowe BH, et al. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitz KH, Holtzman J, Courneya KS, et al. Controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 28.Jones LW, Guill B, Keir ST, et al. Patterns of exercise across the cancer trajectory in brain tumor patients. Cancer. 2006;106:2224–2232. doi: 10.1002/cncr.21858. [DOI] [PubMed] [Google Scholar]
- 29.Peddle CJ, Au HJ, Courneya KS. Associations between exercise, quality of life, and fatigue in colorectal cancer survivors. Dis Colon Rectum. 2008;51:1242–1248. doi: 10.1007/s10350-008-9324-2. [DOI] [PubMed] [Google Scholar]
- 30.Milne HM, Gordon S, Guilfoyle A, et al. Association between physical activity and quality of life among Western Australian breast cancer survivors. Psychooncology. 2007;16:1059–1068. doi: 10.1002/pon.1211. [DOI] [PubMed] [Google Scholar]
- 31.Jones LW, Courneya KS, Vallance JK, et al. Association between exercise and quality of life in multiple myeloma cancer survivors. Support Care Cancer. 2004;12:780–788. doi: 10.1007/s00520-004-0668-4. [DOI] [PubMed] [Google Scholar]