Abstract
The study looks at the high cost of new treatments for chronic lymphocytic leukemia and calls for the consideration of a standardized and transparent method to determine the price of new drugs based on comparative effectiveness.
The last decade has been a time of incredible progress in the treatment of patients with chronic lymphocytic leukemia (CLL), the most common leukemia in the western world. This interval has seen the development and U.S. Food and Drug Administration approval of fludarabine- and rituximab-containing chemoimmunotherapy regimens as well as the approval of alemtuzumab, bendamustine, and ofatumumab for the treatment of CLL patients.
In December 2009, two randomized phase III trials presented at the American Society of Hematology annual meeting demonstrated, for the first time, that the type of first-line therapy administered affects the overall survival of CLL patients. Dr. Kanti Rai reported, on behalf of the North American Intergroup, that first-line treatment with purine nucleoside analogs results in longer survival than first-line chlorambucil [1], and Dr. Michael Hallek reported, on behalf of the German CLL Study Group, that the addition of rituximab to fludarabine and cyclophosphamide led to longer survival than fludarabine and cyclophosphamide alone [2].
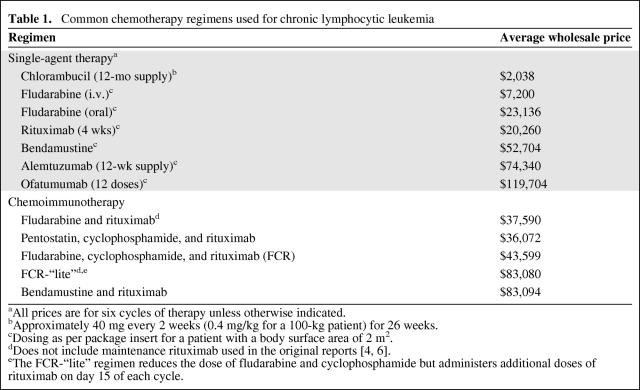
This progress, however, has not come without price. As shown in Table 1, the average wholesale price for a 12-month course of chlorambucil (the historic standard therapy approximately one decade ago) is approximately $2,000. In contrast, the average wholesale prices for six cycles of the chemoimmunotherapy regimens (fludarabine, cyclophosphamide, and rituximab; fludarabine and rituximab; pentostatin, cyclophosphamide, and rituximab) [3–5] that appear to improve survival for CLL patients run at $35,000–$45,000. Six cycles of bendamustine if administered with rituximab cost >$80,000. These average wholesale prices do not include the costs of infusion or other supportive care medications, such as granulocyte-stimulating factors, which are also high-priced medications. Other single-agent approaches recently approved for first-line (bendamustine and alemtuzumab) or salvage (ofatumumab) therapy for patients with CLL cost $50,000–$120,000 for a standard course, where drug pricing is not necessarily proportional to clinical benefit.
Table 1.
Common chemotherapy regimens used for chronic lymphocytic leukemia
aAll prices are for six cycles of therapy unless otherwise indicated.
bApproximately 40 mg every 2 weeks (0.4 mg/kg for a 100-kg patient) for 26 weeks.
cDosing as per package insert for a patient with a body surface area of 2 m2.
eThe FCR-“lite” regimen reduces the dose of fludarabine and cyclophosphamide but administers additional doses of rituximab on day 15 of each cycle.
Given that the average patient age at the time of a CLL diagnosis is ∼70 years and the fact that most patients are observed for several years before they require treatment, most patients with CLL in the U.S. receive reimbursement for chemotherapy via Medicare. Accordingly, the 20- to 40-fold increase in the cost of first-line therapy for patients with CLL is largely being borne at the cost of the U.S. taxpayer. Such increases in the cost of care are obviously not sustainable. It is unclear that time will reduce the price of many of these treatments since generic versions of monoclonal antibodies such as rituximab, alemtuzumab, and ofatumumab may not be available even after they go off patent.
While the progress in the care of patients with CLL over the last decade is encouraging, the cost of these new treatments has unfortunately priced these advances out of reach for CLL patients in developing countries, and these costs are unlikely to be sustainable even in the U.S. In the era of evidence-based medicine, pay for performance, and health care reform, it is time to consider a standardized and transparent method to determine the price of new drugs based on comparative effectiveness (e.g., response/efficacy-based pricing).
Author Contributions
Conception/Design: Tait D. Shanafelt, Timothy G. Call
Collection and/or assembly of data: Tait D. Shanafelt, Heidi Gunderson, Timothy G. Call
Manuscript writing: Tait D. Shanafelt, Heidi Gunderson, Timothy G. Call
Final approval of manuscript: Tait D. Shanafelt, Heidi Gunderson, Timothy G. Call
References
- 1.Rai K, Peterson BL, Appelbaum FR, et al. Long-term survival analysis of the North American Intergroup Study C9011 comparing fludarabine (F) and chlorambucil (C) in previously untreated patients with chronic lymphocytic leukemia (CLL) Blood. 2009;114:536. [Google Scholar]
- 2.Hallek M, Fingerle-Rowson G, Fink A, et al. First-line treatment with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) improves overall survival (OS) in previously untreated patients (pts) with advanced chronic lymphocytic leukemia (CLL): Results of a randomized phase III trial on behalf of an international group of investigators and the German CLL Study Group. Blood. 2009;114:535. [Google Scholar]
- 3.Keating M, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: Results from Cancer and Leukemia Group B 9712 (CALGB 9712) Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- 5.Kay NE, Geyer SM, Call TG, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood. 2007;109:405–411. doi: 10.1182/blood-2006-07-033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foon KA, Boyiadzis M, Land SR, et al. Chemoimmunotherapy with low-dose fludarabine and cyclophosphamide and high dose rituximab in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:498–503. doi: 10.1200/JCO.2008.17.2619. [DOI] [PubMed] [Google Scholar]