This review summarizes the preclinical and clinical pharmacokinetics and pharmacodynamics of pazopanib, as well as data on clinical activity, that ultimately resulted in its recent approval.
Keywords: Pazopanib, Angiogenesis, Tyrosine kinase inhibitor, Renal cell cancer
Abstract
Pazopanib is a recently approved, novel tyrosine kinase inhibitor specifically designed to impair angiogenesis by abrogating vascular endothelial growth factor receptor 2 (VEGFR-2) to exert its function. Pazopanib inhibits VEGF-induced endothelial cell proliferation in vitro and angiogenesis in vivo and demonstrates antitumor activity in mouse models. Furthermore, the pazopanib concentration resulting in maximal inhibition of VEGFR-2 phosphorylation in vivo was in line with the steady-state concentration required to inhibit growth of tumor xenografts, suggesting that pazopanib's mechanism of action is indeed through VEGFR-2 inhibition.
In a phase I trial, a generally well-tolerated dose was identified at which the majority of patients achieved pazopanib plasma concentrations above the concentration required for maximal in vivo inhibition of VEGFR-2 phosphorylation in preclinical models. Administered as monotherapy, evidence of antitumor activity was observed in phase II studies in several tumor types, including soft tissue sarcoma, renal cell cancer (RCC), ovarian cancer, and non-small cell lung cancer. Recently, the U.S. Food and Drug Administration granted approval for treatment with pazopanib in patients with RCC based on the longer progression-free survival time observed with this agent in a placebo-controlled, randomized trial. This review summarizes the preclinical and clinical pharmacokinetics and pharmacodynamics of pazopanib, as well as data on clinical activity, that ultimately resulted in its recent approval.
Introduction
The advent of tyrosine kinase inhibitors (TKIs) has considerably changed the daily practice of oncology. This type of anticancer agent can be classified within the larger group of the so-called cancer(-cell)-specific therapies, and several of these compounds also target infiltrating host cells supporting tumor growth, such as endothelial cells and fibroblasts. Over the last several years, major advances have been made in elucidating the pathogenesis of tumor growth and metastasis. This has resulted in the identification of numerous tumor growth–driving factors, such as vascular endothelial growth factor receptor 2 (VEGFR-2) and platelet-derived growth factor receptor (PDGFR). By inhibiting the activity of these factors, it was aimed to specifically intervene in tumor pathogenesis and to avoid untoward effects on normal cells.
After the introduction of imatinib, the first TKI used in solid tumors, the therapeutic armamentarium in solid malignancies was expanded by registration of several other TKIs. These include the epidermal growth factor receptor (EGFR) inhibitors erlotinib and gefitinib, the dual EGFR and human epidermal growth factor receptor (HER)-2 inhibitor lapatinib, and the VEGFR inhibitors sunitinib, sorafenib, and, recently, pazopanib. Importantly, none of the TKIs are entirely specific for one target. In particular, the VEGFR inhibitors target a wide spectrum of kinases, including the PDGFR and fibroblast growth factor receptor (FGFR). Additionally, sorafenib is also a strong Raf inhibitor.
In addition to different mechanisms of action with regard to antitumor activity, TKIs are also characterized by a toxicity pattern that is substantially different from that of conventional cytotoxic agents. Furthermore, the need to administer these agents more or less continuously necessitates a different assessment of tolerability than with classic cytotoxic drugs. Compliance with anticancer therapy, and therefore its success, is, to a large extent, determined by its toxicity and tolerance. Therefore, getting insight into the mechanisms accounting for TKI-mediated toxicity and its manageability is of great importance.
Pazopanib (GW786034), a synthetic indazolylpyrimidine, is a novel multitargeted TKI targeting several tumor and tumor environment factors thought to play an important role in a broad spectrum of tumor types. The first outcomes of several phase II studies have been reported, suggesting antitumor activity in patients with diverse tumor types and showing that pazopanib is generally well tolerated. Recently, the first phase III data became available, resulting in approval of this agent by the U.S. Food and Drug Administration (FDA) for the treatment of patients with renal cell cancer (RCC). Currently, phase III trials in other tumor entities are ongoing. As described, multiple kinases are inhibited by pazopanib. However, the observation that bevacizumab, a pure VEGF inhibitor, also has activity in patients with RCC strongly suggests that pazopanib's mechanism of action in RCC is largely through VEGFR-2 inhibition. Importantly, it is conceivable that its antitumor effect in other types of cancer depends on inhibition of receptors other than VEGFR-2.
This review summarizes the preclinical and clinical pharmacokinetics (PK) and pharmacodynamics of pazopanib as well as data on its clinical activity.
Preclinical Data
Mechanism of Action
Angiogenesis plays a critical role in the progression of solid malignancies from tumor volumes as small as 1–2 mm3 [1]. Numerous proangiogenic factors are involved in this process, with the VEGF family being the most important. The human VEGF family consists of VEGF-A (referred to as VEGF), VEGF-B, VEGF-C, VEGF-D, and placenta growth factor. Members of the VEGF family bind to the cell surface receptors VEGFR-1, VEGFR-2, and VEGFR-3 on endothelial cells to initiate cellular signaling. Of these, VEGFR-2 is the primary tyrosine kinase receptor mediating VEGF signaling.
Because of its central role in angiogenesis, VEGF is considered a pivotal factor in the pathogenesis of many tumor types. Increased expression of VEGF has been found in many tumor types, including breast cancer (BC), colorectal cancer (CRC), and lung cancer, and is associated with a poor prognosis and response to therapy [2, 3]. Moreover, inhibition of the VEGF pathway has been demonstrated to exhibit antitumor activity in clinical studies in a wide range of tumor types, including RCC, CRC, non-small cell lung cancer (NSCLC), and BC [4–7].
Under physiological conditions, VEGFR is only activated by ligand binding. Subsequently, ATP is recruited and binds in the so-called ATP-binding pocket of the tyrosine kinase region of the receptor. This is followed by the transfer of a phosphate group from ATP to VEGFR itself and to various other substrates in a process called phosphorylation. Through phosphorylation, downstream signaling pathways become activated, ultimately resulting in cellular effects, including proliferation of endothelial cells and recruitment of endothelial progenitor cells derived from the bone marrow, both of which are pivotal for angiogenesis.
Inhibition of VEGF–VEGFR driven processes can be achieved by several approaches. Monoclonal antibodies either target the extracellular domain of the VEGFR or trap VEGF, thereby preventing VEGF binding to VEGFRs. Another mechanism is through TKIs competitively binding to the ATP-binding pocket of the intracellularly located tyrosine kinase domain of VEGFR. Consequently, the binding of ATP to VEGFR is hampered, resulting in inhibition of the signal transduction from VEGFR.
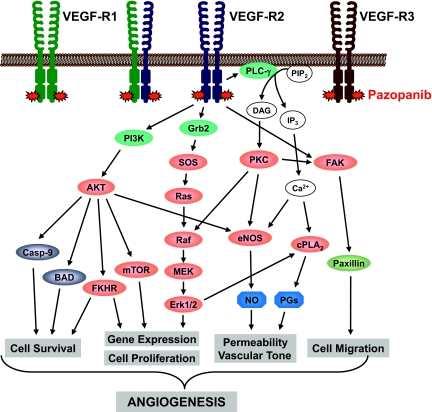
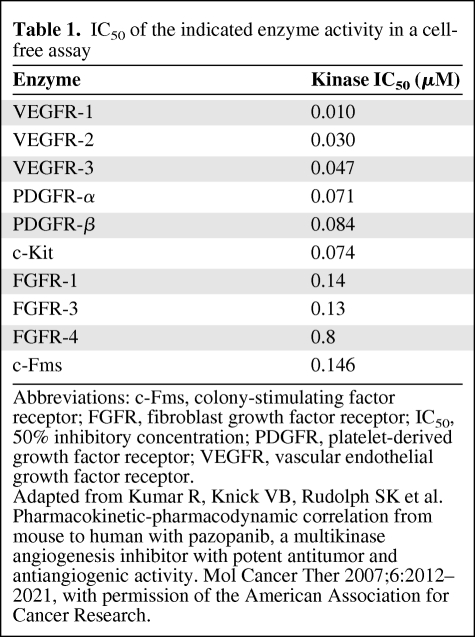
Pazopanib is a TKI designed to inhibit angiogenesis by abrogating VEGFR-2 function (Fig. 1). A widely used parameter reflecting the inhibitory effects of a drug on the kinase activity of a certain factor is the inhibition of the autophosphorylation of that factor in vitro. The pazopanib concentration required to produce 50% inhibition (IC50) of human VEGFR-2 kinase activity is 0.03 μM (Table 1) [8]. In comparison, sorafenib and sunitinib, other TKIs inhibiting VEGFR-2, have IC50 values of 0.09 and 0.009 μM for inhibiting VEGFR-2 activity, respectively [9, 10]. Furthermore, pazopanib inhibited VEGF-induced proliferation in a human umbilical vein endothelial cell (HUVEC) culture in vitro (IC50, 0.02 μM). As observed with other TKIs, pazopanib is not entirely specific for one target. Besides VEGFR-2, comparable inhibitory effects were found against VEGFR-1, VEGFR-3, PDGFR-α, PDGFR-β, and c-Kit (Table 1) [8]. Pazopanib inhibition of VEGF-induced proliferation of HUVECs in vitro was more pronounced than that of basic FGF–induced HUVEC proliferation (IC50, 0.72 μM). Furthermore, VEGF-induced as well as basic FGF–induced angiogenesis in a mouse corneal micropocket model was impaired by pazopanib, although the inhibition was more pronounced when VEGF was used as the stimulant [8].
Figure 1.
VEGFR-2 downstream pathway. By binding to the intracellular domain of VEGFR-2, pazopanib abrogates this pathway.
Abbreviations: BAD, Bcl-2-associated death promoter; Casp-9, caspase 9; cPLA2, cytosolic phospholipases A2; DAG, diacyl glycerol; eNOS, endothelial nitric oxide synthase; Erk, extracellular signal–related kinase; FAK, focal adhesion kinase; FKHR, forkhead box O1; Grb2, growth factor receptor-bound protein 2; IP3, inositol 1,4,5-trisphosphate; MEK, mitogen-activated protein kinase/extracellular signal–related kinase kinase; mTOR, mammalian target of rapamycin; PG, prostaglandin; PIP2, phosphatidylinositol-bisphosphate; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; PLC-γ, phospholipase Cγ; SOS, son of sevenless; VEGFR, vascular endothelial growth factor receptor.
Table 1.
IC50 of the indicated enzyme activity in a cell-free assay
Abbreviations: c-Fms, colony-stimulating factor receptor; FGFR, fibroblast growth factor receptor; IC50, 50% inhibitory concentration; PDGFR, platelet-derived growth factor receptor; VEGFR, vascular endothelial growth factor receptor.
Adapted from Kumar R, Knick VB, Rudolph SK et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther 2007;6:2012–2021, with permission of the American Association for Cancer Research.
Preclinical PK Data
Pazopanib is orally available, with 49% bioavailability in dogs [11]. In mice, the level needed for maximal inhibition of VEGFR-2 phosphorylation occurs in vivo at approximately 40 μM [8]. The discrepancy between in vivo and in vitro requirements can be attributed to >99.9% protein binding for pazopanib. PK analysis showed that tumor growth inhibition in a xenograft model using a CRC cell line is correlated with the steady-state concentration (Ctrough) and not with the peak plasma concentration (Cmax). In addition, the Ctrough required for in vivo inhibition of tumor growth in a xenograft model was almost equivalent to the concentration required for in vivo inhibition of VEGFR-2 phosphorylation (approximately 40 μM), suggesting that the drug concentration of pazopanib required for in vivo VEGFR-2 inhibition can predict the PK requirements for in vivo antitumor activity of pazopanib [8]. In mice, a single dose of 30 mg/kg resulted in plasma concentrations >40 μM for >8 hours [8].
Preclinical Efficacy and Toxicity
The antitumor activity of pazopanib was demonstrated in several human tumor xenograft models in mice, most prominently in an RCC model but also in CRC, NSCLC, and multiple myeloma (MM) models. Less potent inhibition was observed in melanoma, BC, and prostate cancer models [8, 12]. Sunitinib and sorafenib, as antiangiogenic class members of pazopanib, do share preclinical activity in several tumor types, including RCC, thyroid cancer, pancreatic cancer, and hepatocellular cancer (HCC). Furthermore, sorafenib has activity in MM, melanoma, and osteosarcoma, whereas sunitinib also exerts antitumor activity in small cell lung cancer, urothelial cancer, and acute myeloid leukemia models.
After cessation of pazopanib, rapid regrowth of MM cells was seen, underlining the importance of continuous exposure [12]. Because pazopanib has no significant effect on the proliferation of most tumor cell lines in vitro, inhibition of angiogenesis is likely the mechanism underlying the antitumor effects observed in vivo.
In addition to its preclinical efficacy as a single agent, synergistic cytotoxic effects of low-dose pazopanib combined with conventional chemotherapy (melphalan) or other molecular targeted agents (bortezomib) were observed in MM cell lines [12].
Currently, no published data are available on preclinical toxicity with pazopanib.
Clinical Data
PK Data
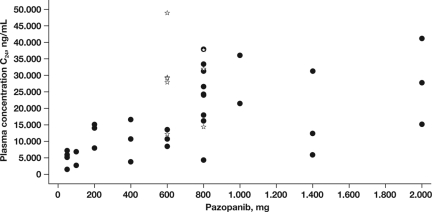
Clinical PK data on single-agent pazopanib are available from 63 patients who were enrolled in a phase I study [13]. Three-times-a-week (50 mg), once-a-day (OD) (50–2,000 mg), and twice-a-day (BID) (300 mg or 400 mg) schedules were evaluated at 13 dose levels. Pazopanib was absorbed orally with median time to maximum concentration (tmax) values in the range of 2.0–4.0 hours and 2.0–8.0 hours following single and multiple dosing, respectively. Although of less relevance because preclinical data strongly suggest that, for antitumor activity Ctrough levels are more important, Cmax increased with higher doses of pazopanib. By comparing Ctrough at day 22 with the concentration 24 hours after a single dose, accumulation appeared to be 1.2- to 4.5-fold [13]. The steady-state exposure was dependent on the dose and frequency of administration (Figure 2). Steady-state exposure plateaued at doses ≥800 mg/day and was ≥15 μg/ml (≈34 μM) in 93% of patients receiving a dose of 800 mg OD. This Ctrough plasma pazopanib concentration of ≥15 μg/ml appeared to correlate with clinical activity in patients with RCC as well as with the pharmacodynamic effect of hypertension [13]. A separate phase I, PK study was performed in HCC patients, showing that, at the maximum-tolerated dose (MTD) of 600 mg daily (QD), Ctrough was ≥15 μg/ml in 67% of patients [14].
Figure 2.
Steady-state exposure to pazopanib in the individual patients in the phase I study. Reprinted from Hurwitz HI, Dowlati A, Saini S et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res 2009;15:4220–4227, with permission of the American Association for Cancer Research.
Pazopanib was eliminated slowly, with mean half-life values in the range of 20.3–52.3 hours [13].
Drug–Drug Interactions
Two interaction studies are available exploring several doses of pazopanib in combination with lapatinib and with paclitaxel. Concurrent administration with lapatinib (750–1,500 mg OD), an orally available potent ErbB-1 and ErbB-2 TKI, alters the PK of pazopanib, given an increased Ctrough of pazopanib. Lapatinib concentrations were similar to those observed after monotherapy [15]. In contrast, pazopanib administered concomitantly led to a higher mean Cmax and area under the curve of paclitaxel, by approximately 40% and 45%, respectively [16]. Whether paclitaxel affects the PK of pazopanib has not yet been reported.
Recommended Dose for Further Studies
In the phase I study, pazopanib was generally well tolerated with continuous daily dosing of pazopanib ≤2,000 mg OD. An MTD was not determined. Four patients experienced dose-limiting toxicities (DLTs) at 50 mg OD (n = 2), 800 mg OD (n =1), and 2,000 mg OD (n = 1). The two DLTs occurring at 50 mg OD were gastrointestinal hemorrhage from a metastatic lesion in the small bowel in a patient with RCC and grade 3 extrapyramidal involuntary movements resulting from a potential drug–drug interaction between trazadone and pazopanib. Grade 3 hypertension and subsequently recurring grade 3 proteinuria were seen at the 800-mg pazopanib OD dose despite dose reductions, whereas a DLT comprising grade 3 fatigue occurred at the 2,000-mg OD dose, which improved to grade 1 after a dose reduction to 800 mg OD [13]. Despite the DLTs at 50 mg OD and 800 mg OD, dose escalation to 2,000 mg was feasible. In the absence of a MTD, the choice of the 800-mg dose as the recommended dose for further studies was based on the observation of a plateau in Ctrough at doses ≥800 mg/day, significant changes in dynamic contrast–enhanced magnetic resonance imaging (DCE-MRI) at doses of 300–400 mg BID, a threshold concentration that correlates with preclinical activity in patients, and pharmacodynamic effects of hypertension, as discussed below. OD administration was recommended for further studies because the fluctuation between Cmax and Ctrough with OD dosing was low (∼2), rendering drug exposure similar to that with continuous infusion [13].
In contrast to pazopanib, for which no MTD has been defined based on toxicity, the MTD of sunitinib was set at 50 mg daily for 28 days every 6 weeks, given an excess in toxicity (grade 3 asthenia and grade 3 hypertension) observed at doses >50 mg/day [17]. For sorafenib, skin and gastrointestinal toxicities were dose limiting, rendering an MTD of 400 mg BID [18].
The MTD of pazopanib in patients with HCC has been determined to be 600 mg QD, although the observed toxicity has not been reported yet [19].
Antitumor Activity
One phase III trial showed a beneficial effect of pazopanib in patients with RCC [19] and resulted in approval by the FDA. In other tumor types, some interesting signs of antitumor activity with pazopanib were observed, though it should kept in mind that activity data from phase I/II trials should be interpreted with extreme caution.
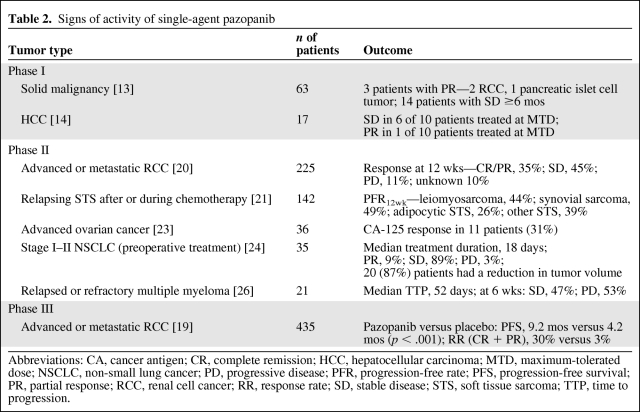
In the phase I study, of the 63 patients included, a partial response (PR) was observed in three patients and 14 patients achieved stable disease (SD) for >6 months (Table 2) [13]. Of interest is the activity seen in the 10 included patients with RCC: two patients achieved a PR (at the 300-mg BID and 1,400-mg OD doses), SD was observed in four patients (at the 300-mg BID, 800-mg OD [n = 2], and 2,000-mg OD doses), and progressive disease (PD) occurred in four patients, all at doses ≤400 mg OD. A Ctrough ≥15 μg/ml was achieved in 83% of patients with RCC who achieved a PR or SD, whereas all four patients experiencing PD had a Ctrough <15 μg/ml [13].
Table 2.
Signs of activity of single-agent pazopanib
Abbreviations: CA, cancer antigen; CR, complete remission; HCC, hepatocellular carcinoma; MTD, maximum-tolerated dose; NSCLC, non-small lung cancer; PD, progressive disease; PFR, progression-free rate; PFS, progression-free survival; PR, partial response; RCC, renal cell cancer; RR, response rate; SD, stable disease; STS, soft tissue sarcoma; TTP, time to progression.
In a randomized discontinuation phase II trial, 225 patients who had received, maximally, one prior line of systemic therapy for RCC received pazopanib at a dose of 800 mg OD for 12 weeks. Pazopanib was continued if a response was achieved at 12 weeks, but in cases of SD at 12 weeks, patients were randomized between placebo and continuation of pazopanib. Following an interim analysis by the independent data monitoring committee that showed a high response rate (38%) in the first 60 patients at week 12, all randomized patients were unblinded and allowed to cross over to pazopanib [20]. Sixty-nine percent of patients had received no prior systemic therapy and 31% had failed one prior systemic therapy (cytokine- or bevacizumab-based therapy) [20]. In all 225 patients, the complete response (CR) + PR rate at 12 weeks was 35%, whereas an additional 45% of patients achieved SD [20]. Subsequently, a placebo-controlled, randomized phase III trial (n = 435) was conducted in therapy-naïve or cytokine-pretreated patients with RCC [18]. At the interim analysis, a significantly longer progression-free survival (PFS) interval was observed (9.2 months versus 4.2 months). The response rate (CR + PR) was also more favorable in the pazopanib-treated patients (30% versus 3%), and a response had a median duration of 59 weeks [19]. The difference in overall survival was statistically not significant, given the interim O'Brien-Fleming boundary [19]. Furthermore, clean survival data will most likely not become available because patients on placebo could, upon progression, receive pazopanib. No worsening of quality of life was observed in the patients treated with pazopanib, versus placebo [19].
The phase II European Organization for Research and Treatment of Cancer 62043 trial explored pazopanib (800 mg OD) in patients with relapsing or refractory soft tissue sarcoma (STS) [21]. The primary endpoint of that study was the 12-week progression-free rate (PFR12wk), because the response rate is thought to not adequately reflect the antitumor activity of many drugs in STS [22]. An interesting PFR12wk, meeting the predefined criteria of a potentially active agent, was found in patients with leiomyosarcomas, patients with synovial sarcomas, and the group of patients with other eligible STSs (44%, 49%, and 39%, respectively). In contrast, there was insufficient activity in adipocytic STS [21].
Furthermore, preliminary data from a small phase II study in women with progressive, platinum-pretreated ovarian cancer showed a cancer antigen 125 response (defined as a confirmed decrease ≥50% from baseline) in 11 (31%) patients, with a median duration of response of 113 days [23].
In preliminary data from a proof-of-concept phase II study evaluating preoperative treatment with pazopanib (800 mg OD; median duration, 18 days) in stage I–II NSCLC patients, 87% of patients had a reduction in tumor volume, with volume changes in the range of −86% to +17%, whereas three patients achieved a PR [24].
In patients with advanced HCC, signs of activity were observed at the MTD, with six of 10 patients achieving prolonged SD and one of 10 patients achieving a PR. Furthermore, using DCE-MRI, a decline of 40% in imaging markers was seen in those 10 patients [15].
No data on single-agent pazopanib in BC patients are available. However, the combination of pazopanib (400 mg OD) and lapatinib (1000 mg/day) was recently compared with single-agent lapatinib (1,500 mg/day) in a randomized, phase II trial in patients with advanced or metastatic HER-2-positive BC. Previous chemotherapy or HER-2–directed therapy for advanced or metastatic disease was not allowed. A predefined interim analysis after 62 patients were accrued showed PD rates at 12 weeks, the primary endpoint, of 19% versus 27%, whereas the response rates were 44% versus 30%, both favoring the combination [26].
Insufficient activity of pazopanib in patients with MM, as shown by a complete lack of response at 6 weeks and a median time to progression of 52 days, resulted in the early termination of the phase II trial [25].
Adverse Events
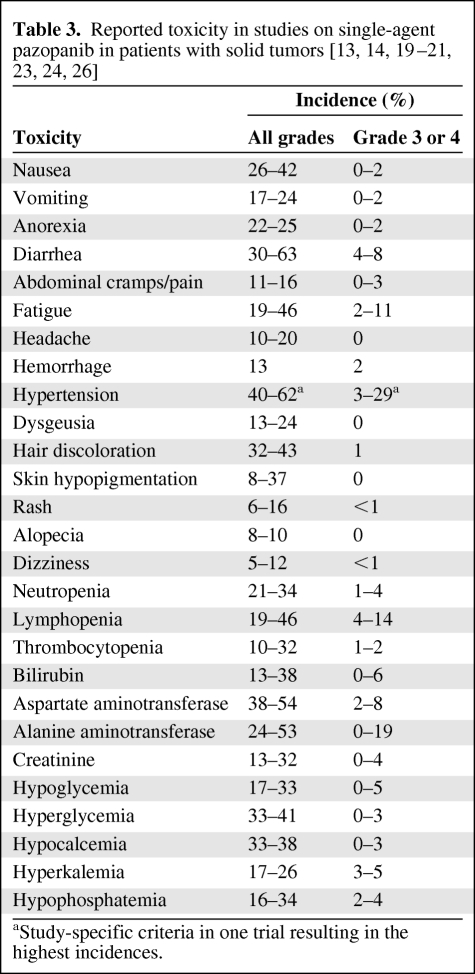
The most common adverse events in the phase I study were hypertension, diarrhea, hair depigmentation, and nausea [13]. A similar toxicity profile was seen in the phase II and phase III studies [19, 20, 21, 23, 24].
Similar to other agents targeting the VEGF–VEGFR pathway, hypertension is frequently reported during pazopanib treatment [6, 13]. In the phase I study, a study-specific hypertension definition was used in order to not underestimate the incidence: ≥15 mmHg rise from baseline in mean arterial blood pressure on three separate occasions and/or the initiation or escalation of antihypertensive medications. Antihypertensive medications were started or increased if blood pressure exceeded 160/100 mmHg on three occasions over any 2-week period. By this definition, the incidence was 62%; according to National Cancer Institute Common Toxicity Criteria, version 2.0, 29% had grade 3 hypertension. The overall incidence of hypertension was similar in patients with and without a history of hypertension (71% versus 62%) [13]. All episodes of hypertension in the phase I study were easily manageable with antihypertensive medication; however, temporary interruption or dose reduction because of hypertension was needed in two patients [13]. Hypertension seems to be correlated with a Ctrough ≥15 μg/ml, because 77% of the patients above this cutoff developed hypertension, versus only 39% of the patients below this level [14]. In the three largest studies conducted to date, the incidence of hypertension was 37%–40% (grade 3 or 4, 4%–8%) [19–21]. Of interest, cumulative incidence analysis revealed that, in general, patients develop hypertension within 4 weeks after treatment initiation, and only a few patients develop it thereafter [21].
Hair depigmentation, as reported for other agents targeting c-Kit, was observed in 32%–38% of patients and seen at pazopanib doses ≥600 mg/day [13, 19, 27]. Other frequent, mostly mild, toxicities comprised fatigue, anorexia, diarrhea, and skin discoloration. Laboratory findings show mild and infrequent bone marrow suppression, whereas elevations in aspartate aminotransferase and/or alanine aminotransferase are relatively common but rarely a reason for treatment discontinuation (Table 3). Moreover, pazopanib-induced isolated hyperbilirubinemia is, in most cases, a benign manifestation of Gilbert's syndrome, as shown by UGT1A1 polymorphism in 84% of patients [28]. Collectively, it appears that pazopanib is generally well tolerated, which is important given the necessity of administering this agent for prolonged periods of time. In the two largest phase II studies of pazopanib, 6%–15% of patients discontinued treatment because of adverse events [20, 21].
Table 3.
Reported toxicity in studies on single-agent pazopanib in patients with solid tumors [13, 14, 19–21, 23, 24, 26]
aStudy-specific criteria in one trial resulting in the highest incidences.
Discussion
Pazopanib is one of the novel drugs belonging to the rapidly expanding class of TKIs and was recently approved for patients with advanced RCC. Although other mechanisms may contribute, the main mechanism underlying its antitumor activity in RCC is thought to be the inhibition of VEGFR-2, although other inhibited factors will play a role as well and might even be of greater relevance in tumor types other than RCC. In vivo, a threshold for inhibition of VEGFR-2 by pazopanib has been established, a level paralleling its antitumor activity in vivo in preclinical models. Furthermore, it was revealed that a Ctrough above the threshold associated with VEGFR-2 inhibition, rather than Cmax, is associated with antitumor activity. In humans, a comparable Ctrough level is reached in the majority of patients treated at pazopanib doses of 800 mg/day. Based on the finding that doses >800 mg do not result in higher Ctrough values, increasing the dose to >800 mg is unlikely to yield better outcomes.
At a dose of 800 mg, pazopanib is well tolerated, even with long-term use. The side-effect profile seems consistent with the tyrosine kinases inhibited. In several phase II trials conducted in STS, RCC, relapsing ovarian cancer, and NSCLC, early hints of antitumor activity of pazopanib were seen. In the first phase III study, a longer PFS time was observed in patients with RCC, resulting in the FDA approval of pazopanib.
Putting pazopanib into perspective and comparing it with other VEGFR TKIs that have been approved for a longer time are difficult and have to be done on the basis of indirect comparisons, given the lack of data from comparative trials among the several treatment options. With respect to the first-line treatment of patients with RCC, with the clear cell subtype and belonging to the so-called good and intermediate prognostic groups, according to the Memorial Sloan-Kettering Cancer Center classification [29], three treatment options are currently approved: sunitinib, pazopanib, and the combination of bevacizumab and interferon. Sorafenib failed to show superiority relative to interferon-α in treatment-naïve patients [30]. Approval of all three treatment options was granted on the basis of longer PFS times; however, in the meantime, sunitinib was shown to lead to longer overall survival than with interferon-α (26.4 months versus 21.8 months) [31]. The two pivotal trials on the combination of bevacizumab and interferon-α showed superiority over monotherapy interferon-α as a result of a longer PFS time, 8.5 months versus 5.2 months and 10.2 months versus 5.4 months, respectively [32, 33]. An indirect comparison was made between sunitinib and the combination of bevacizumab and interferon-α, suggesting superiority for sunitinib; however, no firm conclusions can yet be drawn [34]. In contrast to the other two treatment options, pazopanib has not been compared with interferon-α but with placebo, hindering a direct comparison. This issue soon will be clarified because a phase III study comparing pazopanib with sunitinib is currently enrolling patients. Altogether, based on the current data, pazopanib can be regarded as an alternative first-line therapy for these patients, in particular for those not tolerating sunitinib or the combination of bevacizumab and interferon-α.
For second-line treatment of RCC patients following cytokine-based therapy, three treatment options have gained approval: sunitinib, sorafenib, and pazopanib. Approval for sunitinib was based on two single-arm studies [35, 36], whereas sorafenib and pazopanib were approved on the basis of placebo-controlled trials rendering longer PFS intervals with these agents [19, 37].
In the two single-arm studies of sunitinib in RCC patients pretreated with cytokine-based therapy, sunitinib resulted in objective response rates of 34%–40% and a PFS time of 8.3–8.7 months [35, 36]. Pazopanib in second-line treatment was tested in the placebo-controlled trial that also examined first-line treatment; the proportion of patients who were cytokine pretreated (47%) had a longer PFS duration, 7.4 months versus 4.2 months, while receiving pazopanib as compared to placebo [19]. Sorafenib treatment led to a longer PFS interval, 5.5 months versus 2.8 months, in a cytokine-pretreated population [37]. Indirectly comparing these two trials, neither agent is clearly superior to the other with regard to survival, making arguments like differences in the toxicity profiles more important.
In conclusion, the recent approval of pazopanib for patients with advanced RCC is a result of a rational stepwise development using translational research. Based on preclinical and early clinical data on pazopanib, further exploration of pazopanib is warranted in tumor types other than RCC. The currently ongoing phase III studies and studies examining the feasibility of pazopanib in combination with other antitumor agents will be instrumental in defining the place of pazopanib as a novel antitumor agent.
Author Contributions
Conception/Design: Paul Hamberg, Jaap Verweij, Stefan Sleijfer
Collection and/or assembly of data: Paul Hamberg, Jaap Verweij, Stefan Sleijfer
Data analysis and interpretation: Paul Hamberg, Jaap Verweij, Stefan Sleijfer
Manuscript writing: Paul Hamberg, Jaap Verweij, Stefan Sleijfer
Final approval of manuscript: Paul Hamberg, Jaap Verweij, Stefan Sleijfer
References
- 1.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 2.Foekens JA, Peters HA, Grebenchtchikov N, et al. High tumor levels of vascular endothelial growth factor predict poor response to systemic therapy in advanced breast cancer. Cancer Res. 2001;61:5407–5414. [PubMed] [Google Scholar]
- 3.Toi M, Matsumoto T, Bando H. Vascular endothelial growth factor: Its prognostic, predictive, and therapeutic implications. Lancet Oncol. 2001;2:667–673. doi: 10.1016/S1470-2045(01)00556-3. [DOI] [PubMed] [Google Scholar]
- 4.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 5.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 7.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Knick VB, Rudolph SK, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6:2012–2021. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- 9.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 10.Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 11.Sonpavde G, Hutson TE. Pazopanib: A novel multitargeted tyrosine kinase inhibitor. Curr Oncol Rep. 2007;9:115–119. doi: 10.1007/s11912-007-0007-2. [DOI] [PubMed] [Google Scholar]
- 12.Podar K, Tonon G, Sattler M, et al. The small-molecule VEGF receptor inhibitor pazopanib (GW786034B) targets both tumor and endothelial cells in multiple myeloma. Proc Natl Acad Sci U S A. 2006;103:19478–19483. doi: 10.1073/pnas.0609329103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurwitz HI, Dowlati A, Saini S, et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. 2009;15:4220–4227. doi: 10.1158/1078-0432.CCR-08-2740. [DOI] [PubMed] [Google Scholar]
- 14.Yau CC, Chen PJ, Curtis CM, et al. Phase I study of pazopanib in hepatocellular carcinoma: Evaluation of clinical activity, pharmacokinetics and dynamic contrast enhanced MRI (DCE MRI) Eur J Cancer. 2009;7(suppl 2):122. [Google Scholar]
- 15.De Jonge M, Savage S, Verweij J, et al. A phase I open-label study of the safety and pharmacokinetics of pazopanib and lapatinib administered concurrently [abstract 3088] Proc Am Soc Clin Oncol. 2006;24:142. [Google Scholar]
- 16.Tan AR, Jones SF, Dowlati A, et al. Phase I study of the safety, tolerability, and pharmacokinetics (PK) of weekly paclitaxel administered in combination with pazopanib (GW786034) [abstract 3552] Proc Am Soc Clin Oncol. 2008;26:166. [Google Scholar]
- 17.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 18.Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43–9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 19.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 20.Hutson TE, Davis ID, Machiels JP, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2010;28:475–480. doi: 10.1200/JCO.2008.21.6994. [DOI] [PubMed] [Google Scholar]
- 21.Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase II study from the European Organisation for Research and Treatment of Cancer–Soft Tissue and Bone Sarcoma Group (EORTC study 62043) J Clin Oncol. 2009;27:3126–3132. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 22.Van Glabbeke M, Verweij J, Judson I, et al. EORTC Soft Tissue and Bone Sarcoma Group. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38:543–549. doi: 10.1016/s0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 23.Friedlander M, Hancock KC, Benigno B, et al. Pazopanib (GW786034) is active in women with advanced epithelial ovarian, fallopian tube and peritoneal cancers: Final results of a phase II study. Ann Oncol. 2008;19(suppl 8):viii211. [Google Scholar]
- 24.Altorki N, Heymach J, Guarino M, et al. Phase II study of pazopanib (GW786034) given preoperatively in stage I-II non-small cell lung cancer (NSCLC): A proof-of-concept study. Ann Oncol. 2008;19(suppl 8):viii89. [Google Scholar]
- 25.Slamon D, Gomez H, Kabbinawar F, et al. Randomized study of pazopanib + lapatinib vs. lapatinib alone in patients with HER2 positive advanced or metastatic breast cancer [abstract 1016] Proc Am Soc Clin Oncol. 2008;26:45. [Google Scholar]
- 26.Prince HM, Hönemann D, Spencer A, et al. Vascular endothelial growth factor inhibition is not an effective therapeutic strategy for relapsed or refractory multiple myeloma: A phase 2 study of pazopanib (GW786034) Blood. 2009;113:4819–4820. doi: 10.1182/blood-2009-02-207209. [DOI] [PubMed] [Google Scholar]
- 27.Routhouska S, Gilliam AC, Mirmirani P. Hair depigmentation during chemotherapy with a class III/V receptor tyrosine kinase inhibitor. Arch Dermatol. 2006;142:477–479. doi: 10.1001/archderm.142.11.1477. [DOI] [PubMed] [Google Scholar]
- 28.Xu CF, Reck BH, Xue Z, et al. Pazopanib-induced hyperbilirubinemia is associated with Gilbert's syndrome UGT1A1 polymorphism. Eur J Cancer. 2009;7(suppl 2):119. doi: 10.1038/sj.bjc.6605653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 30.Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1280–1289. doi: 10.1200/JCO.2008.19.3342. [DOI] [PubMed] [Google Scholar]
- 31.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 33.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson Coon JS, Liu Z, Hoyle M, et al. Sunitinib and bevacizumab for first-line treatment of metastatic renal cell carcinoma: A systematic review and indirect comparison of clinical effectiveness. Br J Cancer. 2009;101:238–243. doi: 10.1038/sj.bjc.6605167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 36.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 37.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]