The study used a vignette-type survey to assess preferences and influential factors in geriatric cancer management of U.S. practicing oncologists. Advanced patient age was found to deter oncologists from choosing intensive cancer therapy, even if the patient was highly functional and lacked comorbidities.
Keywords: Age factors, Antineoplastic agents/therapeutic use, Geriatric assessment, Health care disparities, Quality of health care
Abstract
Background.
Over half of new cancer cases occur in patients aged ≥65 years. Many older patients can benefit from intensive cancer therapies, yet evidence suggests that this population is undertreated.
Methods.
To assess preferences and influential factors in geriatric cancer management, practicing U.S. medical oncologists completed a survey containing four detailed vignettes exploring colon, breast, lung, and prostate cancer treatment. Participants were randomly assigned one of two surveys with vignettes that were identical except for patient age (<65 years or >70 years).
Results.
Physicians in each survey group (n = 200) were demographically similar. Intensive therapy was significantly less likely to be recommended for an older than for a younger, but otherwise identical, patient in two of the scenarios. For a woman with metastatic colon cancer (Eastern Cooperative Oncology Group [ECOG] score, 1) for whom chemotherapy was recommended, nearly all oncologists chose an intensive regimen if the patient's age was 63; but if her age was 85, one fourth of the oncologists chose a less intensive treatment. Likewise, for stage IIA breast cancer (ECOG score, 0), 93% recommended intensive adjuvant treatment for a previously healthy patient aged 63; but only 66% said they would do so if the patient's age was 75. Oncologists commonly identified patient age as an influence on treatment choice, but were even more likely to cite performance status as a determining factor.
Conclusions.
Advanced age can deter oncologists from choosing intensive cancer therapy, even if patients are highly functional and lack comorbidities. Education on tailoring cancer treatment and a greater use of comprehensive geriatric assessment may reduce cancer undertreatment in the geriatric population.
Introduction
Population projections suggest that individuals who develop cancer are increasingly likely to be age >65 years [1]. More than half of new cancer cases now involve patients in this age group [2]. This demographic shift reflects an aging baby boom population as well as continued increases in longevity.
Older adults often have distinct characteristics that must be considered when planning cancer treatment [3]. Older patients, compared with their younger counterparts, commonly have more comorbidities and sensory impairments and lower physiologic reserves that may or may not be overt. Although advanced chronologic age is associated with some treatment toxicities [4, 5], these health impairments further increase the risk for adverse events [6]. Many older adults face additional barriers that may threaten treatment outcomes, such as inadequate social supports and limited transportation. These resource deficits must also be factored into treatment planning.
The optimal approach to cancer therapy in older adults is often unclear. Historically, advanced age has been an exclusion criterion in clinical cancer trials, and older adults have been consistently underrepresented [7, 8]. As a result, high-level evidence about treatment efficacy and tolerability in this population is often limited. In recent years, retrospective analyses and modifications to clinical trial designs have attempted to address this gap. The literature emerging from these efforts increasingly indicates that many older adults can both benefit from and tolerate intensive cancer treatments [9–15]. There is also expanding guidance about strategies that reduce treatment risks, yet still maintain efficacy [16, 17].
Despite growing evidence of treatment benefit, cancer in older adults is often undertreated [18–25], which contributes to poor outcomes and can be considered a health care disparity [26, 27]. This finding is concerning, because many older adults diagnosed with cancer are in relatively good health and have potentially many years of life ahead of them [28]. The benefits of standard cancer therapies for these patients can be substantial and often outweigh the potential risks. Many factors contribute to the undertreatment of older adults. Although some of these exert their influence before an oncology consultation even occurs, oncologists' misperceptions about life expectancy, therapeutic benefit, and treatment risks also appear to play a role [18].
The present study further explores the influence of older age on oncologists' treatment selection. The study tested the hypothesis that, all else being equal, standard cancer therapy is less likely to be recommended to a patient with advanced age. The study also sought insight into oncologists' perceptions of chronologic age and its influence on their treatment choices. These issues were examined in the context of lung, breast, prostate, and colorectal cancers, the leading causes of cancer mortality in the U.S.
Methods
To assess the impact of age on cancer treatment decisions, we designed a survey based on vignettes illustrating four detailed cancer patient cases. The survey was modified so that an equal portion of respondents would decide on a treatment approach for two younger and two older cancer patients. Multiple-choice responses of treatment selection and open-ended responses for factors involved in this decision were analyzed to test the hypothesis.
Case Vignette Survey
This study used a survey containing case vignettes to investigate practicing U.S. medical oncologists' current practice patterns in managing elderly patients with cancer. Four vignettes were developed for the survey by an expert in medical oncology and described patients with either non-small cell lung cancer (NSCLC), prostate cancer, breast cancer, or colorectal cancer. Results from recent research demonstrate that case vignettes, compared with other methods of measuring processes of care such as chart review and standardized patients, are a valid, noninvasive, and cost-effective method. The cases portrayed patients at different disease stages and integrated real-world psychosocial complexities that also might influence treatment selection. Each case was developed in two versions that differed with respect to patient age (<65 years or >70 years), but was otherwise identical. Each case was followed by one or two multiple choice questions asking about the next step in cancer management and offered options of varying therapeutic intensities. An open-ended question then asked oncologists which case features most significantly influenced their selected treatment. The cases were distributed among two instruments (versions A and B), with each version containing cases featuring two older (aged 72–85) and two younger (aged 58–63) patients, as shown in Appendix 1. A set of standard demographic questions was added to assess physician and practice characteristics. The study instrument and protocol were approved by the Western Institutional Review Board (Olympia, WA).
Data Collection
Invitations to complete the survey were distributed by e-mail during March 2009 to a nationally representative random sample of physicians specializing in medical oncology or hematology/oncology identified by the American Medical Association (AMA) Physician Masterfile. Invitations were stratified by U.S. Census Bureau–defined regions and were then randomized to direct oncologists to either the A or B version of the online survey. Oncologists were issued up to three invitations and then randomly replaced if they did not respond. A small monetary incentive was offered for participation. Oncologists' responses were considered eligible for inclusion if they saw patients with at least one of the four cancer types of interest, were currently seeing ≥20 patients per week, and estimated that ≥10% of their patients were aged >65 years.
Analysis
Demographics were compared between oncologists responding to the two versions of the survey to ensure the similarity of the two cohorts. Additionally, these demographics were compared with the AMA Physician Masterfile to assess the generalizability of the sample. Descriptive statistics were conducted on all items of the survey, using χ2 analysis to examine differences between the two cohorts (SPSS, version 17.0; SPSS, Inc., Chicago, IL). The Monte Carlo method was used for the χ2 analyses that involved cells with small numbers. The open-ended responses were analyzed by creating dichotomous variables to reflect the number of times that “age” or “performance status” was mentioned as an influence on treatment selection.
Results
Sample Characteristics
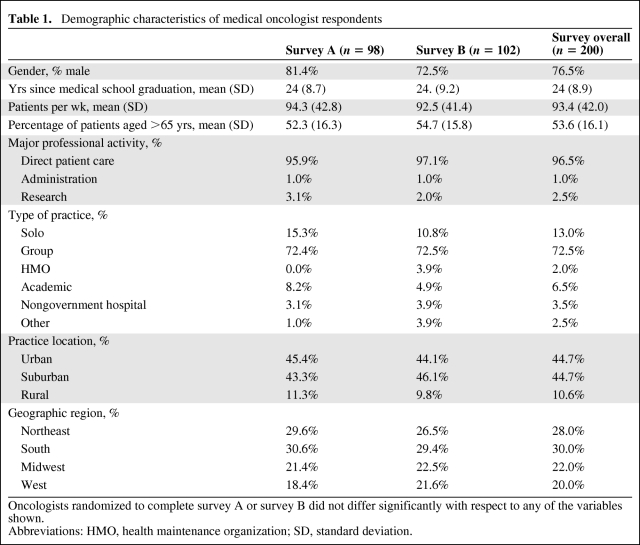
Of the 202 oncologists who responded to the invitation, 200 met the inclusion criteria and were included in the analysis. Sample demographic characteristics are shown in Table 1. Ninety-eight respondents completed survey A and 102 completed survey B. The characteristics of these two groups were similar with respect to their demographic characteristics and scope of practice relative to the four cancer types examined. The overall sample is representative of medical oncologists listed in the AMA Physician Masterfile with respect to gender and years since medical school graduation, but oncology respondents were more likely to be in private practice and primarily engaged in direct patient care (p < .01).
Table 1.
Demographic characteristics of medical oncologist respondents
Oncologists randomized to complete survey A or survey B did not differ significantly with respect to any of the variables shown.
Abbreviations: HMO, health maintenance organization; SD, standard deviation.
Impact of Age on Cancer Treatment Choice
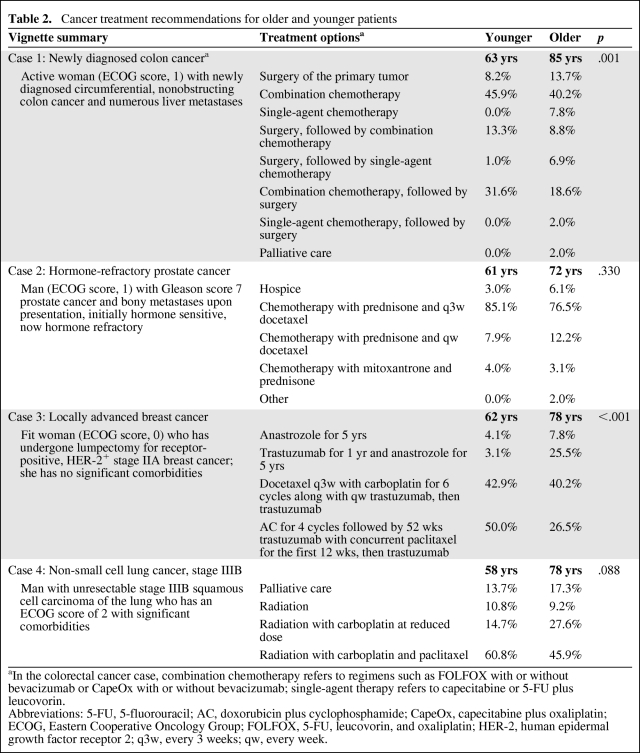
Response patterns showing oncologists' recommended cancer treatments for each of the four vignettes are shown in Table 2. Each vignette conveyed the patient's age and functional status, as well as clinical and psychosocial details. Full descriptions are available in Appendix 1. For two of the four vignettes, the oncologists' selected treatments differed significantly based solely on the age of the patient.
Table 2.
Cancer treatment recommendations for older and younger patients
aIn the colorectal cancer case, combination chemotherapy refers to regimens such as FOLFOX with or without bevacizumab or CapeOx with or without bevacizumab; single-agent therapy refers to capecitabine or 5-FU plus leucovorin.
Abbreviations: 5-FU, 5-fluorouracil; AC, doxorubicin plus cyclophosphamide; CapeOx, capecitabine plus oxaliplatin; ECOG, Eastern Cooperative Oncology Group; FOLFOX, 5-FU, leucovorin, and oxaliplatin; HER-2, human epidermal growth factor receptor 2; q3w, every 3 weeks; qw, every week.
Newly Diagnosed Metastatic Colon Cancer in the Active Patient
The first vignette featured an active woman with newly diagnosed circumferential colon cancer and numerous liver metastases. The patient is minimally symptomatic (Eastern Cooperative Oncology Group [ECOG] score, 1) and her age was varied at either 63 or 85 (see Appendix 1 for full case description). Oncologists were first asked to choose a general treatment strategy and then asked to choose a chemotherapy regimen. The combined responses are shown in Table 2. Oncologists showed a greater tendency toward treatment involving chemotherapy for a patient aged 63 than for a patient aged 85 (92% versus 84%; p = .128). Among the subset choosing to use chemotherapy, treatment intensity differed significantly by patient age (p < .001). These oncologists almost universally recommended a combination therapy (either 5-fluorouracil [5-FU], leucovorin, and oxaliplatin [FOLFOX] with or without bevacizumab or capecitabine plus oxaliplatin [CapeOx] with or without bevacizumab) for the patient if her age was 63, whereas nearly one in four would choose a single-agent regimen (either capecitabine or 5-FU plus leucovorin) if this same patient's age was 85.
Patient Whose Prostate Cancer Has Become Hormone Refractory
In a second vignette, a patient, either 61 or 72 years old, was described who had an initial favorable response to androgen-deprivation therapy for widely metastatic Gleason score 7 prostate cancer. The cancer had become hormone refractory. The patient's ECOG performance status score was 1 and he had transportation limitations. Age was not significantly associated with oncologists' selection among the available treatment options (p = 0.32) in this vignette.
Locally Advanced Breast Cancer in a Previously Healthy Woman
The third case portrayed a woman, aged either 63 or 78 years, who underwent lumpectomy with sentinel node biopsy and was staged as estrogen receptor (ER)+/progesterone receptor (PR)+ human epidermal growth factor receptor (HER)-2+ stage IIA (T1N1M0) breast cancer. The patient was otherwise fit without significant comorbidities (ECOG score, 0) and had strong social support. Adjuvant treatment recommendations differed significantly for the older compared with the younger patient (p < .001). For a 63-year-old patient, 93% of the oncologists said they would recommend intensive adjuvant chemotherapy. If the patient was 78 years old, only 66% would make this recommendation, with 25% of oncologists instead choosing a less toxic regimen with a biologic agent.
Unresectable NSCLC in a Patient with Comorbidities
The lung cancer vignette described a man aged either 58 or 78 with unresectable stage IIIB squamous cell carcinoma (T4N2M0). The patient had significantly diminished functional capacity (ECOG score, 2) and limited English proficiency. Treatment choices varied by patient age, but not significantly (p = .09). This trend was driven primarily by those choosing a combined modality option, with fewer oncologists recommending standard chemotherapy if the patient was 78 years old (46%) rather than 58 years old (61%). In the setting of a poor performance status at either age, more than one in seven oncologists said they would recommend palliative care for this patient.
Self-Identified Influences on Oncologists' Selected Treatment Choice
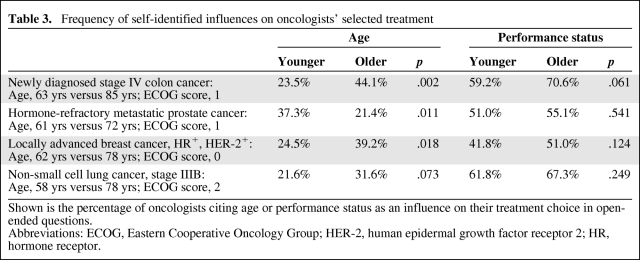
In all four cases, performance status was the most commonly cited influence on oncologists' treatment choices (Table 3). Patient age was also a frequently cited influence and was particularly likely to be mentioned in the colon and breast cancer cases. These cases portrayed active adults with minimal pre-existing impairments and both were associated with significant variation in treatment selections based on patient age. In these cases, age was cited as an influence without reference to other health parameters by 15%–20% of oncologists. These chronologically focused oncologists were less likely to recommend intensive cancer therapies than those who cited favorable health indices as influential.
Table 3.
Frequency of self-identified influences on oncologists' selected treatment
Shown is the percentage of oncologists citing age or performance status as an influence on their treatment choice in open-ended questions.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HER-2, human epidermal growth factor receptor 2; HR, hormone receptor.
Oncologists tended to mention age more frequently as patient age increased, but this was not always the case. In the context of prostate cancer, physicians were more likely to comment on age for a patient aged 61 rather than an older patient, aged 72. This suggests that oncologists may be particularly influenced by a patient's age when it falls outside the usually expected range.
Discussion
Findings from this study confirm our hypothesis and offer further evidence that older adults are less likely than younger patients to be offered standard cancer therapies. The impact of this health care disparity is particularly well illustrated in the two cases describing individuals who were active and relatively healthy at the time of their diagnosis. Although current evidence suggests that older patients with these characteristics should be treated with standard therapies [16], a segment of physicians surveyed favored less intensive treatment options for this population. The age disparities observed in this study are consistent with those from other studies using survey methodology [29, 30], as well as from retrospective studies of cancer care [18, 21, 25].
Oncologists in this study were often overtly aware of age as an influence on their treatment recommendations. However, an even larger portion of oncologists based their treatment recommendations on functional status. The relative importance of age and functional status on clinical decision making appears to be important. When choosing treatment for patients with few limitations, oncologists who focused on chronologic age favored conservative treatment regimens, whereas those who focused more globally on health indices such as performance status or a lack of comorbidities more frequently favored treatments that carried a greater risk but were also more likely to reduce or delay cancer mortality.
The findings from this survey have some limitations. Participating medical oncologists differed somewhat from those in the AMA Physician Masterfile and were more likely to be in private practice, rather than academic settings. Community-based oncologists often work closely with referring physicians and their approach to older adults may be influential within this network. In some studies, these community-based oncologists appear to be less aggressive in treating older adults than academic clinicians [31].
In this study, oncologists were asked about their recommendation, but were only able to offer one option. In clinical practice, however, physicians may commonly discuss the range of available options, noting which seems most appropriate for a given patient. Thus, intensive cancer therapies may not be overtly withheld from older patients. Instead, there is some evidence that patients may receive too little information from their physician to choose among the options that are presented [32]. Further studies examining how patient age influences physician communication when discussing treatment options may be informative.
These findings highlight the critical importance of comprehensive geriatric assessment (CGA) in oncology care, which is advocated by guidelines and provides a more finely detailed measure of functional capacity and health status [33]. CGA can also unmask problems that are relatively unique to older adults, such as dementia and subtle gait disturbances. Collectively, this information can help to identify patients with above average life expectancy who are likely to benefit from standard chemotherapy. By prompting a proactive response to relevant health problems and resource needs during treatment planning, CGA may also improve patient outcomes [34]. Brief screening tests such as the “timed up and go” test or the Vulnerable Elders Survey can be used to rapidly identify patients with significant impairments who may especially benefit from CGA [35, 36]. Patients with abnormal screens can be further evaluated to determine whether they can benefit from treatment if special precautions are taken or whether they are too frail for intensive therapy. Hurria and colleagues developed a CGA instrument based on patient self-report, making a detailed evaluation more feasible for busy oncologists [37].
Conclusion
All else being equal, oncologists are less likely to recommend intensive, but beneficial, cancer therapies to older adults. Treatment planning for older adults must look beyond simply chronologic age and should consider multiple indices of health as well as patient resources to support the cancer treatment process. The use of CGA methods can facilitate this process by improving life-expectancy estimation and identifying subtle gerontologic issues that might influence a patient's treatment experience. Older adults are prevalent in oncology practices, but high-level evidence about how to best care for them is sometimes lacking. Several resources have been developed that seek to address this void. Newer clinical trials are helping to address the current evidence gap and will hopefully identify additional treatment regimens that are both effective and tolerable for older adults.
Acknowledgments
This study was supported by an educational grant from sanofi-aventis. We appreciate Teresa Hayes, M.D., who provided consultation during the study and reviewed the draft of this article.
Appendix 1: Case Vignettes
Case 1: Colon Cancer
The patient is a 63 (85)-year-old white woman who volunteers in her church office. During evaluation of a hemoccult positive stool and increasing fatigue, she was found to have a nonobstructing circumferential mass in the ascending colon, biopsy positive for adenocarcinoma. Further workup revealed multiple inoperable liver metastases. Other than well-controlled hypertension, she had been healthy for most of her life and was still actively taking care of her yard and garden. Her ECOG performance status score is 1. Her husband, who accompanied her to the follow-up visit, is a retired surgeon and asks you several questions on the varying methods of treatment.
-
What initial treatment plan would you recommend to this patient? (select only one)
Surgery of the primary tumor
Chemotherapy
Surgery of the primary tumor, followed by adjuvant chemotherapy
Neoadjuvant chemotherapy, followed by surgery of the primary tumor
Palliative care and hospice referral
-
If chemotherapy was planned, which of the following regimens would you choose? (select only one)
5-FU and leucovorin
Capecitabine
FOLFOX
FOLFOX plus bevacizumab
Other: ________________
What factors in this case most significantly influence how you would treat this patient? (list up to three factors)
Case 2: Prostate Cancer
The patient is a 61 (72)-year-old white man who presented with bone pain and was found to have a prostate-specific antigen level of 124 and multiple blastic metastases throughout the skeleton. His daughter insists on accompanying him to all appointments but works across town and must take time off work to pick her father up for each visit to your office. Biopsy of the prostate revealed Gleason score 7 adenocarcinoma. He was treated with goserelin with a good response. The cancer eventually progressed. He received sequential treatment with bicalutamide, then androgen withdrawal, then ketoconazole and hydrocortisone. Four years after diagnosis, his prostate cancer was deemed to be hormone refractory. His ECOG performance status score was 1. Neither the patient nor his family understand much about the condition and possible treatments, and they prefer not to have an active role in making decisions.
-
4. What would be your next step in this patient's management? (select only one)
Hospice referral
Chemotherapy with prednisone and docetaxel every 3 weeks
Chemotherapy with prednisone and docetaxel once per week
Chemotherapy with mitoxantrone and prednisone
Other: _______________
5. What factors in this case most significantly influence how you would treat this patient? (list up to three factors)
Case 3: Breast Cancer
A 62 (78)-year-old white woman presented with a mammographically detected 2-cm breast mass, which on biopsy was a high grade, ER+/PR+ HER-2+ adenocarcinoma. The patient would like to avoid mastectomy, but is open to all other treatment options. She undergoes lumpectomy and sentinel lymph node biopsy. One lymph node shows a microscopic focus of metastasis measuring 1 mm and her breast cancer is staged as IIA. The patient lives with her daughter, who usually accompanies her to appointments. She exercises every day and seems very fit for her age (ECOG score, 0). She is on multivitamins and has no history of heart disease, diabetes, or hypertension. Her ejection fraction is 68%.
-
6. Would you recommend radiation treatment for this patient?
Yes
No
-
7. Which of the following adjuvant regimens would you consider most appropriate for this patient? (select only one)
Anastrozole for 5 years
Trastuzumab for 1 year and anastrozole for 5 years
Docetaxel given every 3 weeks with carboplatin for six cycles along with weekly trastuzumab, then followed by trastuzumab every 3 weeks for 1 year
Doxorubicin and cyclophosphamide for four cycles followed by 52 weeks of trastuzumab with concurrent paclitaxel for the first 12 weeks, then trastuzumab for up to 1 year and anastrozole for 5 years
8. What factors in this case most significantly influence how you would treat this patient? (list up to three factors)
Case 4: Lung Cancer
The patient is a 78 (58)-year-old Hispanic man who has smoked two packs of cigarettes per day since he was 18. A chest x-ray revealed a large left upper lobe mass. Staging evaluation demonstrated mediastinal adenopathy, and the patient was diagnosed with squamous cell carcinoma of the lung, clinical stage IIIB (T4N2M0). He had a history of myocardial infarct 3 years prior to diagnosis, with stent placement for two-vessel coronary artery disease. His forced expiratory volume was 1.7 l/minute. He lives a sedentary lifestyle in his own home and is able to perform all his own activities of daily living slowly, but independently (ECOG score, 2). On physical exam, he appears his stated age and walks with a cane. He does not speak English well and is assisted by his son for translation as well as mobility. The lung cancer was deemed to be unresectable, and you discuss options of chemotherapy and radiation therapy with the patient and his son.
-
9. What would be your next step for this patient? (select only one)
Palliative care
Radiation
Radiation with carboplatin at a reduce dose
Radiation as well as carboplatin and paclitaxel
10. What factors in this case most significantly influence how you would treat this patient? (list up to three factors)
Author Contributions
Conception/Design: Gregory D. Salinas, Jill A. Foster, James C. Williamson, Linda L. Casebeer
Collection and/or assembly of data: Gregory D. Salinas, Jill A. Foster, James C. Williamson
Data analysis and interpretation: Gregory D. Salinas, Jill A. Foster, Dorcas Mansell, James C. Williamson, Linda L. Casebeer
Manuscript writing: Jill A. Foster, Gregory D. Salinas, Dorcas Mansell, Linda L. Casebeer
Final approval of manuscript: Gregory D. Salinas, Jill A. Foster, Dorcas Mansell, James C. Williamson, Linda L. Casebeer
References
- 1.U. S. Census Bureau PD. 2008 National Population Projections - Table 3. Percent Distribution of the Projected Population by Selected Age Groups and Sex for the United States: 2010 to 2050. [accessed June 15, 2009]. Available at http://www.census.gov/population/www/projections/summarytables.html.
- 2.American Cancer Society SR. Cancer Facts & Figures 2009 Supplemental Data: Cases and Deaths by Age 2009. [accessed July 28, 2009]. Available at http://www.cancer.org/docroot/PRO/content/PRO_1_1_2009_Cases_and_Deaths_Age.asp.
- 3.Blank TO, Bellizzi KM. A gerontologic perspective on cancer and aging. Cancer. 2008;112(11 suppl):2569–2576. doi: 10.1002/cncr.23444. [DOI] [PubMed] [Google Scholar]
- 4.Hurria A, Fleming MT, Baker SD, et al. Pharmacokinetics and toxicity of weekly docetaxel in older patients. Clin Cancer Res. 2006;12:6100–6105. doi: 10.1158/1078-0432.CCR-06-0200. [DOI] [PubMed] [Google Scholar]
- 5.Muss HB, Berry DA, Cirrincione C, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: The Cancer and Leukemia Group B experience. J Clin Oncol. 2007;25:3699–3704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine. Cancer in Elderly People: Workshop Proceedings; Washington, D.C.: National Academies Press; 2007. pp. 1–107. [Google Scholar]
- 7.Hurria A. Clinical trials in older adults with cancer: Past and future. Oncology (Williston Park) 2007;21:351–358. discussion 363–364, 367. [PubMed] [Google Scholar]
- 8.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer EL, Tew WP, Levine DA, et al. Response and outcomes in elderly patients with stages IIIC-IV ovarian cancer receiving platinum-taxane chemotherapy. Gynecol Oncol. 2007;106:381–387. doi: 10.1016/j.ygyno.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Elkin EB, Hurria A, Mitra N, et al. Adjuvant chemotherapy and survival in older women with hormone receptor-negative breast cancer: Assessing outcome in a population-based, observational cohort. J Clin Oncol. 2006;24:2757–2764. doi: 10.1200/JCO.2005.03.6053. [DOI] [PubMed] [Google Scholar]
- 12.Giordano SH, Duan Z, Kuo YF, et al. Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol. 2006;24:2750–2756. doi: 10.1200/JCO.2005.02.3028. [DOI] [PubMed] [Google Scholar]
- 13.Hesketh PJ, Lilenbaum RC, Chansky K, et al. Chemotherapy in patients ≥ 80 with advanced non-small cell lung cancer: Combined results from SWOG 0027 and LUN 6. J Thorac Oncol. 2007;2:494–498. doi: 10.1097/JTO.0b013e318060097e. [DOI] [PubMed] [Google Scholar]
- 14.Langer CJ. Neglected and underrepresented subpopulations: Elderly and performance status 2 patients with advanced-stage non-small-cell lung cancer. Clin Lung Cancer. 2006;7(suppl 4):S126–S137. doi: 10.3816/clc.2006.s.004. [DOI] [PubMed] [Google Scholar]
- 15.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wedding U, Honecker F, Bokemeyer C, et al. Tolerance to chemotherapy in elderly patients with cancer. Cancer Control. 2007;14:44–56. doi: 10.1177/107327480701400106. [DOI] [PubMed] [Google Scholar]
- 17.Wildiers H, Kunkler I, Biganzoli L, et al. Management of breast cancer in elderly individuals: Recommendations of the International Society of Geriatric Oncology. Lancet Oncol. 2007;8:1101–1115. doi: 10.1016/S1470-2045(07)70378-9. [DOI] [PubMed] [Google Scholar]
- 18.Bouchardy C, Rapiti E, Blagojevic S, et al. Older female cancer patients: Importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25:1858–1869. doi: 10.1200/JCO.2006.10.4208. [DOI] [PubMed] [Google Scholar]
- 19.Earle CC, Neumann PJ, Gelber RD, et al. Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol. 2002;20:1786–1792. doi: 10.1200/JCO.2002.07.142. [DOI] [PubMed] [Google Scholar]
- 20.Enger SM, Thwin SS, Buist DS, et al. Breast cancer treatment of older women in integrated health care settings. J Clin Oncol. 2006;24:4377–4383. doi: 10.1200/JCO.2006.06.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Firat S, Pleister A, Byhardt RW, et al. Age is independent of comorbidity influencing patient selection for combined modality therapy for treatment of stage III nonsmall cell lung cancer (NSCLC) Am J Clin Oncol. 2006;29:252–257. doi: 10.1097/01.coc.0000217824.20290.ab. [DOI] [PubMed] [Google Scholar]
- 22.Hurria A, Leung D, Trainor K, et al. Factors influencing treatment patterns of breast cancer patients age 75 and older. Crit Rev Oncol Hematol. 2003;46:121–126. doi: 10.1016/s1040-8428(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 23.Movsas B, Moughan J, Komaki R, et al. Radiotherapy patterns of care study in lung carcinoma. J Clin Oncol. 2003;21:4553–4559. doi: 10.1200/JCO.2003.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Potosky AL, Saxman S, Wallace RB, et al. Population variations in the initial treatment of non-small-cell lung cancer. J Clin Oncol. 2004;22:3261–3268. doi: 10.1200/JCO.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 25.Zeber JE, Copeland LA, Hosek BJ, et al. Cancer rates, medical comorbidities, and treatment modalities in the oldest patients. Crit Rev Oncol Hematol. 2008;67:237–242. doi: 10.1016/j.critrevonc.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Cronin DP, Harlan LC, Potosky AL, et al. Patterns of care for adjuvant therapy in a random population-based sample of patients diagnosed with colorectal cancer. Am J Gastroenterol. 2006;101:2308–2318. doi: 10.1111/j.1572-0241.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- 27.Etzioni DA, El-Khoueiry AB, Beart RW., Jr Rates and predictors of chemotherapy use for stage III colon cancer: A systematic review. Cancer. 2008;113:3279–3289. doi: 10.1002/cncr.23958. [DOI] [PubMed] [Google Scholar]
- 28.Walter LC, Covinsky KE. Cancer screening in elderly patients: A framework for individualized decision making. JAMA. 2001;285:2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 29.Hurria A, Wong FL, Villaluna D, et al. Role of age and health in treatment recommendations for older adults with breast cancer: The perspective of oncologists and primary care providers. J Clin Oncol. 2008;26:5386–5392. doi: 10.1200/JCO.2008.17.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krzyzanowska MK, Regan MM, Powell M, et al. Impact of patient age and comorbidity on surgeon versus oncologist preferences for adjuvant chemotherapy for stage III colon cancer. J Am Coll Surg. 2009;208:202–209. doi: 10.1016/j.jamcollsurg.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Keating NL, Landrum MB, Klabunde CN, et al. Adjuvant chemotherapy for stage III colon cancer: Do physicians agree about the importance of patient age and comorbidity? J Clin Oncol. 2008;26:2532–2537. doi: 10.1200/JCO.2007.15.9434. [DOI] [PubMed] [Google Scholar]
- 32.Maly RC, Leake B, Silliman RA. Health care disparities in older patients with breast carcinoma: Informational support from physicians. Cancer. 2003;97:1517–1527. doi: 10.1002/cncr.11211. [DOI] [PubMed] [Google Scholar]
- 33.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Senior Adult Oncology (V1.2009) [accessed June 15, 2009]. Available at http://www.nccn.org.
- 34.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 35.Podsiadlo D, Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 36.Saliba D, Elliott M, Rubenstein LZ, et al. The Vulnerable Elders Survey: A tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49:1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 37.Hurria A, Lachs MS, Cohen HJ, et al. Geriatric assessment for oncologists: Rationale and future directions. Crit Rev Oncol Hematol. 2006;59:211–217. doi: 10.1016/j.critrevonc.2006.03.007. [DOI] [PubMed] [Google Scholar]