Abstract
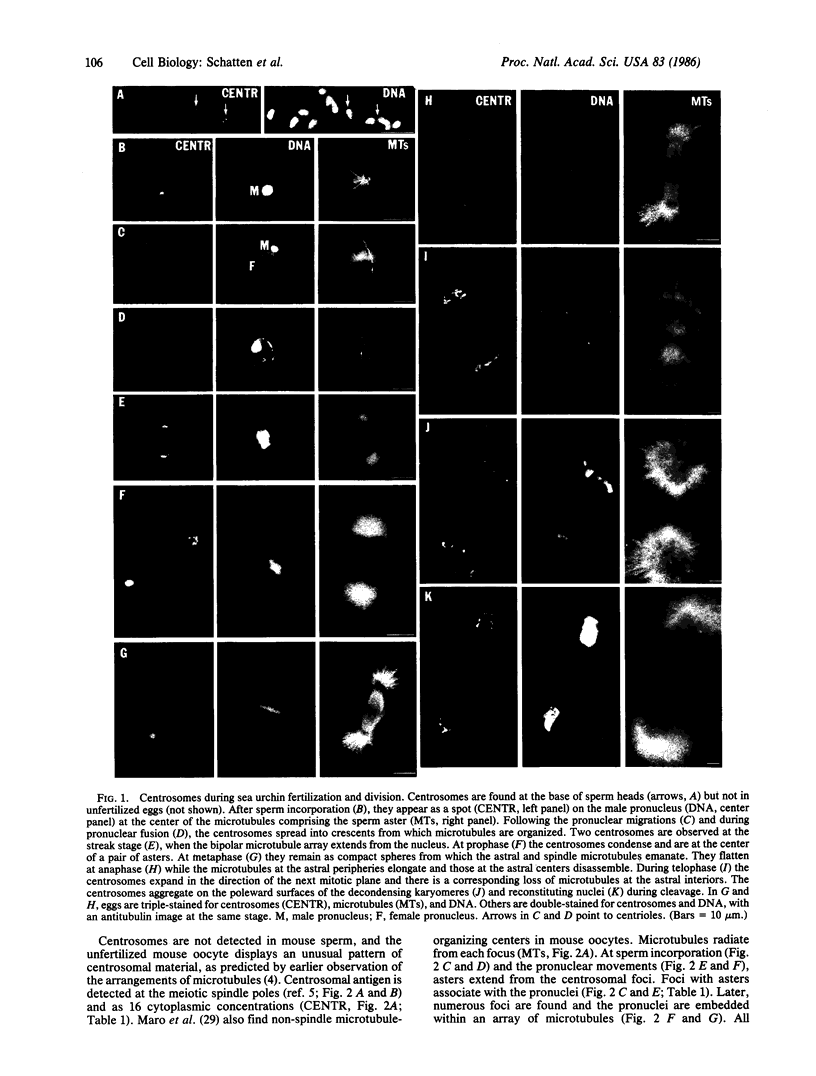
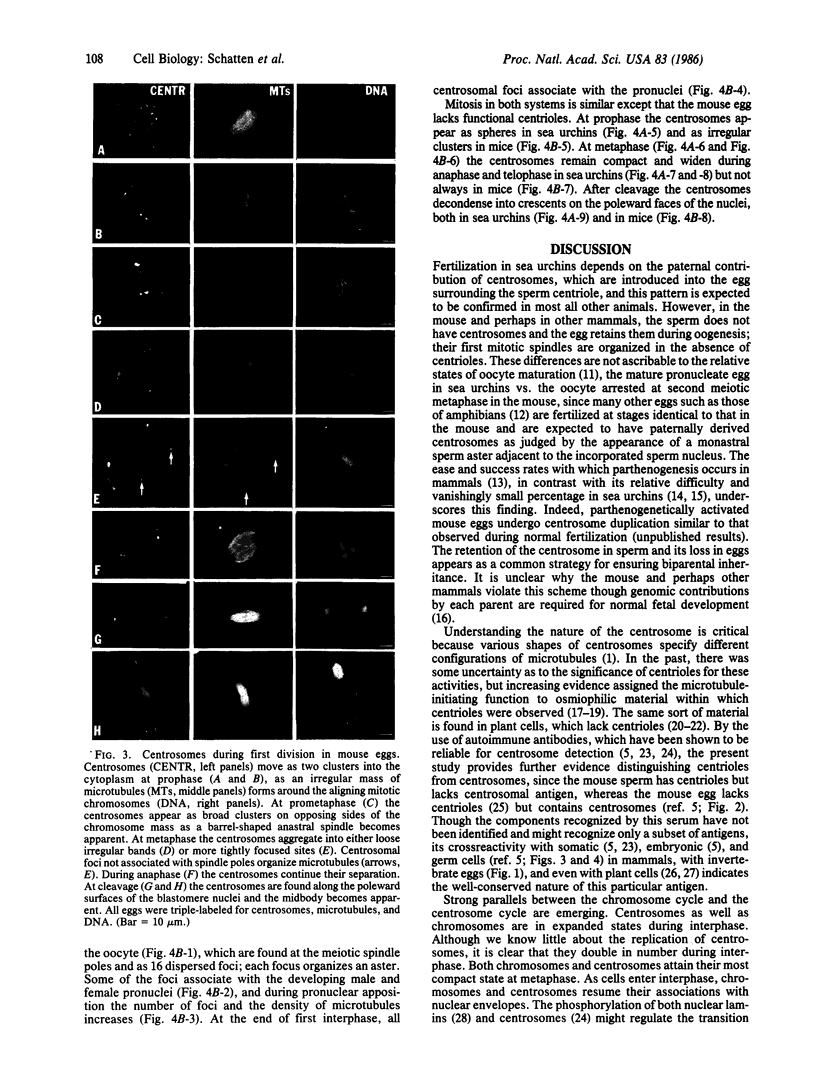
The forms and locations of centrosomes in mouse oocytes and in sea urchin eggs were followed through the whole course of fertilization and first cleavage by immunofluorescence microscopy. Centrosomes were identified with an autoimmune antiserum to centrosomal material. Staining of the same preparations with tubulin antibody and with the DNA dye Hoechst 33258 allowed the correlation of the forms of the centrosomes with the microtubule structures that they generate and with the stages of meiosis, syngamy, and mitosis. The results with sea urchin eggs conform to Boveri's view on the paternal origin of the functional centrosomes. Centrosomes are seen in spermatozoa and enter the egg at fertilization. Initially, the centrosomes are compact, but as the eggs enter the mitotic cycle the forms of the centrosomes go through a cycle in which they spread during interphase, apparently divide, and condense into two compact poles by metaphase. In anaphase, they spread to form flat poles. In telophase and during reconstitution of the daughter nuclei, the centrosomal material is disposed as hemispherical caps around the poleward surfaces of the nuclei. Mouse sperm lack centrosomal antigen. In the unfertilized mouse oocyte, the meiotic spindle poles are displayed as broad-beaded centrosomes. In addition, centrosomal material is detected in the cytoplasm as particles, about 16 in number, which are foci of small aster-like arrays of microtubules. The length and number of astral microtubules correlate with the size of the centrosomal foci. After sperm incorporation, as the pronuclei develop and more cytoplasmic microtubules assemble, a few of the foci associate with the peripheries of the nuclei. The number of foci multiplies during the first cell cycle. At the end of interphase, all of the centrosomal foci have concentrated on the nuclear peripheries and the cytoplasmic microtubules have disappeared. At prophase, the centrosomes are seen as two irregular clusters, marking the poles which, at metaphase and anaphase, appear as rough bands with foci, and the spindle is typically barrel-shaped. At telophase, the centrosomes are seen as arcs that lie on the nuclear peripheries after cleavage. The ordering of microtubules in all the stages reflects the shapes of the centrosomes. The findings on the sea urchin confirm the classical theory of the paternal origin of centrosomes and contrast with observations tracing the mitotic poles of the mouse egg to maternal centrosomal material. This evidence strengthens the conclusion that mouse centrosomes derive from the oocyte.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajer A. S., Molè-Bajer J. Asters, poles, and transport properties within spindlelike microtubule arrays. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):263–283. doi: 10.1101/sqb.1982.046.01.029. [DOI] [PubMed] [Google Scholar]
- Brenner S. L., Brinkley B. R. Tubulin assembly sites and the organization of microtubule arrays in mammalian cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):241–254. doi: 10.1101/sqb.1982.046.01.027. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R., Fistel S. H., Marcum J. M., Pardue R. L. Microtubules in cultured cells; indirect immunofluorescent staining with tubulin antibody. Int Rev Cytol. 1980;63:59–95. doi: 10.1016/s0074-7696(08)61757-x. [DOI] [PubMed] [Google Scholar]
- Calarco-Gillam P. D., Siebert M. C., Hubble R., Mitchison T., Kirschner M. Centrosome development in early mouse embryos as defined by an autoantibody against pericentriolar material. Cell. 1983 Dec;35(3 Pt 2):621–629. doi: 10.1016/0092-8674(83)90094-6. [DOI] [PubMed] [Google Scholar]
- Clayton L., Black C. M., Lloyd C. W. Microtubule nucleating sites in higher plant cells identified by an auto-antibody against pericentriolar material. J Cell Biol. 1985 Jul;101(1):319–324. doi: 10.1083/jcb.101.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Gould R. R., Borisy G. G. The pericentriolar material in Chinese hamster ovary cells nucleates microtubule formation. J Cell Biol. 1977 Jun;73(3):601–615. doi: 10.1083/jcb.73.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenbach R. J., Mazia D. Origin and maturation of centrioles in association with the nuclear envelope in hypertonic-stressed sea urchin eggs. Eur J Cell Biol. 1982 Aug;28(1):68–76. [PubMed] [Google Scholar]
- Mazia D. Centrosomes and mitotic poles. Exp Cell Res. 1984 Jul;153(1):1–15. doi: 10.1016/0014-4827(84)90442-7. [DOI] [PubMed] [Google Scholar]
- Mazia D., Schatten G., Sale W. Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J Cell Biol. 1975 Jul;66(1):198–200. doi: 10.1083/jcb.66.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miake-Lye R., Kirschner M. W. Induction of early mitotic events in a cell-free system. Cell. 1985 May;41(1):165–175. doi: 10.1016/0092-8674(85)90071-6. [DOI] [PubMed] [Google Scholar]
- Paweletz N., Mazia D., Finze E. M. The centrosome cycle in the mitotic cycle of sea urchin eggs. Exp Cell Res. 1984 May;152(1):47–65. doi: 10.1016/0014-4827(84)90229-5. [DOI] [PubMed] [Google Scholar]
- Pickett-Heaps J. D., Northcote D. H. Organization of microtubules and endoplasmic reticulum during mitosis and cytokinesis in wheat meristems. J Cell Sci. 1966 Mar;1(1):109–120. doi: 10.1242/jcs.1.1.109. [DOI] [PubMed] [Google Scholar]
- Schatten G., Maul G. G., Schatten H., Chaly N., Simerly C., Balczon R., Brown D. L. Nuclear lamins and peripheral nuclear antigens during fertilization and embryogenesis in mice and sea urchins. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4727–4731. doi: 10.1073/pnas.82.14.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten G., Simerly C., Schatten H. Microtubule configurations during fertilization, mitosis, and early development in the mouse and the requirement for egg microtubule-mediated motility during mammalian fertilization. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4152–4156. doi: 10.1073/pnas.82.12.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surani M. A., Barton S. C., Norris M. L. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature. 1984 Apr 5;308(5959):548–550. doi: 10.1038/308548a0. [DOI] [PubMed] [Google Scholar]
- Szollosi D., Calarco P., Donahue R. P. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J Cell Sci. 1972 Sep;11(2):521–541. doi: 10.1242/jcs.11.2.521. [DOI] [PubMed] [Google Scholar]
- Vandre D. D., Davis F. M., Rao P. N., Borisy G. G. Phosphoproteins are components of mitotic microtubule organizing centers. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4439–4443. doi: 10.1073/pnas.81.14.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick S. M. Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. III. Transition between mitotic/cytokinetic and interphase microtubule arrays. Cell Biol Int Rep. 1985 Apr;9(4):357–371. doi: 10.1016/0309-1651(85)90031-1. [DOI] [PubMed] [Google Scholar]
- Wick S. M., Seagull R. W., Osborn M., Weber K., Gunning B. E. Immunofluorescence microscopy of organized microtubule arrays in structurally stabilized meristematic plant cells. J Cell Biol. 1981 Jun;89(3):685–690. doi: 10.1083/jcb.89.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]