This article examines how discouraging the use of anthracyclines in combination with trastuzumab in patients with human epidermal growth factor receptor 2 positive metastatic breast cancer because of fears of cardiotoxicity has influenced the use of these agents in this patient setting.
Keywords: Breast neoplasm, Neoplasm metastasis, Anthracycline, HER-2, Trastuzumab
Abstract
Anthracyclines are among the most active drugs in breast cancer. Because of excessive cardiotoxicity, their use in combination with trastuzumab has been discouraged in patients with human epidermal growth factor receptor (HER)-2+ metastatic breast cancer. We sought to describe how this treatment paradigm influenced the use of anthracyclines in this patient setting.
We analyzed a multi-institutional database containing the treatment history of 450 patients who received at least one trastuzumab-based regimen for HER-2+ metastatic breast cancer. Patients were considered eligible for anthracyclines for metastatic disease if they were never exposed (NE) or had been previously exposed (PE) to an anthracycline in the neoadjuvant or adjuvant setting and had relapsed after 12 months from the last dose. We then assessed the use of anthracycline-based therapy after failure with the first trastuzumab-based regimen in eligible patients.
Three-hundred twenty-one patients were considered eligible for anthracyclines. In total, 190 eligible patients developing disease progression during the initial trastuzumab-based therapy were analyzed. An anthracycline was administered as first salvage treatment in 14 NE and two PE patients. Another 15 NE and nine PE patients received an anthracycline as a further line of therapy. Of 119 eligible patients who died from breast cancer, only 30 received an anthracycline for metastatic disease.
In conclusion, despite the fact that two thirds of the patients receiving trastuzumab-based therapy for HER-2 metastatic breast cancer are eligible for anthracyclines, these drugs are infrequently used nowadays to treat trastuzumab-refractory disease. A role for these compounds should be redefined in this patient subset.
Introduction
Anthracyclines have been the mainstay of the treatment of metastatic breast cancer for the last three decades. Clinical trials in the adjuvant setting have demonstrated that anthracycline-based combinations yield superior outcomes compared with cyclophosphamide, methotrexate, and 5-fluorouracil–like regimens [1]. A recent meta-analysis of three randomized studies in metastatic breast cancer patients showed that the antitumor activity of single-agent doxorubicin is comparable with that of single-agent taxanes, which are now considered the first-line agents of choice [2]. Previous exposure to neoadjuvant or adjuvant anthracyclines does not preclude meaningful rates of tumor control when patients with features of potential sensitivity (i.e., metastatic progression occurring beyond 12 months from the completion of adjuvant anthracycline-based therapy) are rechallenged with these drugs [3]. A steep increase in the risk for irreversible cardiotoxicity for cumulative doses of doxorubicin and epidoxorubicin >550 mg/m2 and >1,000 mg/m2, respectively, represents the main limitation to rechallenge with these drugs [4, 5]. Liposome-encapsulated doxorubicins, which are much less cardiotoxic than the traditional compound, can be safely administered to previously exposed patients, allowing higher cumulative doses [6–8].
A high rate of clinical cardiotoxicity was observed with the combination of doxorubicin and the monoclonal antibody trastuzumab in a pivotal randomized trial conducted in patients with human epidermal growth factor receptor (HER)-2+ advanced breast cancer [9]. The overall results of that trial and of another randomized phase II study [10] showed that the addition of trastuzumab to chemotherapy resulted in a dramatic improvement in the prognosis of these patients. Based on these results, the upfront use of trastuzumab with nonanthracycline chemotherapy and continuous HER-2 targeting with trastuzumab or newer anti–HER-2 compounds when tumor progression occurs have become solid treatment paradigms [11–13]. As a consequence, a reduction in the use of anthracyclines for the treatment of advanced HER-2+ breast cancer is expected to have occurred since the adoption of trastuzumab in clinical practice. Interestingly, preclinical data suggest that, for a proportion of patients with HER-2+ breast cancer, anthracyclines may act as “biologically targeted therapy.” Genetic alterations in the topoisomerase IIα gene (TOP2A) (amplification or deletions), which are found in ∼40% of HER-2–amplified tumors and are virtually absent in tumors with normal HER-2 gene status, confer sensitivity to anthracyclines [14]. Indeed, in the pivotal trial by Slamon et al. [9], the anthracycline-based control arm yielded a 42% overall response rate, a median progression-free survival interval of 6.1 months, and an overall survival duration of 21 months. These results suggest that anthracycline-based chemotherapy may still represent a valuable therapeutic option in HER-2+ metastatic breast cancer patients whose disease is resistant to anti–HER-2 therapy.
On these premises, we sought to quantify the use of anthracyclines in patients with HER-2+ advanced breast cancer receiving trastuzumab-based therapy between September 1999 and November 2008. This time interval started approximately when trastuzumab became available in some European countries, including Italy. In the context of the current controversy around the role of anthracyclines in the treatment of early breast cancer [15], our analysis provides a framework for possible strategies to reintegrate these active agents in the management of patients with HER-2+ advanced breast cancer.
Patients and Methods
Patients for this analysis were selected from a multi-institutional database containing clinical data from women with HER-2+ breast cancer receiving trastuzumab-based therapy for metastatic disease. For each patient, we collected clinical and pathological characteristics, prior treatments for breast cancer, and details of the first trastuzumab-based treatment and of subsequent lines of treatment until death (drugs and doses, best tumor response according to the World Health Organization criteria as assessed by investigators at each site, date of further progression, and date of death or of last follow-up visit). As of August 31, 2009, the database contained clinical data from 450 patients. Eligibility to receive anthracyclines in the metastatic setting was retrospectively ascertained for each patient and was defined as follows: (a) no exposure to these drugs in the neoadjuvant or adjuvant setting, (b) no exposure to anthracyclines as treatment for metastatic disease prior to or with the first-trastuzumab-based treatment, and (c) prior exposure to an anthracycline in the neoadjuvant or adjuvant setting and metastatic progression occurring beyond 12 months from treatment completion. This latter definition did not include dose thresholds of prior exposure because, with the availability of liposome encapsulated doxorubicin, the cumulative dose of anthracyclines could no longer be considered a contraindication to rechallenge [8]. An ad hoc analysis focused on a subset of patients treated with trastuzumab-based therapy at two institutions (European Institute of Oncology, Milan, Italy and Institute for Cancer Research and Treatment, Candiolo, Italy) for whom we could collect additional detailed information on the doses of prior anthracycline exposure. For these patients, we applied a more stringent definition of eligibility [16]: cumulative dose ≤300 mg/m2 of doxorubicin or ≤720 mg/m2 of epidoxorubicin and the development of metastatic disease beyond 12 months from the last dose of adjuvant chemotherapy. Patients developing clinically significant cardiac dysfunction during trastuzumab-based therapy were considered no longer eligible for anthracyclines.
Being a retrospective analysis, no specific written informed consent was required for this study. However, the process of data collection was conducted in compliance with the ethical requirements of each of the participating institutions.
Statistical Analysis
Descriptive statistics are reported using the median (range) or frequency (adjusted Wald 95% confidence interval [CI]) where appropriate. Proportions were compared using the χ2 test and trends in proportions over time were analyzed using the χ2 test for trends (Armitage test). Univariate and multivariate logistic regression models were fitted to the data in order to analyze factors associated with the use of anthracyclines in patients developing disease progression after the initial trastuzumab-based regimen. Results are reported with odds ratios (ORs) and 95% CIs. p-values < .05 were considered statistically significant. Analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL).
Results
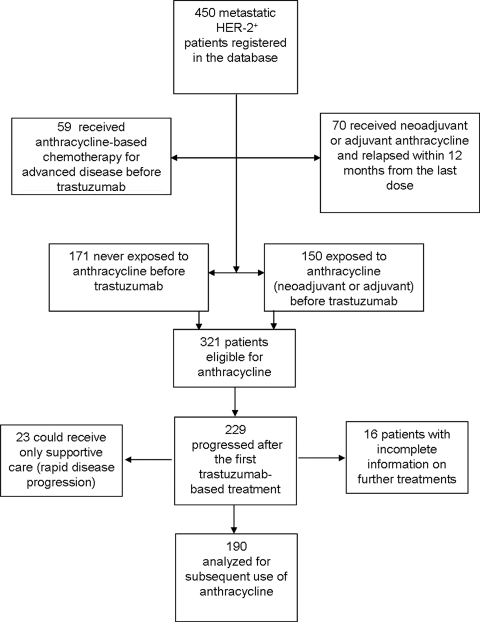
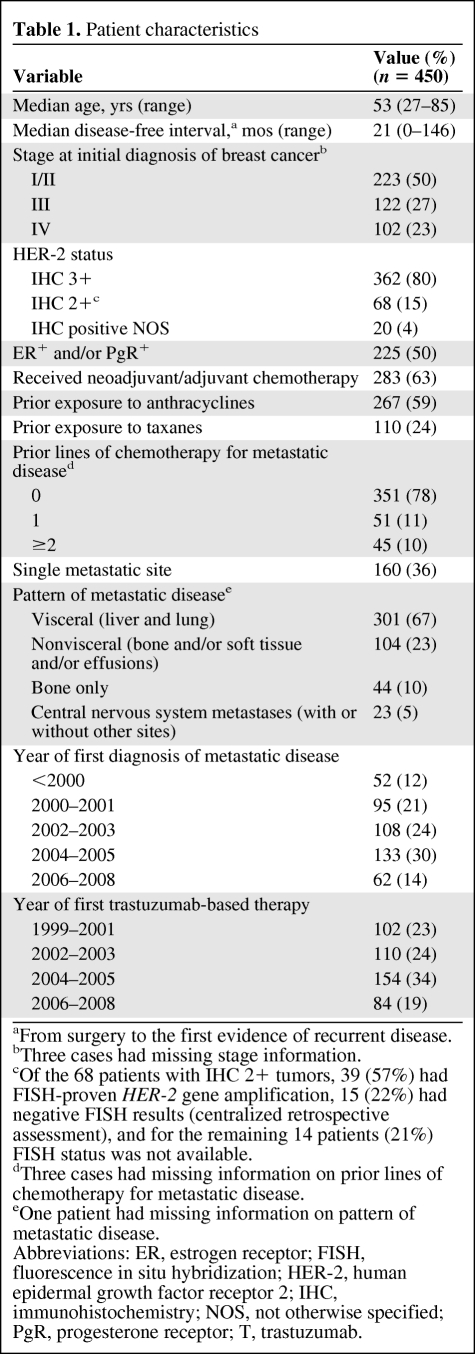
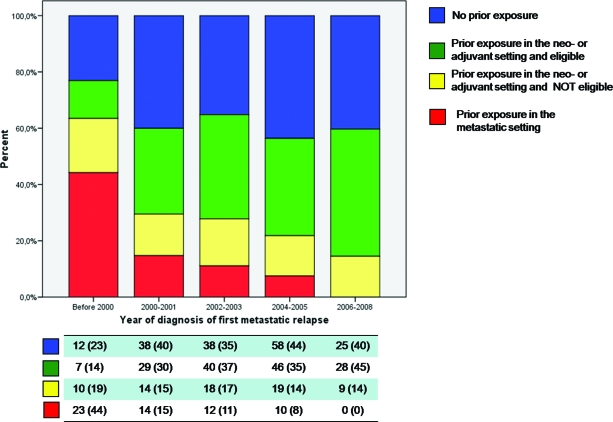
The process of patient selection for this analysis is represented in Figure 1. The demographic characteristics of the 450 patients registered in the database are summarized in Table 1. In total, 321 patients (171 with no prior exposure and 150 exposed only in the neoadjuvant or adjuvant setting), representing 71% (95% CI, 67%–75%) of the total, were deemed to be eligible for anthracyclines for the treatment of metastatic disease (Fig. 1). Percentages of patients in each category of eligibility or noneligibility for anthracyclines in the metastatic setting before the first trastuzumab-based treatment were calculated by year of first diagnosis of metastatic disease (Fig. 2). The use of anthracyclines before trastuzumab as treatment for metastatic disease declined sharply for patients diagnosed with metastatic disease in the year 2000 and onward, with no patients treated in the years 2006–2008 (χ2 test for trends in proportions, p < .01). The proportion of patients receiving an anthracycline in the adjuvant setting and found to be eligible for retreatment increased significantly over the time periods analyzed (χ2 test for trends in proportions, p = .01).
Figure 1.
Flow chart of the selection patients for the main analysis.
Abbreviation: HER-2, human epidermal growth factor receptor 2.
Table 1.
Patient characteristics
aFrom surgery to the first evidence of recurrent disease.
bThree cases had missing stage information.
cOf the 68 patients with IHC 2+ tumors, 39 (57%) had FISH-proven HER-2 gene amplification, 15 (22%) had negative FISH results (centralized retrospective assessment), and for the remaining 14 patients (21%) FISH status was not available.
dThree cases had missing information on prior lines of chemotherapy for metastatic disease.
eOne patient had missing information on pattern of metastatic disease.
Abbreviations: ER, estrogen receptor; FISH, fluorescence in situ hybridization; HER-2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; NOS, not otherwise specified; PgR, progesterone receptor; T, trastuzumab.
Figure 2.
Proportion of patients fulfilling each definition for eligibility or noneligibility for treatment with anthracyclines for metastatic disease by year of diagnosis of first metastasis. Below each bar of the graph is summarized, by each time period, the number of patients in each category of eligibility. Numbers in parentheses represent column percentages.
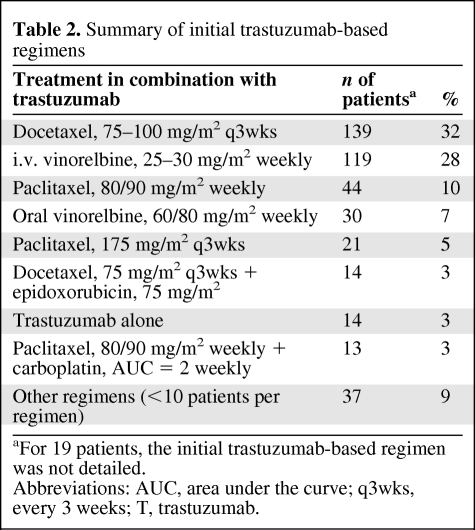
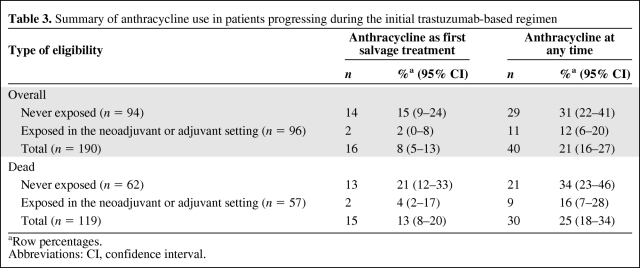
As expected, the first trastuzumab-based therapy did not include an anthracycline in most of the patients (Table 2). Overall, at the time of data cutoff, 365 patients had experienced disease progression during the initial trastuzumab-based treatment and 254 had died. Considering the initial criteria for retreatment and excluding 14 patients who received trastuzumab combined with an anthracycline and another 11 patients developing cardiotoxicity requiring trastuzumab discontinuation, the number of patients potentially eligible for anthracyclines after the initial trastuzumab-based regimen was 229. Twenty-three of these patients could receive only supportive care because of rapid disease progression and death. For another 16 patients, we could not retrieve information on additional anticancer treatment received after the initial trastuzumab-based therapy. In total, 190 patients, 94 of whom had no prior exposure to anthracyclines and 96 who were exposed only in the neoadjuvant or adjuvant setting, could be analyzed for subsequent treatments after progression. Only 16 (8%; 95% CI, 5%–13%) of those patients received an anthracycline as part of the first salvage treatment (Table 3). Another 24 patients (cumulative proportion, 21%; 95% CI, 16%–27%) received an anthracycline-containing regimen as a further line of therapy. Furthermore, of a total of 119 patients eligible for anthracyclines who died and for whom we could retrieve complete information on treatments, only 30 (25%; 95% CI, 18%–34%) ever received an anthracycline for the treatment of metastatic disease (Table 3).
Table 2.
Summary of initial trastuzumab-based regimens
aFor 19 patients, the initial trastuzumab-based regimen was not detailed.
Abbreviations: AUC, area under the curve; q3wks, every 3 weeks; T, trastuzumab.
Table 3.
Summary of anthracycline use in patients progressing during the initial trastuzumab-based regimen
aRow percentages.
Abbreviations: CI, confidence interval.
Anthracycline-based therapies were heterogeneous and included a liposomal compound in about half the cases. The ad hoc analysis, including 247 patients treated at two institutions for whom we could retrieve treatment details, did not differ from the main analysis (data not shown).
Logistic regression analysis was used to study the impact of the following variables on the use of anthracyclines after the initial trastuzumab-based therapy: patient age, which was dichotomized around its median value of 53 years; type of eligibility for anthracyclines (never exposed versus exposed in the neoadjuvant or adjuvant setting and having relapsed beyond 12 months from the completion of therapy); continuation of trastuzumab beyond the initial trastuzumab-based regimen (versus no continuation); institution; and year of initial trastuzumab-based treatment (1999–2001 versus 2002–2003 versus 2004–2005 versus 2006–2008). Because of the heterogeneous number of patient records provided by each participant (see Appendix 1), the variable “institution” was recoded into four groups, keeping separate the three institutions providing >60 patient records and combining all the remaining records into a single group.
In the 190 patients progressing during the initial trastuzumab-based regimen, having received prior anthracyclines in the neoadjuvant or adjuvant setting (OR, 0.272; 95% CI, 0.124–0.596; p = .01) and continuous administration of trastuzumab beyond disease progression (OR, 0.360; 95% CI, 0.171–0.759; p < .01) were significantly associated with a lower probability of receiving an anthracycline. The same results were obtained when restricting the analysis to the 119 patients who died from progressive breast cancer (not shown).
Discussion
Our study shows that, despite the fact that two thirds of the patients receiving trastuzumab-based therapy for HER-2+ metastatic breast cancer are eligible for anthracyclines, these drugs are infrequently used to treat trastuzumab-refractory disease nowadays. The administration of anthracyclines for the treatment of metastatic disease before a trastuzumab-based regimen declined sharply after the introduction of this monoclonal antibody in clinical practice (Fig. 2). This trend is justified by the rapid adoption by oncologists of the paradigm that anthracycline-free combinations of trastuzumab with chemotherapy should be administered at the onset of HER-2+ metastatic breast cancer [9]. After trastuzumab failure, a minority of eligible patients received an anthracycline, either as first-salvage treatment or at any time for further tumor progression. Use of an anthracycline was particularly infrequent in patients who had been exposed to these drugs in the adjuvant setting (cumulative proportion, 12%). The frequency of anthracycline use was higher in anthracycline-naïve patients, but in this group the majority also received alternative treatments. In fact, about one third of the dying patients never received an anthracycline throughout their entire disease course (Table 3).
Before discussing the potential relevance of our findings, we must point out some obvious limitations of our analysis. The first issue is with the number of patients involved: The analysis of patterns of anthracycline use was restricted to only 190 patients who were considered eligible for anthracyclines, had experienced disease progression during the initial trastuzumab-based regimen, and could receive additional anticancer therapy. A further analysis focused on 119 of these patients who died during the observation period. Therefore, the generalizability of our results is limited by wide CIs around the point estimates and by restrictions in the number of potential predictors that could be studied by multivariate analysis. Second, this was a retrospective analysis of data initially collected to test hypotheses other than the use of anthracyclines [17]. Third, our definition of eligibility for anthracyclines for patients pretreated in the neoadjuvant or adjuvant setting could be criticized because we did not take into account dose thresholds of prior exposure to conventional anthracyclines. However, the ad hoc analysis, in which the dose of prior anthracyclines was included as a criterion to establish eligibility to rechallenge, provided similar results [16].
Most of the patients were treated in the context of the availability of liposomal doxorubicins, which renders prior cumulative doses of anthracyclines approaching the thresholds for cardiotoxicity no longer a contraindication to retreatment [8]. In fact, in Italy both nonpegylated and pegylated doxorubicin have been available for compassionate use in breast cancer since the late 1990s and were registered for this indication in mid-2001 and in the early 2003, respectively.
Despite these limitations, we believe that our analysis provides a realistic picture of how the current use of anthracyclines in HER-2+ metastatic breast cancer patients has declined since the introduction of trastuzumab. This monoclonal antibody has caused a dramatic change in the natural history of HER-2+ advanced breast cancer. Encouraging results with newer compounds reinforce the concept that continued HER-2 targeting is the backbone of treatment for these patients [12, 13]. Indeed, the multivariate analysis showed that the policy of continuing trastuzumab beyond tumor progression was significantly associated with a lower probability of receiving an anthracycline. Trastuzumab-related left ventricular ejection fraction is reversible and probably a result of HER-2–HER-4 signaling inhibition in myocardial cells [18, 19]. Normally, this pathway is elicited through the expression of neuregulins when the myocardium encounters adverse hemodynamic stress or other stresses like those induced by anthracyclines. Neuregulins are natural ligands of HER-3 and HER-4 and induce their heterodimerization with HER-2 [20]. Interference with this relevant cell survival and growth-promoting pathway sensitizes myocardial cells to the irreversible toxicity caused by anthracyclines. For this reason, concerns about combinations of anthracyclines and trastuzumab have been translated to other anti–HER-2 compounds, which are usually studied in the context of anthracycline-free regimens.
The multivariate analysis also revealed that patients exposed in the adjuvant setting and relapsing 12 months after the last dose were less likely to receive an anthracycline despite being eligible for rechallenge. In general, aside from concerns regarding cardiotoxicity, oncologists are reluctant to rechallenge patients with anthracyclines because there are limited prospective data on the efficacy of this approach. However, a recent phase II clinical trial with a combination of liposomal doxorubicin and cyclophosphamide demonstrated a 38% overall response rate and a 71% clinical benefit rate in 70 women with advanced breast cancer previously exposed to anthracyclines in the adjuvant setting and relapsing beyond 12 months of the last dose [8]. These figures are comparable with those from other first-line combinations in unselected metastatic breast cancer patients.
In recent years, the importance of anthracyclines in adjuvant chemotherapy regimens has been debated on the basis of the observation that HER-2 and TOP2A seem to predict their efficacy [21, 22]. These findings point to a limited role of adjuvant anthracycline-based regimens in patients with HER-2− breast cancer. Furthermore, a still unpublished large clinical trial suggests that, if the anti–HER-2 monoclonal antibody trastuzumab is part of adjuvant treatment, anthracyclines also may be omitted in patients with HER-2+ operable breast cancer [23]. At this time, these results are not considered conclusive [15], but prospective trials are ongoing that will provide potentially practice changing findings (ClinicalTrials.gov identifier, NCT00365365). One of the possible implications is that the proportion of anthracycline-naïve patients who develop HER-2+ metastatic disease will increase significantly in the near future. Paradoxically, because of concomitant TOP2A genetic alterations (amplification and deletion in 40% of the patients) in tumors harboring HER-2 amplification, anthracyclines may be particularly active in a relevant subset of these patients [23].
Conclusion
In summary, our retrospective analysis shows that anthracyclines are infrequently used to treat HER-2+ advanced breast cancer. Alternatives to rechallenge with an anthracycline and cardiac safety concerns related to the combination of these compounds with HER-2–targeting drugs are two plausible explanations. The activity and tolerability of the newer less cardiotoxic liposomal anthracyclines in combination with trastuzumab are being studied, but no randomized comparisons with non–anthracycline-based regimens are available [24–27]. Another possibility may arise from a more precise definition, within the HER-2+ subset, of factors for susceptibility or resistance to anti–HER-2 therapy [28]. In fact, despite results confirming that continuous HER-2 targeting with alternative compounds also yields meaningful clinical activity in patients resistant to a previously used anti–HER-2 compound (at this time, mainly trastuzumab), the absolute rates of tumor response are in the range of 5%–25% [29]. It is possible, therefore, that despite HER-2 amplification and/or overexpression, there may be tumors that lose or do not possess biological addiction to this oncogene. Markers of absolute HER-2 independence, which imply refractoriness to anti–HER-2 therapy, regardless of the agent used, may identify patients for whom anthracyclines may represent a valuable therapeutic option among those that are currently available.
Acknowledgment
Supported in part by “Ricerca Sanitaria Finalizzata 2008” Piedmont Region.
Appendix 1.
Participating institutions and relative contribution in patient records
Author Contributions
Conception/Design: Filippo Montemurro, Valentina Rossi, Massimo Aglietta
Provision of study material or patients: Franco Nolè, Michela Donadio, Giorgio Valabrega, Maria Cossu Rocca, Maria Elena Jacomuzzi, Giuseppe Viale, Anna Sapino
Collection and/or assembly of data: Valentina Rossi, Franco Nolè, Stefania Redana, Michela Donadio, Maria Cossu Rocca, Maria Elena Jacomuzzi, Giuseppe Viale, Rossella Martinello, Anna Sapino, Elena Verri
Data analysis and interpretation: Filippo Montemurro
Manuscript writing: Filippo Montemurro, Valentina Rossi
Final approval of manuscript: Franco Nolè, Stefania Redana, Michela Donadio, Giorgio Valabrega, Massimo Aglietta, Giuseppe Viale, Rossella Martinello, Anna Sapino, Elena Verri
References
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Piccart-Gebhart MJ, Burzykowski T, Buyse M, et al. Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol. 2008;26:1980–1986. doi: 10.1200/JCO.2007.10.8399. [DOI] [PubMed] [Google Scholar]
- 3.Buzdar AU, Legha SS, Hortobagyi GN, et al. Management of breast cancer patients failing adjuvant chemotherapy with adriamycin-containing regimens. Cancer. 1981;47:2798–2802. doi: 10.1002/1097-0142(19810615)47:12<2798::aid-cncr2820471207>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 4.Jensen BV. Cardiotoxic consequences of anthracycline-containing therapy in patients with breast cancer. Semin Oncol. 2006;33(suppl 8):S15–S21. doi: 10.1053/j.seminoncol.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Floyd JD, Nguyen DT, Lobins RL, et al. Cardiotoxicity of cancer therapy. J Clin Oncol. 2005;23:7685–7696. doi: 10.1200/JCO.2005.08.789. [DOI] [PubMed] [Google Scholar]
- 6.Harris L, Batist G, Belt R, et al. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer. 2002;94:25–36. doi: 10.1002/cncr.10201. [DOI] [PubMed] [Google Scholar]
- 7.O'Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 8.Trudeau ME, Clemons MJ, Provencher L, et al. Phase II multicenter trial of anthracycline rechallenge with pegylated liposomal doxorubicin plus cyclophosphamide for first-line therapy of metastatic breast cancer previously treated with adjuvant anthracyclines. J Clin Oncol. 2009;27:5906–5910. doi: 10.1200/JCO.2009.22.7504. [DOI] [PubMed] [Google Scholar]
- 9.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 11.Montemurro F, Valabrega G, Aglietta M. Trastuzumab-based combination therapy for breast cancer. Expert Opin Pharmacother. 2004;5:81–96. doi: 10.1517/14656566.5.1.81. [DOI] [PubMed] [Google Scholar]
- 12.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: A German Breast Group 26/Breast International Group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 13.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 14.Järvinen TA, Tanner M, Rantanen V, et al. Amplification and deletion of topoisomerase IIα associate with ErbB-2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. Am J Pathol. 2000;156:839–847. doi: 10.1016/s0002-9440(10)64952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gianni L, Norton L, Wolmark N, et al. Role of anthracyclines in the treatment of early breast cancer. J Clin Oncol. 2009;27:4798–4808. doi: 10.1200/JCO.2008.21.4791. [DOI] [PubMed] [Google Scholar]
- 16.Sparano JA, Makhson AN, Semiglazov VF, et al. Pegylated liposomal doxorubicin plus docetaxel significantly improves time to progression without additive cardiotoxicity compared with docetaxel monotherapy in patients with advanced breast cancer Previously treated with neoadjuvant-adjuvant anthracycline therapy: Results from a randomized phase III study. J Clin Oncol. 2009;27:4522–4529. doi: 10.1200/JCO.2008.20.5013. [DOI] [PubMed] [Google Scholar]
- 17.Montemurro F, Donadio M, Clavarezza M, et al. Outcome of patients with HER2-positive advanced breast cancer progressing during trastuzumab-based therapy. The Oncologist. 2006;11:318–324. doi: 10.1634/theoncologist.11-4-318. [DOI] [PubMed] [Google Scholar]
- 18.Schneider JW, Chang AY, Rocco TP. Cardiotoxicity in signal transduction therapeutics: erbB2 antibodies and the heart. Semin Oncol. 2001;28(suppl 16):18–26. doi: 10.1016/s0093-7754(01)90278-7. [DOI] [PubMed] [Google Scholar]
- 19.Sawyer DB, Zuppinger C, Miller TA, et al. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1β and anti-erbB2: Potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551–1554. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]
- 20.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 21.Pritchard KI, Shepherd LE, O'Malley FP, et al. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006;354:2103–2111. doi: 10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 22.Gennari A, Sormani MP, Pronzato P, et al. HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: A pooled analysis of randomized trials. J Natl Cancer Inst. 2008;100:14–20. doi: 10.1093/jnci/djm252. [DOI] [PubMed] [Google Scholar]
- 23.Slamon D, Eiermann W, Robert N, et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients: BCIRG 006 study. Cancer Res. 2009;69:77. [Google Scholar]
- 24.Chia S, Clemons M, Martin LA, et al. Pegylated liposomal doxorubicin and trastuzumab in HER-2 overexpressing metastatic breast cancer: A multicenter phase II trial. J Clin Oncol. 2006;24:2773–2778. doi: 10.1200/JCO.2005.03.8331. [DOI] [PubMed] [Google Scholar]
- 25.Cortes J, Di Cosimo S, Climent MA, et al. Nonpegylated liposomal doxorubicin (TLC-D99), paclitaxel, and trastuzumab in HER-2-overexpressing breast cancer: A multicenter phase I/II study. Clin Cancer Res. 2009;15:307–314. doi: 10.1158/1078-0432.CCR-08-1113. [DOI] [PubMed] [Google Scholar]
- 26.Stickeler E, Klar M, Watermann D, et al. Pegylated liposomal doxorubicin and trastuzumab as 1st and 2nd line therapy in her2/neu positive metastatic breast cancer: A multicenter phase II trial. Breast Cancer Res Treat. 2009;117:591–598. doi: 10.1007/s10549-008-0306-9. [DOI] [PubMed] [Google Scholar]
- 27.Rayson D, Richel D, Chia S, et al. Anthracycline-trastuzumab regimens for HER2/neu-overexpressing breast cancer: Current experience and future strategies. Ann Oncol. 2008;19:1530–1539. doi: 10.1093/annonc/mdn292. [DOI] [PubMed] [Google Scholar]
- 28.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: Mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977–984. doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 29.Baselga J, Swain SM. Novel anticancer targets: Revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]