Peritumoral colony-stimulating factor 1 receptor (CSF-1R) is associated with intrahepatic metastasis, tumor recurrence, and patient survival after hepatectomy, highlighting the critical role of the peritumoral liver milieu in hepatocellular carcinoma progression. CSF-1R may become a potential therapeutic target for postoperative adjuvant treatment.
Keywords: Macrophage colony-stimulating factor-1 receptor, Hepatocellular carcinoma, Microenvironment, Peritumoral liver tissue, Prognosis
Abstract
Background.
Macrophage colony-stimulating factor 1 receptor (CSF-1R) expression in hepatocellular carcinoma (HCC) and its prognostic values are unclear. This study evaluated the prognostic values of the intratumoral and peritumoral expression of CSF-1R in HCC patients after curative resection.
Methods.
Tissue microarrays containing material from cohort 1 (105 patients) and cohort 2 (32 patients) were constructed. Immunohistochemistry was performed and prognostic values of these and other clinicopathological data were evaluated. The CSF-1R mRNA level was assessed by quantitative real-time polymerase chain reaction in cohort 3 (52 patients).
Results.
Both the CSF-1R density and its mRNA level were significantly higher in peritumoral liver tissue than in the corresponding tumor tissue. CSF-1R was distributed in a gradient in the long-distance peritumoral tissue microarray, with its density decreasing as the distance from the tumor margin increased. High peritumoral CSF-1R was significantly associated with more intrahepatic metastases and poorer survival. Peritumoral CSF-1R was an independent prognostic factor for both overall survival and time to recurrence and affected the incidence of early recurrence. However, intratumoral CSF-1R did not correlate with any clinicopathological feature. Peritumoral CSF-1R was also associated with both overall survival and time to recurrence in a subgroup with small HCCs (≤5 cm).
Conclusions.
Peritumoral CSF-1R is associated with intrahepatic metastasis, tumor recurrence, and patient survival after hepatectomy, highlighting the critical role of the peritumoral liver milieu in HCC progression. CSF-1R may become a potential therapeutic target for postoperative adjuvant treatment.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third most common cause of death from cancer worldwide [1, 2]. Although hepatectomy or radiofrequency ablation are the best methods for prolonging patient survival [2], a high postoperative recurrence rate is a major problem [3]. Conventional tumor–node–metastasis (TNM) classification is usually insufficient to predict HCC recurrence. Although biomarkers from HCC tumors have been extensively studied [4–7], the results have not been satisfying until recently.
Our recent studies found that a high expression level of peritumoral colony-stimulating factor 1 (CSF-1), also known as macrophage colony-stimulating factor, and/or macrophage infiltration was associated with poor survival after hepatectomy [8, 9]. Together with other reports [10, 11], these findings imply that the peritumoral liver tissue might be a favorable substrate for intrahepatic micrometastasis and HCC progression. However, whether or not the receptor for CSF-1 (CSF-1R) expression is also enriched in the peritumoral liver tissue as well as the clinical significance of CSF-1R remain unclear.
CSF-1R, which is encoded by c-fms proto-oncogene, is a transmembrane receptor tyrosine kinase (RTK) [12]. Its ligand, CSF-1, is a cytokine mainly produced by tumor cells [13]. Expression of CSF-1R is found in primitive multipotent hematopoietic cells [14], mononuclear phagocytic lineage cells [15–17], the testis, uterus, ovary, placenta, and mammary glands, and the early phase of the developing prostate [18, 19]. CSF-1 binding to CSF-1R results in dimerization, autophosphorylation, and activation of signal transduction, and it causes monocytes and macrophages to differentiate, trophoblastic implantation, and mammary gland development [18, 20–22]. In pathological conditions, macrophages can be recruited by CSF-1. When appropriately activated through the CSF-1 and CSF-1R combination, macrophages secrete growth factors that are essential for the growth of a premetastatic niche and helpful for tumor growth or metastasis, resulting in a higher recurrence rate [23–25]. Inhibition of CSF-1R activity could therefore reduce macrophage infiltration and cause a delay in tumor progression, whereas macrophage recruitment is enhanced by overexpression of CSF-1 in the tumor, which correlates with accelerated tumor malignant transformation and angiogenesis [26–31]. High CSF-1R expression correlates with aggressive behavior in a variety of epithelial malignancies, including breast cancer, progressing prostate cancer, and ovarian cancer [18, 19, 31–35]. But the distribution of CSF-1R in HCC and its prognostic value are still unclear.
In this study, we investigated the distribution of CSF-1R in specimens obtained from HCC patients. The relationships among CSF-1R, macrophages, immunopositive-staining cells, and other clinicopathological variables and outcome in patients were studied as well.
Materials and Methods
Patients, Specimens, Follow-up, and Postoperative Treatment
In total, 105 patients (cohort 1) who underwent curative liver resection for pathology-proven HCC were examined in the present and previous studies [8, 36]. None of these patients received any preoperative anticancer treatment. Entire tumors with corresponding peritumoral liver tissues were collected. Tumor stage was determined according to the 2002 International Union Against Cancer TNM Classification system (Sixth Edition). The study was approved by the Zhongshan Hospital Research Ethics Committee. Informed consent was obtained from each patient according to the committee's regulations.
All patients were monitored until March 2008, with a median follow-up time of 31.7 months. The surgical techniques for liver resection were described previously [36–38]. Treatment modalities after relapse were administered according to a uniform guideline [37, 39, 40]. The overall survival (OS) time and time to recurrence (TTR) were defined as the interval between the dates of surgery and death or recurrence, respectively. If recurrence was suspected, computerized tomography scanning or magnetic resonance imaging was performed immediately; if recurrence was not diagnosed, patients were observed until death or the last follow-up. The detailed clinicopathological features of cohort 1 are listed in Table 1. At the time of the last follow-up, 49 patients had tumor recurrence and 38 patients had died, including 10 patients who died from liver failure without a record of tumor recurrence. The 1-, 3-, and 5-year OS rates were 83%, 59%, and 45%, respectively, and the 1-, 3-, and 5-year TTR rates were 73%, 55%, and 43%, respectively.
Table 1.
The clinicopathological features of 105 patients from cohort 1
Abbreviations: ALT, alanine aminotransferase; SD, standard deviation; TNM, tumor–node–metastasis; UICC, International Union Against Cancer Classification.
Based on Zhu XD, Zhang JB, Zhuang PY et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma.
J Clin Oncol 2008;26:2707–2716.
Tissue Microarray Construction, Immunohistochemistry, and Evaluation
Tissue microarray (TMA) construction was described in our previous reports [8, 36, 38]. Briefly, to construct TMA slides, two cores were taken from each representative formalin-fixed, paraffin-embedded tumor tissue and from tissue adjacent to the tumor within a distance of 10 mm. Cohort 1 TMA slides with 105 pairs of tumorous and matched peritumoral samples were constructed. Another 32 specimens including tumor and long-distance peritumoral liver tissue (at distances of 10 mm, 20 mm, and 30 mm from the tumor margin) were collected and constructed into a long-distance peritumoral TMA chip (cohort 2).
Immunohistochemistry was performed by a two-step method, as described in our previous reports [8, 36, 38]. The primary antibody (1:200 polyclonal rabbit anti-human CSF-1R; Santa Cruz Biotechnology, Santa Cruz, CA) was added, and the slides were incubated overnight at 4°C in a wet chamber. The components of the EnVision+ detection system were applied with an anti-rabbit polymer (EnVision+/HRP/Mo; Dako, Glostrup, Denmark). Reaction products were visualized by incubation with 3,3′-diaminobenzidine. Negative controls were treated identically but with the primary antibody omitted.
The intensity of positive CSF-1R staining in the whole view was measured using a computerized image system composed of a Leica charged-coupled device camera, DFC500, connected to a Leica DM IRE2 microscope (Leica Microsystems Imaging Solutions Ltd., Cambridge, U.K.). Under 200× magnification, images of four representative fields were captured using Leica QWin Plus Version 3 software. An identical setting was used for all images. The density of immunostaining was measured using Image-Pro Plus Version 6.2 software (Media Cybernetics Inc., Bethesda, MD), as described previously [8]. The integrated optical density (IOD) in each image was measured, and CSF-1R density was calculated as IOD/total area of each image. The TMAs were assessed independently by two observers (W.Q. Wang and X.D. Zhu), with a high correlation between them (p < .001).
Because it was impossible to separate the expression of CSF-1R on hepatocytes and macrophages by the computerized imaging system, the regions of most intense staining were scored by eye. The intensity of staining in the cytoplasm of hepatocytes was graded as 0, 1, 2, and 3 and then classified into low expression (grades 0 and 1) and high expression (grades 2 and 3). The number of infiltrated macrophages with positive CSF-1R staining in peritumoral liver tissue was also counted by eye. The TMAs were scored or counted independently by two observers, as described earlier.
Immunofluorescence Double-Staining of CSF-1R and CD68
Eight HCC samples were collected and frozen sections were made. Sections were rinsed three times in phosphate-buffered saline (PBS). Goat serum (10%) in PBS was used to block nonspecific protein binding. Sections were then incubated overnight at 4°C with CSF-1R antibody (1:50 polyclonal rabbit anti-human CSF-1R) and CD68 antibody (1:50 monoclonal mouse anti-human CD68; Abcam, Cambridge, MA). After rinsing to remove excess antibody, sections were incubated in both Cy3-conjugated affinipure goat anti-mouse IgG antibody (1:100 dilution) and Cy5-conjugated affinipure goat anti-rabbit IgG antibody (1:100 dilution) (Amersham Biosciences, Ltd., Little Chalfont, U.K.) and counterstained with 10 μg/ml 4′,6-diamidine-2′-phenylindole dihydrochloride to stain nuclei. Representative fields were acquired with a computerized imaging system as described earlier. Negative controls were treated identically but with the primary antibody omitted.
Quantitative Real-Time Polymerase Chain Reaction
CSF-1R and hypoxia-inducing factor (HIF)-1α mRNA levels were assessed by quantitative real-time polymerase chain reaction (qRT-PCR) in frozen paired specimens obtained from cohort 3, which contained 52 HCC patients who underwent curative resection in 2008–2009. Total RNA was retracted by Trizol according to the protocol recommended by the manufacturer. Briefly, specimens were obtained during surgery as blocks of 1 cm3, snap frozen in liquid nitrogen, and stored at −80°C. Then, CSF-1R mRNA was reverse transcribed and amplified using a SYBR PrimeScript RT-PCR kit (Invitrogen Life Technologies, Gaithersburg, MD). The primer sequences were as follows: CSF-1R forward, 5′-ACACTAAGCTCGCAATCCC-3′; CSF-1R reverse, 5′-GTATCGAAGGGTGAGCTCAAA-3′; HIF-1α forward, 5′-GCTGACCCTGCACTCAAT-3′; HIF-1α reverse, 5′-GATCGAAGGAAGGTAACTGG-3′; β-actin forward, 5′-CATCTCTTGCTCGAAGTCCA-3′; β-actin reverse, 5′-ATCATGTTTGAGACCTTCAACA-3′. Gene expression was quantified by RT-PCR and relative gene expression values were calculated by the ΔΔCt method [41] using Sequence Detection System 2.1 software (Applied Biosystems, Foster City, CA). The result of CSF-1R or HIF-1α mRNA was normalized to the corresponding β-actin signal (ΔCt). Measurements were performed in triplicate.
Statistical Analysis
All statistical analyses were performed with SPSS 13.0 for Windows (SPSS, Inc., Chicago, IL). Pearson's χ2 and Fisher's exact tests were applied to compare qualitative variables, and quantitative variables were analyzed by Student's t-test or Spearman's ρ rank correlation coefficient determination. Clinicopathological features were compared between the two risk groups using the Mann-Whitney test. One-way analysis of variance (ANOVA) was applied to determine the distributional characteristics of the biomarker in peritumoral liver tissue. Kaplan–Meier analysis was applied to determine survival. The log-rank test was applied to compare patient survival between subgroups, and the Cox proportional hazard regression model was applied to perform the multivariate analysis. Receiver operating characteristic (ROC) curve analysis was applied to determine predictive value among parameters. p < .05 was considered statistically significant. X-tile Plots Version 3.6.1 (Yale University, New Haven, CT) [42] was used to find an optimal cutoff point for CSF-1R expression.
Results
Patterns of CSF-1R Expression and Distribution
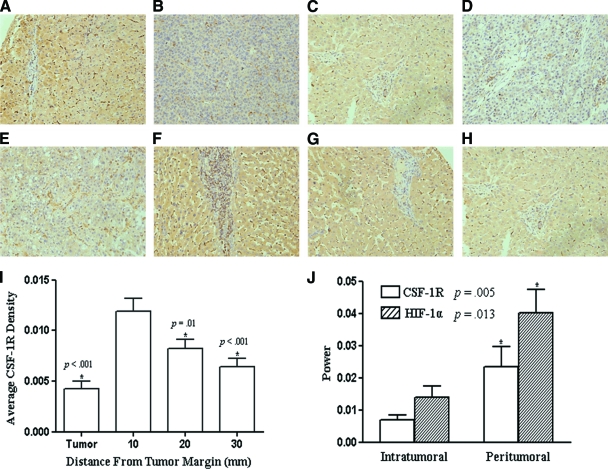
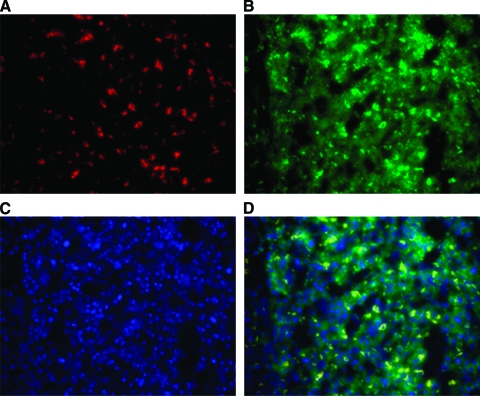
As shown in Figure 1, CSF-1R staining was mainly in the cytoplasm and cytomembrane of tumor cells or hepatocytes. Sporadic stroma cells were also observed with positive staining. Some cells with darker staining were observed in between tumor cells or hepatocytes. Most of these cells also stained positive for CD68 (Fig. 2). The CSF-1R densities of intratumoral and peritumoral tissues were 0.011 ± 0.008 (range, 0.000–0.042) and 0.091 ± 0.019 (range, 0.007–0.688), respectively. CSF-1R expression was significantly higher in peritumoral liver tissue than in tumor tissue (p < .001).
Figure 1.
Patterns of CSF-1R expression and distribution. Representative strong (case 20, (A), (B)) and mild (case 60, (C), (D)) immunostaining of colony-stimulating factor 1 receptor (CSF-1R) in peritumoral (A, C) and intratumoral (B, D) tissue microarray (TMA) chips (200×). (E–H): Immunostaining of intratumoral (E) and peritumoral (F–H) CSF-1R in a long-distance peritumoral TMA chip. (I): CSF-1R expression differed in tumor and peritumoral liver tissue and presented a gradient distribution pattern in the latter. *p < .05, one-way analysis of variance, compared with proximal peritumoral CSF-1R density. (J): Quantitative real-time polymerase chain reaction analyses of CSF-1R and hypoxia-inducing factor 1α gene expression in intratumoral and peritumoral specimens. *p < .05, paired-samples t-test between the two intratumoral or peritumoral subgroups.
Figure 2.
Immunofluorescence double staining of CD68 and colony-stimulating factor 1 receptor (CSF-1R) in eight hepatocellular carcinoma samples. CD68 staining used Cy3 ((A), red), CSF-1R staining used Cy5 ((B), green), and nucleus staining used 4′,6-diamidine-2′-phenylindole dihydrochloride ((C), blue) as substrates. (D) was merged from (A), (B), and (C).
As shown in Figure 1J, both the CSF-1R and HIF-1α mRNA levels were significantly higher in peritumoral liver tissue than in the corresponding intratumoral tissue (p = .005 and p = .013, respectively). The average power (2−ΔCt) was 0.014 ± 0.003 and 0.007 ± 0.002 (mean ± standard deviation) for peritumoral and intratumoral CSF-1R, respectively, and 0.040 ± 0.007 and 0.024 ± 0.006 for peritumoral and intratumoral HIF-1α, respectively. CSF-1R expression correlated with HIF-1α expression in both intratumoral and peritumoral tissues (r = 0.478, p = .001 and r = 0.397, p = .008, respectively).
A gradient distribution of CSF-1R expression in peritumoral liver tissue was observed in the long-distance peritumoral TMA, with the expression of CSF-1R decreasing as the distance from the tumor margin increased (Fig. 1F–1I). The CSF-1R densities of the proximal, median, and distant peritumoral liver tissues and the matched intratumoral tissue were 0.012 ± 0.008 (range, 0.000–0.029), 0.008 ± 0.006 (range, 0.000–0.026), 0.006 ± 0.005 (range, 0.000–0.028), and 0.004 ± 0.004 (range, 0.000–0.025), respectively. CSF-1R expression differed significantly among the proximal, median, and distant distances of peritumoral liver tissue (one-way ANOVA, compared with proximal peritumoral CSF-1R expression; p < .001, p = .01, p < .001, respectively). The CSF-1R expression level at 10 mm correlated positively with those at 20 mm and 30 mm (r = 0.825, p < .001 and r = 0.689, p < .001, respectively).
Correlations with Clinicopathological Factors
The 85% value as the cutoff point for peritumoral CSF-1R density was obtained with an optimal p value, using the OS time as the marker, and it was located at the position of 0.08 (supplemental online Fig. S1A). The range of low CSF-1R density was 0.0065–0.076 and the range of high CSF-1R density was 0.13–0.69. There was not an optimal cutoff point for intratumoral CSF-1R expression (online supplemental Fig. S1B); therefore, the cutoff point was set at the median value. The distribution of peritumoral hepatocyte scoring is shown in supplemental online Figure S1C. The 66% value as the cutoff point for peritumoral macrophage counting was obtained with an optimal p-value, using the OS time as the endpoint (supplemental online Fig. S1D).
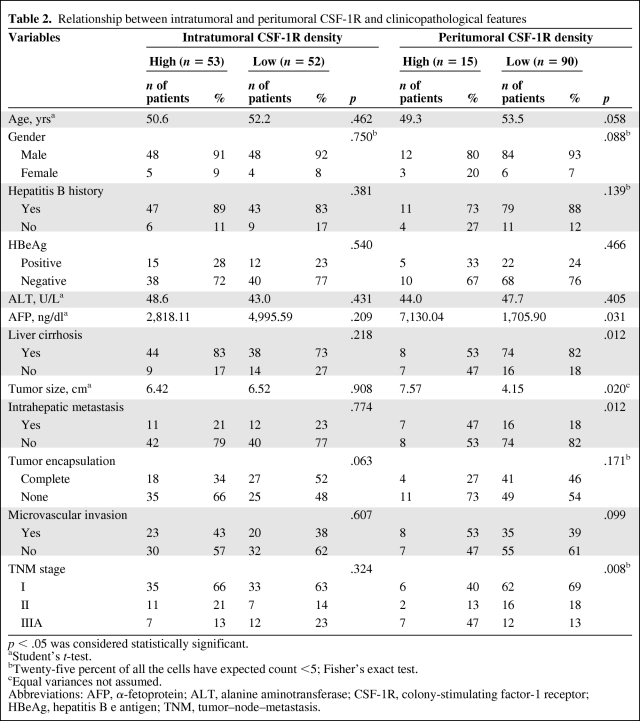
As shown in Table 2 (and supplemental online Tables S1 and S2), patients with a high peritumoral CSF-1R expression level were prone to have a large tumor size, high serum α-fetoprotein concentration, high TNM stage, and the presence of intrahepatic metastasis, whereas intratumoral CSF-1R expression did not correlate with any clinicopathological feature. Patients with cirrhosis (stage 4, n = 38) had a significantly lower peritumoral CSF-1R density than patients without cirrhosis (stages 1–3, n = 62; 0.044 versus 0.074, respectively; p = .009).
Table 2.
Relationship between intratumoral and peritumoral CSF-1R and clinicopathological features
p < .05 was considered statistically significant.
aStudent's t-test.
bTwenty-five percent of all the cells have expected count <5; Fisher's exact test.
cEqual variances not assumed.
Abbreviations: AFP, α-fetoprotein; ALT, alanine aminotransferase; CSF-1R, colony-stimulating factor-1 receptor; HBeAg, hepatitis B e antigen; TNM, tumor–node–metastasis.
Correlation with Patient Outcome
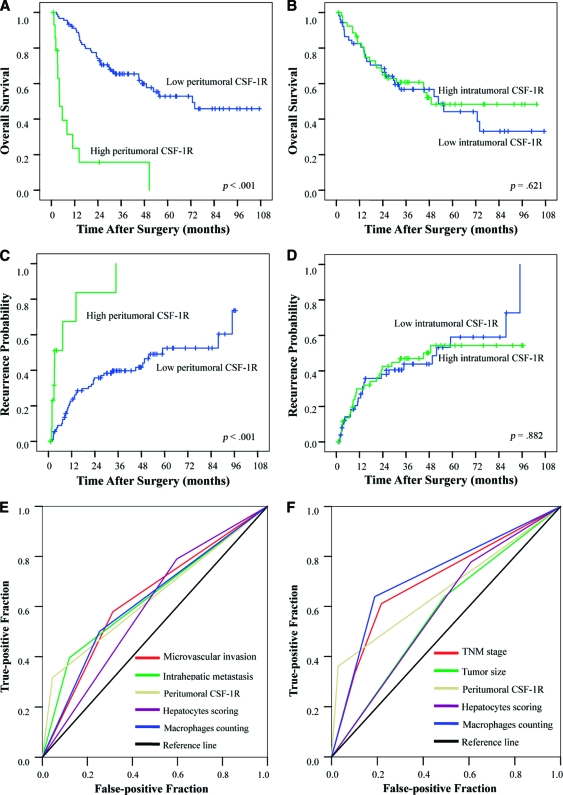
On univariate analysis, tumor size, the presence of intrahepatic metastasis, and TNM stage were associated with the OS time and TTR. The presence of microvascular invasion, liver cirrhosis, and tumor differentiation was also associated with OS (Table 3). The median OS time and TTR for patients with a high peritumoral CSF-1R expression level were 8.4 months and 10.3 months, respectively, and these periods were significantly shorter than those for patients with a low CSF-1R expression level (>72 months and 75.3 months, respectively; p < .001 for both) (Fig. 3A, 3C). However, intratumoral CSF-1R expression was not associated with the OS time or TTR (p = .621 and p = .882, respectively) (Fig. 3B, 3D). Low peritumoral hepatocyte expression and macrophage count was also associated with a longer OS time (p = .001 and p < .001, respectively) (supplemental online Fig. S2A, S2B, S2E) and TTR (p = .011 and p < .001, respectively) (Fig. S2C, S2D, S2F).
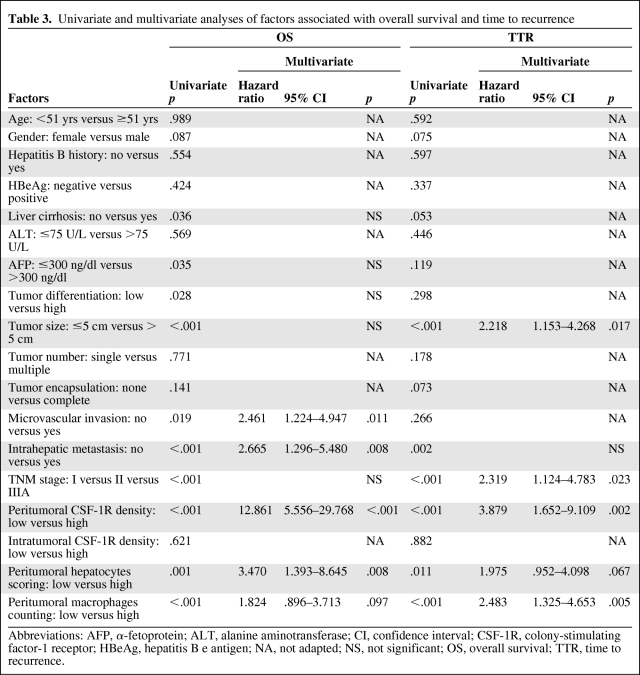
Table 3.
Univariate and multivariate analyses of factors associated with overall survival and time to recurrence
Abbreviations: AFP, α-fetoprotein; ALT, alanine aminotransferase; CI, confidence interval; CSF-1R, colony-stimulating factor-1 receptor; HBeAg, hepatitis B e antigen; NA, not adapted; NS, not significant; OS, overall survival; TTR, time to recurrence.
Figure 3.
Cumulative overall survival (OS) and time to recurrence (TTR) curves of patients (cohort 1) with low or high macrophage colony-stimulating factor-1 receptor (CSF-1R) density. Low peritumoral CSF-1R density was associated with a longer OS time and TTR (A, C), whereas intratumoral CSF-1R density was associated with neither the OS time nor the TTR (B, D). (E, F): Receiver operating characteristic curve: All the other adopted factors predicted death and 1-year recurrence precisely (p < .05 for all), whereas microvascular invasion could not predict death (p = .143).
The risk factors identified by univariate analysis were adopted in a multivariate Cox proportional hazard analysis. High peritumoral CSF-1R expression was an independent risk factor for OS (hazard ratio [HR], 12.861; p < .001) and TTR (HR, 3.879; p = .002) (Table 3). A high peritumoral hepatocyte expression level was an independent risk factor for OS (HR, 3.470; p = .008), and a high peritumoral macrophage count was an independent risk factor for TTR (HR, 2.483; p = .005) (Table 3).
We then used 12 months as the cutoff value to categorize tumor recurrences into early recurrence and late recurrence, as previously reported [43]. More patients with a high peritumoral CSF-1R density (than patients with a low peritumoral CSF-1R density) had an early recurrence (13 of 15 versus 23 of 90 patients, respectively; p < .001) rather than a late recurrence (1 of 15 versus 6 of 90 patients, respectively; p = .300). To reduce the impact of tumor size on patient survival, we further investigated prognostic factors in the small HCC subgroup (maximum diameter ≤5 cm, n = 57). The peritumoral CSF-1R level also correlated with the OS time and TTR (p = .027 and p = .007, respectively) in this subgroup (supplemental online Fig. S3). Positive hepatitis B e antigen (HBeAg) correlated with TTR (p = .013), whereas it did not correlate with the OS time (p = .130).
ROC Analysis
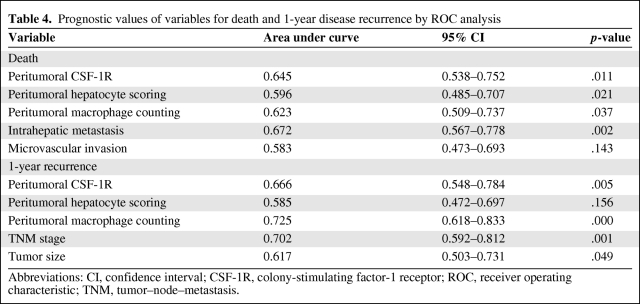
Risk factors identified by multivariate analysis were adopted, and their predictive values were studied by ROC analysis (Table 4). Peritumoral CSF-1R, hepatocyte scoring, macrophage count, and intrahepatic metastasis precisely predicted death, and peritumoral CSF-1R, macrophage count, TNM stage, and tumor size precisely predicted early recurrence (p < .05 for all). However, microvascular invasion did not predict death (p = .143). The area under the curve of peritumoral CSF-1R was 0.645 (95% confidence interval [CI], 0.538–0.752; p = .011) for death and 0.666 (95% CI, 0.548–0.784; p = .005) for 1-year recurrence (Fig. 3E, 3F), and both the predictive values were >0.600.
Table 4.
Prognostic values of variables for death and 1-year disease recurrence by ROC analysis
Abbreviations: CI, confidence interval; CSF-1R, colony-stimulating factor-1 receptor; ROC, receiver operating characteristic; TNM, tumor–node–metastasis.
Discussion
CSF-1R, as a member of the class III RTKs, is important in the malignant transformation of a tumor [44]. The extent of CSF-1R expression in some types of tumor is associated with high TNM grade and poor prognosis [18, 19]. However, the CSF-1R expression level in HCC is still unknown. Moreover, previous studies of CSF-1R mostly focused on tumor tissue, whereas its expression and significance in peritumoral tissue are not fully understood.
Our results revealed that expression of CSF-1R in peritumoral liver tissue was higher than that in the corresponding tumor tissue, and it was associated with poor outcome after resection of the primary tumor. We also found that CSF-1R was expressed not only in macrophages but also in peritumoral hepatocytes and HCC cells; the peritumoral hepatocyte expression of CSF-1R and macrophage infiltration were also associated with poor outcome for HCC patients. Overexpression of CSF-1R in the tumor is common in many types of cancer and correlates with a poor prognosis [18, 19, 31, 34, 35]. However, Kluger et al. [33] found that intratumoral CSF-1R expression was not an independent predictor of survival in breast cancer within a node-positive subset of patients. Our studies showed that there was significant correlation between peritumoral CSF-1R and a high incidence of intrahepatic metastasis; however, there was no correlation between intratumoral CSF-1R and any clinicopathological feature.
Recently, we reported that the expression levels of several angiogenesis factors and CSF-1 as well as macrophage infiltration are higher in peritumoral liver tissues [8, 9, 36]. Together with findings reported by other authors [10, 11], we propose that a unique condition in peritumoral liver tissue, which is associated with a specific type of HCC, provides a suitable substrate for the development and growth of intrahepatic metastasis or tumor recurrence.
The present study revealed that neither intratumoral nor peritumoral CSF-1R expression correlated with features related to hepatitis, including hepatitis B history, serum alanine aminotransferase, and the presence of HBeAg, except for cirrhosis scores. The possible reason may lie in the heterogeneous baseline expression level of CSF-1R, which is associated with other genetic heterogeneity among individuals. However, the centripetal distribution of CSF-1R expression in peritumoral liver tissue implies that the tumor may be a stimulator.
This peritumoral condition may be caused by hypoxia in hepatocytes resulting from direct compression by the primary tumor. Another possibility is that free radicals created by tumor hypoxia penetrate into the peritumoral liver milieu, strongly influencing the HIF-1α activity of hepatocytes [45], and then promote CSF-1R expression. The qRT-PCR results for HIF-1α suggest that a hypoxic microenvironment might explain CSF-1R expression in both intratumoral and peritumoral tissues. However, the tumor itself can be regarded as a stimulator of chronic inflammation as a nonhealing wound that causes a reaction in peritumoral hepatocytes, which produce a number of cytokines and recruit macrophages to the tumor margin. Therefore, the higher expression level of CSF-1R in peritumoral tissue may represent more macrophage infiltration and a more intense reaction of hepatocytes responding to hypoxia or “inflammation stimulation.” This hypothesis is supported by a study that showed strong expression of CSF-1R by macrophages infiltrating near prostate cancers and that the most intense and uniform staining for lesions within the prostate occurred in areas of high-grade carcinoma [19], which is similar to our observation. Recently, it was reported that both the paracrine and autocrine loops involving CSF-1R contribute to a more aggressive phenotype in human breast and ovarian cancer [31, 46], which is consistent with our results.
Although conflicting results on the clinical significance of intratumoral CSF-1R have been reported, the present findings suggest that CSF-1/CSF-1R may be a good target to block to prevent intrahepatic metastasis of HCC, especially in the adjuvant setting, because peritumoral liver tissue remains and becomes a major site for postoperative recurrence.
In summary, our present study shows that a high expression level of CSF-1R in peritumoral liver tissue is a strong predictor of postoperative survival in patients with HCC. Thus, this marker may be clinically useful for evaluating prognosis. Moreover, the expression of CSF-1R in residual liver after hepatectomy may play a critical role in promoting recurrence, invasion, and metastasis. With the recent development of new inhibitors that target RTKs and monoclonal antibodies to these receptors, CSF-1R may become a new target of therapy in HCC.
Supplementary Material
Acknowledgments
We thank Mr. Wei-De Zhang for assistance in collecting patient data. This study was jointly supported by a China Postdoctoral Science Foundation funded project (No. 20080440077) and National Key Science & Technology Specific project (2008ZX10002-019, 021).
Author Contributions
Conception/Design: Jin-Bin Jia, Wen-Quan Wang, Xiao-Dong Zhu, Hui- Chuan Sun, Zhao-You Tang
Financial support: Jin-Bin Jia, Hui-Chuan Sun, Zhao-You Tang
Administrative support: Hui-Chuan Sun, Zhao-You Tang, Wei-Zhong Wu, Lu Wang
Provision of study material or patients: Wen-Quan Wang, Xiao-Dong Zhu, Liang Liu, Peng-Yuan Zhuang, Ju-Bo Zhang, Wei Zhang, Hua-Xiang Xu, Ling-Qun Kong, Lu Lu
Collection and/or assembly of data: Jin-Bin Jia, Wen-Quan Wang, Xiao- Dong Zhu, Liang Liu, Peng-Yuan Zhuang
Data analysis and interpretation: Wen-Quan Wang, Xiao-Dong Zhu, Liang Liu
Manuscript writing: Jin-Bin Jia, Wen-Quan Wang, Hui-Chuan Sun, Zhao- You Tang
Final approval of manuscript: Zhao-You Tang
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Poon RT, Fan ST, Tsang FH, et al. Locoregional therapies for hepatocellular carcinoma: A critical review from the surgeon's perspective. Ann Surg. 2002;235:466–486. doi: 10.1097/00000658-200204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang ZY, Ye SL, Liu YK, et al. A decade's studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–196. doi: 10.1007/s00432-003-0511-1. [DOI] [PubMed] [Google Scholar]
- 5.Ding Y, Chen B, Wang S, et al. Overexpression of Tiam1 in hepatocellular carcinomas predicts poor prognosis of HCC patients. Int J Cancer. 2009;124:653–658. doi: 10.1002/ijc.23954. [DOI] [PubMed] [Google Scholar]
- 6.Ye QH, Qin LX, Forgues M, et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9:416–423. doi: 10.1038/nm843. [DOI] [PubMed] [Google Scholar]
- 7.Ke AW, Shi GM, Zhou J, et al. Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma. Hepatology. 2009;49:491–503. doi: 10.1002/hep.22639. [DOI] [PubMed] [Google Scholar]
- 8.Zhu XD, Zhang JB, Zhuang PY, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707–2716. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 9.Ju MJ, Qiu SJ, Fan J, et al. Peritumoral activated hepatic stellate cells predict poor clinical outcome in hepatocellular carcinoma after curative resection. Am J Clin Pathol. 2009;131:498–510. doi: 10.1309/AJCP86PPBNGOHNNL. [DOI] [PubMed] [Google Scholar]
- 10.Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budhu A, Forgues M, Ye QH, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Rettenmier CW, Chen JH, Roussel MF, et al. The product of the c-fms proto-oncogene: A glycoprotein with associated tyrosine kinase activity. Science. 1985;228:320–322. doi: 10.1126/science.2580348. [DOI] [PubMed] [Google Scholar]
- 13.Wiktor-Jedrzejczak W, Gordon S. Cytokine regulation of the macrophage (M phi) system studied using the colony stimulating factor-1-deficient op/op mouse. Physiol Rev. 1996;76:927–947. doi: 10.1152/physrev.1996.76.4.927. [DOI] [PubMed] [Google Scholar]
- 14.Bartelmez SH, Stanley ER. Synergism between hemopoietic growth factors (HGFs) detected by their effects on cells bearing receptors for a lineage specific HGF: Assay of hemopoietin-1. J Cell Physiol. 1985;122:370–378. doi: 10.1002/jcp.1041220306. [DOI] [PubMed] [Google Scholar]
- 15.Suzumura A, Sawada M, Yamamoto H, et al. Effects of colony stimulating factors on isolated microglia in vitro. J Neuroimmunol. 1990;30:111–120. doi: 10.1016/0165-5728(90)90094-4. [DOI] [PubMed] [Google Scholar]
- 16.Byrne PV, Guilbert LJ, Stanley ER. Distribution of cells bearing receptors for a colony-stimulating factor (CSF-1) in murine tissues. J Cell Biol. 1981;91:848–853. doi: 10.1083/jcb.91.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tushinski RJ, Oliver IT, Guilbert LJ, et al. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982;28:71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- 18.Sapi E, Kacinski BM. The role of CSF-1 in normal and neoplastic breast physiology. Proc Soc Exp Biol Med. 1999;220:1–8. doi: 10.1046/j.1525-1373.1999.d01-1.x. [DOI] [PubMed] [Google Scholar]
- 19.Ide H, Seligson DB, Memarzadeh S, et al. Expression of colony-stimulating factor 1 receptor during prostate development and prostate cancer progression. Proc Natl Acad Sci U S A. 2002;99:14404–14409. doi: 10.1073/pnas.222537099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai XM, Ryan GR, Hapel AJ, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 21.Woolford J, Rothwell V, Rohrschneider L. Characterization of the human c-fms gene product and its expression in cells of the monocyte-macrophage lineage. Mol Cell Biol. 1985;5:3458–3466. doi: 10.1128/mcb.5.12.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sariban E, Mitchell T, Kufe D. Expression of the c-fms proto-oncogene during human monocytic differentiation. Nature. 1985;316:64–66. doi: 10.1038/316064a0. [DOI] [PubMed] [Google Scholar]
- 23.Zins K, Abraham D, Sioud M, et al. Colon cancer cell-derived tumor necrosis factor-alpha mediates the tumor growth-promoting response in macrophages by up-regulating the colony-stimulating factor-1 pathway. Cancer Res. 2007;67:1038–1045. doi: 10.1158/0008-5472.CAN-06-2295. [DOI] [PubMed] [Google Scholar]
- 24.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 25.Condeelis J, Pollard JW. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Murray LJ, Abrams TJ, Long KR, et al. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis. 2003;20:757–766. doi: 10.1023/b:clin.0000006873.65590.68. [DOI] [PubMed] [Google Scholar]
- 27.Scott DA, Aquila BM, Bebernitz GA, et al. Pyridyl and thiazolyl bisamide CSF-1R inhibitors for the treatment of cancer. Bioorg Med Chem Lett. 2008;18:4794–4797. doi: 10.1016/j.bmcl.2008.07.093. [DOI] [PubMed] [Google Scholar]
- 28.Wei S, Dai XM, Stanley ER. Transgenic expression of CSF-1 in CSF-1 receptor-expressing cells leads to macrophage activation, osteoporosis, and early death. J Leukoc Biol. 2006;80:1445–1453. doi: 10.1189/jlb.0506304. [DOI] [PubMed] [Google Scholar]
- 29.Lin EY, Nguyen AV, Russell RG, et al. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–5066. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 31.Toy EP, Azodi M, Folk NL, et al. Enhanced ovarian cancer tumorigenesis and metastasis by the macrophage colony-stimulating factor. Neoplasia. 2009;11:136–144. doi: 10.1593/neo.81150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kacinski BM, Scata KA, Carter D, et al. FMS (CSF-1 receptor) and CSF-1 transcripts and protein are expressed by human breast carcinomas in vivo and in vitro. Oncogene. 1991;6:941–952. [PubMed] [Google Scholar]
- 33.Kluger HM, Dolled-Filhart M, Rodov S, et al. Macrophage colony-stimulating factor-1 receptor expression is associated with poor outcome in breast cancer by large cohort tissue microarray analysis. Clin Cancer Res. 2004;10:173–177. doi: 10.1158/1078-0432.ccr-0699-3. [DOI] [PubMed] [Google Scholar]
- 34.Richardsen E, Uglehus RD, Due J, et al. The prognostic impact of M-CSF, CSF-1 receptor, CD68 and CD3 in prostatic carcinoma. Histopathology. 2008;53:30–38. doi: 10.1111/j.1365-2559.2008.03058.x. [DOI] [PubMed] [Google Scholar]
- 35.Toy EP, Chambers JT, Kacinski BM, et al. The activated macrophage colony-stimulating factor (CSF-1) receptor as a predictor of poor outcome in advanced epithelial ovarian carcinoma. Gynecol Oncol. 2001;80:194–200. doi: 10.1006/gyno.2000.6070. [DOI] [PubMed] [Google Scholar]
- 36.Jia JB, Zhuang PY, Sun HC, et al. Protein expression profiling of vascular endothelial growth factor and its receptors identifies subclasses of hepatocellular carcinoma and predicts survival. J Cancer Res Clin Oncol. 2009;135:847–854. doi: 10.1007/s00432-008-0521-0. [DOI] [PubMed] [Google Scholar]
- 37.Sun HC, Zhang W, Qin LX, et al. Positive serum hepatitis B e antigen is associated with higher risk of early recurrence and poorer survival in patients after curative resection of hepatitis B-related hepatocellular carcinoma. J Hepatol. 2007;47:684–690. doi: 10.1016/j.jhep.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 38.Qian YB, Zhang JB, Wu WZ, et al. P48 is a predictive marker for outcome of postoperative interferon-alpha treatment in patients with hepatitis B virus infection-related hepatocellular carcinoma. Cancer. 2006;107:1562–1569. doi: 10.1002/cncr.22206. [DOI] [PubMed] [Google Scholar]
- 39.Sun HC, Zhuang PY, Qin LX, et al. Incidence and prognostic values of lymph node metastasis in operable hepatocellular carcinoma and evaluation of routine complete lymphadenectomy. J Surg Oncol. 2007;96:37–45. doi: 10.1002/jso.20772. [DOI] [PubMed] [Google Scholar]
- 40.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 41.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 42.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 43.Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507. [PubMed] [Google Scholar]
- 44.Kacinski BM. CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann Med. 1995;27:79–85. doi: 10.3109/07853899509031941. [DOI] [PubMed] [Google Scholar]
- 45.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patsialou A, Wyckoff J, Wang Y, et al. Invasion of human breast cancer cells in vivo requires both paracrine and autocrine loops involving the colony-stimulating factor-1 receptor. Cancer Res. 2009;69:9498–9506. doi: 10.1158/0008-5472.CAN-09-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.