The most frequent cardiovascular toxicities seen during treatment with the angiogenesis inhibitors bevacizumab, sunitinib, and sorafenib and their underlying mechanisms are investigated, with a view to providing indications for effective patient management.
Keywords: Angiogenesis, Bevacizumab, Sunitinib, Sorafenib, Cardiovascular toxicity
Abstract
Treatment with the angiogenesis inhibitors bevacizumab, sunitinib, and sorafenib as single agents or in combination with conventional chemotherapy is becoming a cornerstone of modern anticancer therapy. However, the potential toxicity of these drugs, mainly to the cardiovascular system, is still being investigated. Patient assessment at baseline, of crucial importance in candidates for treatment, involves the evaluation of risk factors and screening for past or present cardiovascular disease. Strict monitoring of treatment-related adverse effects must be conducted in order to allow the early detection of cardiovascular toxicities and their prompt medication. In the present paper, the most frequent cardiovascular toxicities and their underlying mechanisms are investigated, with a view to providing indications for effective patient management.
Introduction
Since 2004, when a randomized phase III trial on first-line treatment for patients with metastatic colorectal cancer indicated a survival benefit gained by administering bevacizumab combined with conventional chemotherapy, tumor angiogenesis inhibition has played a key role in the treatment of cancer [1].
Vascular endothelial growth factor (VEGF) and its downstream signaling network are now recognized as putative targets of new anticancer drugs. Both VEGF-neutralizing antibodies and small molecules binding to the intracellular domain of the VEGF receptor have been investigated. Three antiangiogenic drugs, bevacizumab, sunitinib, and sorafenib, are currently used in clinical practice, and clinical studies are under way to study several other compounds.
Bevacizumab, a humanized recombinant monoclonal antibody, binds to VEGF. Sunitinib and sorafenib, which are oral small molecule receptor tyrosine kinase inhibitors (RTKIs), mainly target VEGF receptor and platelet-derived growth factor (PDGF) receptor, as well as other tyrosine kinases involved in the angiogenic cascade. Although both sunitinib and sorafenib cause caspase activation and apoptosis, they differ because the former interferes with the members of the B-cell lymphoma 2 (BCL2) family [2], whereas the latter acts by disrupting the extracellular signal–regulated kinase (ERK)/v-raf-1 murine leukemia viral oncogene homolog 1 (RAF1) pathway [2]. Although they do not incur the common chemotherapy-related adverse effects such as myelosuppression, nausea, and vomiting, these drugs are not devoid of potential toxicities. The present paper therefore focuses on the currently available data regarding their cardiovascular effects, and possible strategies for the prevention of cardiovascular toxicities and their early detection.
Methods
A systematic review was made of information available in the literature on the cardiovascular safety profile of bevacizumab, sunitinib, and sorafenib. In order to retrieve information on the incidence of adverse effects, data were obtained from PubMed and the Cochrane Library, using the key words “bevacizumab,” “sunitinib,” and “sorafenib,” with each item being matched with “phase 3 trial,” “expanded access,” and “meta-analysis.” All available randomized phase III trials, expanded access programs, and meta-analyses were selected in order to search for detailed data on safety, without regard for efficacy. To provide a reliable analysis, the results obtained were compared with those appearing in U.S. Food and Drug Administration (FDA) approval reports; of all the papers published by the FDA, the first safety report made on the major efficacy study was chosen for evaluation.
Role of the VEGF Family in the Tumor Microenvironment
The VEGF family, a broad group of structurally related molecules, includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor [3]. VEGF-A, the most powerful known angiogenesis inducer, acts through VEGF receptor 2 (VEGFR-2) and is highly expressed by endothelial cells and bone marrow–derived endothelial progenitor cells. Although our understanding of VEGFR-1 is incomplete, we do know that it can bind its ligand with a tenfold higher affinity than VEGFR-2, but it is actually a poor transducer [4]. Tumor cells produce VEGF, which acts in a paracrine way on nearby endothelial cells: whereas the former lacks the receptor, the latter does not produce the ligand. However, it is now believed that cancer cells also express VEGFR [5]. The end result is the proliferation and survival of endothelial cells and the migration and homing of endothelial progenitor cells [6]. VEGF production depends on several epigenetic factors. Inflammatory cytokines such as interleukin-6 and environmental conditions such as low pH are important inducers [6]. Hypoxia, which frequently characterizes solid tumors, acts through hypoxia inducible factor (HIF)-1α and HIF-2α [7]. HIF is a substrate of the von Hippel Lindau tumor suppressor gene product (pVHL), and individuals who lack a VHL tumor suppressor gene allele in their germline (VHL disease) have an elevated risk for clear cell carcinoma of the kidney and hemangioblastoma, with the onset of disease resulting from the spontaneous inactivation of the remaining allele. The recruitment of pVHL to HIF-α leads to its polyubiquitylation and proteosomal degradation. When oxygen levels are low, pVHL is inactivated and the HIF-α that accumulates binds to hypoxia response elements, promoting the expression of up to 200 genes [8]. One of the targets is VEGF, thus explaining the density of vessels found in kidney cancer and the sensitivity of this form of cancer to antiangiogenic drugs.
Cardiovascular Effects of Angiogenesis Inhibitors
Hypertension
Pathogenesis
Hypertension is the most frequent adverse effect of the administration of angiogenesis inhibitors [9–12], and VEGF plays a key role in the maintenance of vascular homeostasis. The i.v. injection of VEGF in rats causes a dose-related decrease in mean arterial blood pressure [13]. This effect is probably a result of VEGF-mediated phosphorylation of endothelial nitric oxide synthase (eNOS). This, in turn, leads to an increase in the production of nitric oxide, which directly dilates vessels. Based upon this evidence, it is has been argued that VEGF antagonism might lead to an inhibition of eNOS, with a consequent vasoconstriction and decrease in sodium excretion. Alternatively, according to some authors [14], vascular rarefaction, with a subsequent increase in peripheral vascular resistance, would explain drug-induced hypertension. This hypothesis contradicts the evidence that average arterial pressure increases within hours following drug administration and is reversed soon after treatment is discontinued. However, it appears likely that there is a relationship between hypertension and vascular rarefaction in view of, for example, the finding that the capillary density of nondiabetic patients with untreated essential hypertension is significantly lower than that of normotensive subjects [15]. This datum suggests that capillary rarefaction is a primary defect in essential hypertension. Nitric oxide, which plays an important role in vascular homeostasis, is not merely a vasorelaxant, but directly drives new vessels that develop during the process of wound healing and stimulates the production of VEGF [16]; the latter, in turn, acts on eNOS.
High blood pressure has been proposed as a surrogate biomarker of antitumoral activity. In a recent study, Scartozzi and coworkers [17] investigated patients with metastatic colorectal cancer, treated with irinotecan, 5-fluorouracil, and leucovorin (the FOLFIRI regimen) plus bevacizumab. The patients were divided into two groups according to blood pressure data obtained from a series of recordings made before, during, immediately after, and 1 hour after infusion of bevacizumab. The criterion used for classifying patients was the development of grade 2–3 hypertension, according to the National Cancer Institute (NCI) Common Toxicity Criteria. Interestingly, patients with bevacizumab-related hypertension had a better outcome than normotensive patients in terms of the response rate (75% versus 32%) and the progression-free survival interval (14.5 months versus 3.1 months); no difference was observed between the groups in terms of overall survival. The series was small, but the evidence obtained suggests that clinically relevant hypertension might be used as a reliable and cost-free marker of antitumor activity.
Assessment and Treatment
The definition of hypertension, and the indications for its management, may vary according to different staging systems; we, however, consider the Common Terminology Criteria for Adverse Events (CTCAE) of NCI, version 3.0 [18] and version 4.0 [19], and the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) guidelines [20]. The latter states that treatment should be started as soon as prehypertension is recorded, if cardiovascular risk factors such as diabetes mellitus and obesity are present, or if there is evidence of organ damage, such as left ventricular hypertrophy, chronic kidney disease, and/or peripheral arterial disease. The lack of concordance between the classifications was recently solved by the latest version of the CTCAE, issued by the NCI in 2009; this update uses the same cutoff blood pressure levels as the JNC7 for grading hypertension. Although these recommendations have not been validated for cancer patients, the JNC7 suggests that, in cancer patients with chronic kidney disease, the target blood pressure is <135/85 mmHg [20]. Moreover, lifestyle modifications, such as dietary sodium reduction and weight loss, are of crucial importance, but may be inappropriate for patients with cancer-related impaired performance status. Despite its frequency, hypertension caused by angiogenesis inhibitors is usually reversible and mostly managed successfully with standard medications [1, 21, 22]. The commonly used antihypertensive agents are: diuretics, angiotensin-converting enzyme inhibitors (ACEIs), beta-blockers, calcium channel blockers (CCBs), and angiotensin receptor blockers (ARBs). Because hypertension often coexists with proteinuria, a condition similar to diabetic nephropathy, the American Diabetes Association [23] has recommended the preferential use of ACEIs and ARBs, because they effectively delay any deterioration in the glomerular filtration rate, the worsening of albuminuria, and the consequent progression of chronic kidney disease. Therefore, all patients undergoing treatment with angiogenic inhibitors should be screened for proteinuria. Finally, there is evidence that angiotensin II is a powerful mitogen and promoter of angiogenesis; this implies that treatment with ACEIs and ARBs may have an anticancer effect [24]. However, not all antihypertensive agents can be used safely: Angiogenic inhibitors are extensively metabolized by hepatic cytochrome P450 3A4, and the nondyhydropyridine CCBs, verapamil and diltiazem, inhibit this enzymatic pathway. Therefore, until further data are available, nondihydropyridine CCBs should be administered with caution [21].
Left Ventricular Dysfunction
Pathogenesis
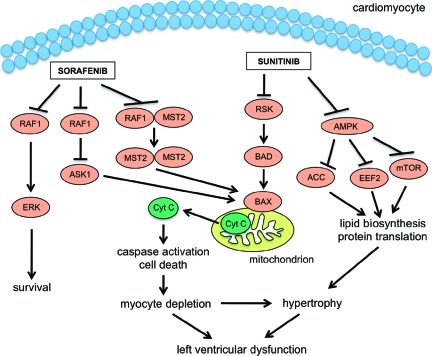
It has been observed that not only hypertension but also another serious adverse effect, a decline in left ventricular ejection fraction (LVEF), can be caused by angiogenic inhibitor administration, mainly during treatment with sunitinib [12, 25, 26]. The mechanism underlying this effect is not well understood; among the several targets of sunitinib, only the PDGF receptor is expressed in cardiomyocytes and, although PDGF overexpression appears to promote cardiomyocyte survival in rats [27], this action is absent in endogenous PDGF and its receptor. Therefore, sunitinib probably has an off-target effect [2]. Two possible candidates have been identified: the ribosomal S6 kinase (RSK) and the adenosine monophosphate activated protein kinase (AMPK) [28, 29]. According to this hypothesis, sunitinib, by inhibiting RSK, allows the release of the proapoptotic factor BCL2-associated death promoter (BAD) from RSK inactivation. BAD thus becomes available for interaction with BCL2-associated X protein (BAX), which in turn causes the release of cytochrome c, leading to caspase activation, apoptosis, and probably ATP depletion with left ventricular dysfunction. Moreover, under normal conditions, AMPK inhibits protein translation and lipid biosynthesis, which are energy-consuming processes, thus counteracting eukaryotic elongation factor 2 (EEF2), the mammalian target of rapamycin (mTOR), and/or acetyl coenzyme A carboxylase (ACC). By inhibiting AMPK, sunitinib fosters energy consumption, exacerbating ATP depletion. Importantly, AMPK is a key factor in cellular response to hypoxia, as occurs during ischemia. Furthermore, hypothyroidism and hypertension, which are sunitinib-related adverse effects, may worsen left ventricular function, eventually leading to heart failure [2].
Treatment with sorafenib is mostly associated with acute coronary syndrome [12]; still, there is evidence that it may interfere with the signal transduction cascade regulating cardiac homeostasis. As with sunitinib, the exact mechanism underlying its cardiotoxicity is not clear. However, a likely target has been identified: RAF1/B-Raf proto-oncogene serine/threonine protein kinase 1 (BRAF1). RAF1, a mitogen-activated protein kinase, is involved in the ERK-mediated prosurvival pathway [30]. Sorafenib disrupts this cascade, thus preventing RAF1/ERK formation. RAF1 has an ERK-independent effect, inhibiting two proapoptotic kinases—apoptosis signal-regulating kinase 1 (ASK1) [31] and mammalian sterile 20 kinase 2 (MST2) [32]—the mechanism underlying RAF1 inhibition is still a matter of debate. It has been shown that RAF1 deletion in murine models leads to dilated and hypocontractile hearts; this effect is reversed by ASK1 ablation [33]. Moreover, murine carriers of kinase-dead and dominant-negative RAF1 had normal hearts but were found to have a defective response to pressure overload [34]. These data suggest that RAF1 kinase activity is of critical importance and that RAF1 counteracts the proapoptotic cascade by means of protein–protein interaction, thus preventing the activation of downstream signals leading to caspase activation and apoptosis. It has therefore been suggested that sorafenib exerts its effect by blocking protein–protein interaction [2].
Available evidence shows that an intact VEGF-mediated angiogenic response is of crucial importance in allowing the heart to adapt to conditions of stress, such as pressure load. If this adaptation does not occur, the heart rapidly switches from compensation to failure (Fig. 1).
Figure 1.
Signaling pathways of sunitinib and sorafenib cardiotoxicity. Sunitinib, by inhibiting the ribosomal S6 kinase (RSK), allows the release of the proapoptotic factor BCL2-associated death promoter (BAD) from RSK inactivation; BAD interacts with BCL2 associated X protein (BAX), which, in turn, causes the release of cytochrome c (Cyt C), leading to caspase activation and cell death. Moreover, sunitinib, counteracting adenosine monophosphate activated protein kinase (AMPK), fosters protein translation and lipid biosynthesis through eukaryotic elongation factor 2 (EEF2), mammalian target of rapamycin (mTOR), and acetyl coenzyme A carboxylase (ACC). Sorafenib, preventing v-raf-1 murine leukemia viral oncogene homolog 1 (RAF1)/extracellular signal regulated kinase (ERK) formation, disrupts the ERK-mediated prosurvival pathway; furthermore, sorafenib, inhibiting the proapoptotic kinases apoptosis signal-regulating kinase 1 (ASK1) and mammalian sterile 20 kinase 2 (MST2), through RAF1, triggers cell death.
Early Detection
The way in which early treatment-induced cardiotoxicity is best detected is still a matter of debate. LVEF, the most commonly used parameter for patient monitoring, may underestimate real cardiac damage because the compensatory reserve of the myocardium allows adequate ventricular output despite the presence of subclinical dysfunction [35]. The currently available methods for cardiac assessment include multiple-gated acquisition (MUGA) scintigraphy, echocardiography, and electrocardiography. Although magnetic resonance imaging provides detailed information by providing high-resolution images, an effective strategy for the detection of early cardiac damage has yet to be established. Besides MUGA scintigraphy, tracers such as indium-111 antimyosin and 123-iodine-metaiobenzylguanidine, specific for necrotic tissue and the myocardial adrenergic neurotransmitter system, respectively, are sensitive, promising techniques. Investigations are under way to evaluate the most widely used serum biomarkers, such as natriuretic peptides (n-terminal probrain natriuretic peptide, brain natriuretic peptide, atrial natriuretic peptide) and troponins T and I, as well as markers of endothelial damage, such as tissue-type plasminogen activator and plasminogen activator inhibitor type 1. Finally, studies on genetic polymorphism are also ongoing. Importantly, current strategies for the early detection of cardiac damage are derived from medium-level evidence [36]. Encouraging results are being achieved with echocardiography because newly developed techniques allow the assessment of diastolic impairment as an early predictive marker of cardiac damage [36, 37].
Assessment and Treatment
In 2007, Schmidinger and coworkers analyzed 74 consecutive patients treated with sunitinib or sorafenib for metastatic renal cell carcinoma (RCC) [38]. Before treatment was started, patients were screened for coronary artery disease (CAD) risk factors, a history or evidence of hypertension, CAD, and rhythm disturbances. In all patients, assessment through biochemical markers (creatine phosphokinase and troponin T), electrocardiography, blood pressure measurement, and echocardiography (in selected patients) was made, and cardiac symptoms such as dyspnea on exertion, typical angina, and dizziness were also evaluated. Twenty-five patients (11 on sunitinib and 14 on sorafenib) had a cardiovascular event; the median duration of TKI administration at the time of the event was 8 weeks. Twelve of the 25 patients presented ECG changes; creatine phosphokinase and troponin T were elevated in 17 and nine patients, respectively; and echocardiographic findings were abnormal in 10 patients. No statistically significant differences were found between sunitinib and sorafenib patients in cardiovascular events. Once they had recovered, all patients were considered eligible for RTKI continuation. The incidence of cardiovascular events recorded in that study was higher than that reported in clinical trials. As suggested by the authors, this apparent discrepancy might have been a result of their broader definition of cardiac events and the closer patient monitoring that was conducted in the search for overt and minor signs of cardiac damage. Moreover, the authors suggest that, despite the antihypertensive medication provided, some of the events may have been related to overlooked episodes of high blood pressure. They, therefore, proposed pre-emptive therapy with ACEIs as a reasonable measure.
Currently there are no guidelines for the management of heart failure (HF) specific for cancer patients. The indications provided by the American College of Cardiology/American Heart Association should be followed [39]. Patients with HF should be managed with a combination of three types of drugs: a diuretic, an ACEI or an ARB, and a beta-blocker. Patients with evidence of fluid retention should take a diuretic until the euvolemic state is restored, and diuretic therapy should be continued to prevent the recurrence. Loop diuretics are the preferred agents because they are highly active, enhance free water clearance, and maintain their effectiveness even with impaired renal function; however, thiazide diuretics may be preferred in patients affected by hypertensive HF with mild fluid retention because they give a longer antihypertensive effect. Generally ACEIs are used together with a beta-blocker. Beta-blockers act mainly by inhibiting the adverse effects of the sympathetic nervous system, and this benefit exceeds their negative inotropic effect. Patients with a history of fluid retention should not be given beta-blockers without diuretics, because diuretics maintain fluid balance and prevent the exacerbation of fluid retention that may occur when beta-blocker therapy is initiated. There is evidence that long-term treatment with beta-blockers and ACEIs can improve the symptoms of HF and the clinical status. Therapy with digoxin as a fourth agent may be considered at any time to reduce symptoms, control rhythm, and enhance exercise tolerance [12].
Arterial and Venous Thromboembolism
Arterial thromboembolism, in particular, cardiac ischemia, has been observed in patients receiving bevacizumab [22, 40–44] and sorafenib [45–48]. Furthermore, treatment with bevacizumab entails an elevated risk for venous thromboembolism, mostly deep vein thrombosis [49, 50]. These drugs may have a direct toxic effect. In fact, VEGF seems to be important to maintain normal endothelial function; the apoptosis of endothelial cells causes the exposure of the highly prothrombotic basement membrane [51]. Platelets, which are carriers of VEGF, may also play a role [52]. However, the higher frequency of these events may, to some extent, be explained by the higher risk for uncontrolled hypertension or pre-existing progressive vascular damage. Whether patients can be safely treated with prophylactic or full-dose anticoagulants to reduce any elevated risk for bleeding is still a matter of debate. Although available data indicate that full-dose anticoagulants are also safe in patients without a concurrent elevated risk for bleeding [51, 53], a large randomized trial should be conducted to confirm the reported findings.
Safety Data: Phase III Trials and Expanded Access Programs
Bevacizumab
Colon Cancer
In 2004, Hurwitz and coworkers first published data on the efficacy of bevacizumab combined with chemotherapy in patients with metastatic colon cancer [1]. Eight hundred thirteen patients with previously untreated metastatic colon cancer were randomly assigned to receive irinotecan, bolus fluorouracil, and leucovorin (the IFL regimen) plus bevacizumab (5 mg/kg) or IFL plus placebo. With regard to cardiovascular toxicities, the experimental arm had a significantly higher incidence of hypertension (any grade, 22.4% versus 8.4%; grade 3 requiring treatment, 11.0% versus 2.3%). All episodes of hypertension were manageable with standard oral antihypertensive medications, and none called for discontinuation of bevacizumab. There were no hypertensive crises or deaths resulting from bevacizumab administration. There was no greater incidence of thrombotic events, deep thrombophlebitis, or pulmonary embolus in the bevacizumab study group. In Eastern Cooperative Oncology Group (ECOG) Study E3200 [54], 829 patients with colorectal cancer previously treated with a fluoropyrimidine and irinotecan were randomized to receive oxaliplatin, 5-fluorouracil, and leucovorin (the FOLFOX regimen), FOLFOX plus bevacizumab (10 mg/kg) or bevacizumab alone. The incidence of grade 3 hypertension was higher in the bevacizumab alone arm (5.2% versus 1.4% versus 7.3%, respectively). No significant difference was found among the groups for the incidence of grade 4 hypertension (1% versus 0.4% versus 0%, respectively), nor were any significant differences found in the incidences of thromboembolism, cardiac ischemia, and cerebrovascular ischemia. Allegra and coworkers recently published a safety report of the National Surgical Adjuvant Breast and Bowel Project C 08 trial [55]. That trial investigated the effectiveness of adding bevacizumab (5 mg/kg) to a modified FOLFOX regimen for patients with stage II or III colon cancer. In total, 2,710 patients were evaluated, with treatment being administered every 2 weeks for a total of 12 doses (duration, 6 months), and the experimental arm received bevacizumab alone for a total of 26 doses (1 year). The incidence of grade 3 hypertension was higher in the bevacizumab arm than in the placebo arm (12% versus 1.8%). Toxicity occurred during the 6 months of treatment with bevacizumab alone after completion of chemotherapy, and the incidence of grade 3 hypertension was higher in the treated than in the control arm (5.9% versus 0.7%). No significant difference was found between the two arms for cardiac/central nervous system ischemia and venous thrombosis. Saltz and coworkers tested the efficacy and safety of bevacizumab combined with first-line oxaliplatin-based chemotherapy (either capecitabine plus oxaliplatin [XELOX] or FOLFOX) in patients with metastatic colon cancer [40]. In total, 1,401 patients were randomized, in a 2×2 factorial design, to receive XELOX plus bevacizumab (7.5 mg/kg), XELOX plus placebo, FOLFOX plus bevacizumab (5 mg/kg), or FOLFOX plus placebo. The incidence of grade 3 or 4 adverse events was about 5% higher in the bevacizumab arm than in the placebo arm. Grade 3 or 4 hypertension was more frequent in the former than in the latter group (4% versus 1%), and the authors reported a difference between the bevacizumab and placebo arms (4% versus <1%) in terms of cardiac disorders, accounted for by arterial thromboembolic events including cardiac ischemia (2% versus 1%); patients who experienced these events had no underlying pattern in common.
Renal Cancer
In a randomized phase III trial, 649 patients with previously untreated metastatic RCC received interferon-α2a and bevacizumab (10 mg/kg) or interferon-α2a and placebo [22]. The incidence of hypertension of all grades was higher in the bevacizumab arm (26% versus 9%). Eleven patients (3%) had grade 3 hypertension and seven patients (2%) discontinued treatment because of hypertension. There were 10 grade 3 or 4 thromboembolic events (3%), six of which were venous and four of which were arterial. Of these, four were grade 4. Death resulting from adverse events was reported in eight patients who received bevacizumab and in seven of those who did not receive the drug; only three deaths, all as a result of bleeding and gastrointestinal perforation, were considered bevacizumab related. In the Cancer and Leukemia Group B 90206 study, which was a similarly designed multicenter trial, 732 patients with metastatic RCC were randomly assigned to receive either bevacizumab (10 mg/kg) plus interferon-α or interferon monotherapy [56]. Patients on bevacizumab had a significantly higher incidence of grade 3 hypertension (9% versus 0%); however, no excess cardiac ischemia/infarction, left ventricular dysfunction, or thrombosis/embolism was observed in the bevacizumab group.
Breast Cancer
In 2006, findings were reported from a randomized phase III trial on 462 patients with metastatic breast cancer who received capecitabine monotherapy or capecitabine plus bevacizumab (15 mg/kg) [57]. Hypertension was more frequent in the combination group (23.5% versus 2.3%); no case of grade 4 hypertension was reported, and four patients discontinued treatment with bevacizumab because of increased blood pressure. No difference was found between groups in the incidence of thrombotic events, pulmonary embolism, congestive HF, or cardiomyopathy. In 2007, the same authors published the results of an open-label, phase III trial in which 722 patients with metastatic breast cancer were randomized to receive either paclitaxel alone or paclitaxel plus bevacizumab (10 mg/kg) [41]. Grade 3 or 4 hypertension was more frequent in the combination group (14.8% versus 0%); only one patient developed grade 4 hypertension, which called for discontinuation of bevacizumab. Overall, thromboembolic events and left ventricular dysfunction were infrequent, but a significantly higher incidence of cerebrovascular ischemia occurred among patients receiving paclitaxel plus bevacizumab (1.9% versus 0%).
Lung Cancer
Between 2001 and 2004 the ECOG conducted a randomized phase III trial in which 878 patients with non-small cell lung carcinoma (NSCLC), stage IIIB or IV, were assigned to chemotherapy with paclitaxel and carboplatin or paclitaxel and carboplatin plus bevacizumab (15 mg/kg) [42]. The incidence of grade ≥3 adverse effects was significantly higher in the paclitaxel–carboplatin–bevacizumab group than in the paclitaxel–carboplatin group. Grade 3 or 4 hypertension was more frequent in the experimental arm (7% versus 0.7%); grade 3 or 4 hypertension occurred in 12 (5.6%) of the 215 patients receiving bevacizumab monotherapy. Of 17 reported treatment-related deaths, 15 occurred in the paclitaxel–carboplatin–bevacizumab arm (p = .001), two of which were a result of cerebrovascular events and one of which was caused by a pulmonary embolus. Three patients receiving paclitaxel–carboplatin–bevacizumab died as a result of cardiac events, although none of those deaths was considered treatment related—one was a result of a myocardial infarction, another was attributed to sudden death, and another was caused by cardiac arrest with bradycardia.
In the Avastin in Lung (AVAiL) trial, 1,043 patients with nonsquamous NSCLC were assigned to receive cisplatin and gemcitabine plus low-dose bevacizumab (7.5 mg/kg), high-dose bevacizumab (15 mg/kg), or placebo [58]. The overall incidence of grades ≥3 adverse effects were similar across the three arms. The incidence of grade 3 or 4 hypertension was slightly higher in the bevacizumab arms than in the placebo arm (6% and 9% versus 2%, respectively). None of these cases were clinically significant. No excess risk for ischemic and/or venous thromboembolic events resulting from bevacizumab was reported.
Pancreatic Cancer
In a phase III trial enrolling patients with metastatic pancreatic adenocarcinoma, 301 patients were randomized to receive either gemcitabine, erlotinib, and bevacizumab (5 mg/kg) or gemcitabine, erlotinib, and placebo [59]. Hypertension was more frequent in the bevacizumab arm (any grade, 20% versus 9%; grade ≥3, 3% versus 1%), whereas there was no difference in the incidences of arterial and venous thromboembolic events. The rates of cardiovascular adverse effects in phase III trials with bevacizumab are listed in Table 1.
Table 1.
Incidence of cardiovascular adverse effects in phase III trials evaluating bevacizumab
Abbreviations: Bev, bevacizumab; FDA, Food and Drug Administration; FOLFOX, oxaliplatin, 5-fluorouracil, and leucovorin; IFL, irinotecan, bolus fluorouracil, and leucovorin; LVEF, left ventricular ejection fraction; NOS, not otherwise specified; NSCLC, non-small cell lung carcinoma; q2wk, every 2 weeks; q3wk, every 3 weeks; XELOX, capecitabine plus oxaliplatin.
Once the improvement in clinical outcomes given by bevacizumab when combined with standard chemotherapy had been established, efficacy and safety were evaluated in a broader population of patients affected by metastatic colorectal cancer. The Bevacizumab Expanded Access Trial considered ∼2,000 patients, with a median follow-up of 21.1 months. Any grade hypertension occurred in 30% of patients (grade 3 or 4, 5%) and arterial thromboembolic events were overall reported in 2% of patients (grade 3 or 4, 1%). Grade 5 events resulting from bevacizumab (38 patients) included cardiac disorders (cardiac arrest, myocardial infarction; three patients, <0.5%) and venous embolism (11 patients, <0.5%) [60].
Sunitinib
The efficacy of sunitinib was first tested in a phase III trial for patients with unresectable imatinib-resistant gastrointestinal stromal tumor [61]. Three hundred twelve patients were randomized in a 2:1 ratio to receive either sunitinib (50 mg daily, 4 weeks on/2 weeks off) or a placebo; dose reductions were planned in cases of grade 3 or 4 adverse effects. In patients given sunitinib, the incidences of grade 1 or 2 hypertension and grade 3 hypertension were higher than in patients given placebo (8% versus 4% and 4% versus 0%, respectively). Neither of the groups reported grade 4 hypertension, nor was either a decrease in LVEF or congestive HF reported. In 2007, Motzer and coworkers published data on the efficacy of sunitinib as first-line therapy in 750 patients with metastatic RCC [25] randomly assigned to receive sunitinib (50 mg daily, 4 weeks on/2 weeks off) or interferon-α (9 MU three times weekly). Reductions in sunitinib (from 37.5 mg to 25 mg) and in interferon-α (from 6 MU to 3 MU) were made to allow for the management of adverse effects, according to a protocol nomogram. With regard to cardiovascular safety, there was a significantly higher incidence of any grade hypertension in the sunitinib group (24% versus 1%). Grade 3 hypertension was present in 8% of cases, whereas no case of grade 4 hypertension was reported. In 10% of patients, sunitinib caused a decline in LVEF (grade 3, 2%), compared with 3% in the interferon-α group (grade 3, 1%). Cardiac function was promptly restored by dose adjustment or discontinuation of treatment. No grade 4 decline in LVEF was observed. Rates of cardiovascular adverse effects in phase III trials with sunitinib are listed in Table 2.
Table 2.
Incidence of cardiovascular adverse effects in phase III trials evaluating sunitinib
Abbreviations: FDA, Food and Drug Administration; LVEF, left ventricular ejection fraction; NO, not observed; NOS, not otherwise specified.
The results of the expanded access program, which provided sunitinib to 4,564 patients, were published recently. With respect to cardiac disorders, 12 patients (<1%) experienced grade ≥3 ventricular dysfunction. Three treatment-related deaths resulted from cardiac failure. Grade 1 or 2 and grade 3 or 4 hypertension was reported in 16% and 5% of patients, respectively [62].
Sorafenib
In 2007, Escudier and coworkers published the results of a phase III trial, carried out by the Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET) study group, on the efficacy of sorafenib in metastatic renal-cell clear-cell carcinoma patients who had progressed after one course of systemic treatment [45]. Nine hundred three patients were randomly assigned to receive oral sorafenib (400 mg, twice daily) or placebo. Dose reductions were allowed in case of clinically significant hematologic and nonhematologic adverse effects (to 400 mg once daily and then to 400 mg every other day; if a further reduction was required, the patient was withdrawn from treatment). Any grade hypertension was more frequent in the sorafenib group (17% versus 2%). The incidence of grade 3 or 4 hypertension was 4% among patients on sorafenib and <1% in the placebo group (p < .001). However, in only 1% of patients, hypertension called for treatment discontinuation. Cardiac ischemia/infarction occurred in 12 patients in the sorafenib group (3%) and in two patients in the placebo group (<1%) (p = .01); among these cases, 11 (including two deaths in the sorafenib group and one death in the placebo group) were considered treatment related. The efficacy of sorafenib was also investigated in patients affected by unresectable stage III or IV melanoma, with progression during or after receiving at least one cycle with a regimen containing dacarbazine or temozolomide [63]. Patients were randomly assigned to receive either paclitaxel and carboplatin plus sorafenib (400 mg twice daily) or paclitaxel and carboplatin plus placebo. The authors did not report on the incidence of cardiovascular adverse effects, but they concluded that there was no significant difference between the two groups in the occurrence of adverse effects, except for thrombocytopenia. Furthermore, in the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial, 602 patients affected by hepatocellular carcinoma without previous systemic treatment were randomly assigned to receive sorafenib (400 mg twice daily) or placebo [46]. The incidences of grade 3 hypertension were similar in the sorafenib and placebo groups (2% versus 1%, respectively). Cardiac ischemia/infarction occurred in 3% of patients on sorafenib and in 1% of patients on placebo. A parallel trial was carried out in the Asia-Pacific region [64]. Two hundred seventy-one patients received either sorafenib (400 mg twice daily) or placebo. Any grade hypertension was more frequent in the treatment than in the placebo arm (18.8% versus 1.3%), whereas the incidences of grade 3 or 4 hypertension were similar in the two groups (2% versus 0%). No difference in serious adverse effects emerged. Rates of cardiovascular adverse effects in phase III trials with sorafenib are listed in Table 3.
Table 3.
Incidence of cardiovascular adverse effects in phase III trials evaluating sorafenib
Abbreviations: bid, twice daily; FDA, Food and Drug Administration; LVEF, left ventricular ejection fraction; NA, not available; NO, not observed; NOS, not otherwise specified.
The advanced RCC sorafenib (ARCCS) expanded access program made sorafenib available to 2,504 patients with advanced RCC before regulatory approval. Grade 3 treatment-related hypertension was reported in 5% of patients; arterial thromboembolic events, including cardiac ischemia, were not reported [65].
Safety Data: Meta-Analyses
Thromboembolism
The study by Choueiri et al. [48] determined the incidence of arterial thromboembolic events (ATEs) in patients receiving sorafenib and sunitinib; the incidence for sorafenib was 1.7% and the incidence for sunitinib was 1.3%. The difference between sunitinib and sorafenib was not statistically significant. The relative risk (RR) for ATEs for sunitinib and sorafenib was 3.03, indicating a threefold greater risk. The study by Ranpura et al. [43] assessed the incidence of ATEs in patients receiving bevacizumab. The incidences of all-grade and high-grade ATEs were 3.3% and 2%, respectively. The RR for ATEs associated with bevacizumab was 2.08. No difference between high-dose and low-dose bevacizumab was detected. The RRs for cardiac ischemia and ischemic stroke were 2.14 and 1.37, respectively, suggesting that bevacizumab entails a higher risk for cardiac ischemia but not for stroke. Furthermore, the Cochrane Collaboration [44] reported a statistically significant greater risk for ATEs in patients with metastatic colorectal cancer receiving bevacizumab. Nalluri determined the risk for venous thromboembolism associated with bevacizumab [50]. The incidences of all grade and grade ≥3 venous thromboembolism were 11.9% and 6.3%, respectively; the RR for all grade and grade ≥3 venous thromboembolism were 1.29 and 1.38, respectively, indicating a higher risk for developing high-grade venous thromboembolism with bevacizumab than in the control group.
Hypertension
According to Zhu et al. [10], the overall incidences of all-grade and high-grade hypertension with sunitinib were 21.6% and 6.8%, respectively. The RRs for all-grade and high-grade hypertension were 3.44 and 22.72, respectively, indicating that sunitinib is associated with a significantly greater risk for high-grade hypertension. A similar meta-analysis was made for sorafenib [11]. The incidences of high-grade and low-grade hypertension were 23.4% and 5.7%, respectively; the overall RR, calculated on RCC patients, was 6.11, suggesting a higher risk for hypertension during treatment with sorafenib. Ranpura et al. [9] assessed the risk for hypertension associated with bevacizumab. The incidence of high-grade hypertension was 7.9%; the RRs for all-grade and high-grade hypertension were 3.02 and 5.28, respectively. The authors did not find any significant difference in the risk for high-grade hypertension between low-dose and high-dose bevacizumab.
Conclusions
The development of new drugs, such as monoclonal antibodies and small molecules, has enabled us to treat advanced cancer refractory to conventional chemotherapy, thus prolonging survival; however, these compounds incur toxicity, although the incidence of serious adverse effects is low. Management of the most frequent effect, hypertension, is relatively straightforward, and discontinuation of treatment is hardly ever required when this effect does occur. Sunitinib is associated with a significantly higher risk for left ventricular dysfunction, which is reversible after dose reductions or temporary interruption of treatment, whereas both sorafenib and bevacizumab entail an enhanced thrombotic risk.
The choice of a compound tailored to patient comorbidities is riddled with difficulties. This observation does not apply to patients with hepatocellular carcinoma or gastrointestinal stromal tumors after failure of imatinib, for which sorafenib and sunitinib, respectively, are the only available therapeutic options. But it does apply to patients with advanced or metastatic RCC, for whom several first-line treatments are available. Patients in the poor risk category (according to the Memorial Sloan-Kettering Cancer Center risk model, those with more than three poor-prognosis pretreatment features) may benefit from treatment with temsirolimus, an mTOR inhibitor almost devoid of cardiovascular toxicity, whereas those in the favorable and intermediate risk categories may be given either bevacizumab combined with interferon-α or sunitinib. The different ways of administration and treatment schedules, and the fact that the former is a combination therapy and the latter is a single medication therapy, could compromise tolerability. Yet, in spite of these considerations, it is important to bear in mind that the efficacies of these compounds in the treatment of advanced or metastatic RCC are identical. Therefore, it is more appropriate to prescribe bevacizumab for patients with decreased LVEF or symptomatic chronic HF, and, while the patient is on treatment, to conduct a close echocardiographic follow-up together with the periodic dosing of cardiac serum biomarkers and careful blood pressure control. On the other hand, patients with inadequately controlled hypertension and/or those receiving more than two active drugs, in whom cardiac function is preserved, may be eligible for sunitinib.
Finally, whichever the treatment chosen, every attempt should be made to detect possible signs of cardiovascular damage in each and every candidate, with careful assessment at baseline and close monitoring in order to ensure that prompt measures are taken to reduce the likelihood of treatment-related adverse effects. New concerns are being raised regarding the best possible way to manage adverse events, and clinicians are now expected to improve their awareness of the currently available methods for patient assessment and early detection.
Author Contributions
Conception/Design: Alba A. Brandes, Enrico Franceschi, Fabio Girardi
Provision of study material or patients: Alba A. Brandes
Collection and/or assembly of data: Fabio Girardi
Data analysis and interpretation: Alba A. Brandes, Enrico Franceschi, Fabio Girardi
Manuscript writing: Alba A. Brandes, Fabio Girardi
Final approval of manuscript: Alba A. Brandes
References
- 1.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7:332–344. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 4.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Dong X, Han ZC, Yang R. Angiogenesis and antiangiogenic therapy in hematologic malignancies. Crit Rev Oncol Hematol. 2007;62:105–118. doi: 10.1016/j.critrevonc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 8.Kaelin WG., Jr Treatment of kidney cancer: Insights provided by the VHL tumor-suppressor protein. Cancer. 2009;115:2262–2272. doi: 10.1002/cncr.24232. [DOI] [PubMed] [Google Scholar]
- 9.Ranpura V, Pulipati B, Chu D, et al. Increased risk of high-grade hypertension with bevacizumab in cancer patients: A meta-analysis. Am J Hypertens. 2010;23:460–468. doi: 10.1038/ajh.2010.25. [DOI] [PubMed] [Google Scholar]
- 10.Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: Systematic review and meta-analysis. Acta Oncol. 2009;48:9–17. doi: 10.1080/02841860802314720. [DOI] [PubMed] [Google Scholar]
- 11.Wu S, Chen JJ, Kudelka A, et al. Incidence and risk of hypertension with sorafenib in patients with cancer: A systematic review and meta-analysis. Lancet Oncol. 2008;9:117–123. doi: 10.1016/S1470-2045(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 12.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 13.Yang R, Thomas GR, Bunting S, et al. Effects of vascular endothelial growth factor on hemodynamics and cardiac performance. J Cardiovasc Pharmacol. 1996;27:838–844. doi: 10.1097/00005344-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Sane DC, Anton L, Brosnihan KB. Angiogenic growth factors and hypertension. Angiogenesis. 2004;7:193–201. doi: 10.1007/s10456-004-2699-3. [DOI] [PubMed] [Google Scholar]
- 15.Serné EH, Gans RO, ter Maaten JC, et al. Impaired skin capillary recruitment in essential hypertension is caused by both functional and structural capillary rarefaction. Hypertension. 2001;38:238–242. doi: 10.1161/01.hyp.38.2.238. [DOI] [PubMed] [Google Scholar]
- 16.Lee PC, Salyapongse AN, Bragdon GA, et al. Impaired wound healing and angiogenesis in eNOS-deficient mice. Am J Physiol. 1999;277:H1600–H1608. doi: 10.1152/ajpheart.1999.277.4.H1600. [DOI] [PubMed] [Google Scholar]
- 17.Scartozzi M, Galizia E, Chiorrini S, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20:227–230. doi: 10.1093/annonc/mdn637. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) Bethesda, MD: National Cancer Institute; 2006. [accessed July 15, 2009]. Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_30. [Google Scholar]
- 19.National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0 (CTCAE) Bethesda, MD: National Cancer Institute; 2009. [accessed July 15, 2009]. Available at http://evs.nci.nih.gov/ftp1/CTCAE/About.html. [Google Scholar]
- 20.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 21.Izzedine H, Ederhy S, Goldwasser F, et al. Management of hypertension in angiogenesis inhibitor-treated patients. Ann Oncol. 2009;20:807–815. doi: 10.1093/annonc/mdn713. [DOI] [PubMed] [Google Scholar]
- 22.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 23.Remuzzi G, Schieppati A, Ruggenenti P. Clinical practice Nephropathy in patients with type 2 diabetes. N Engl J Med. 2002;346:1145–1151. doi: 10.1056/NEJMcp011773. [DOI] [PubMed] [Google Scholar]
- 24.Molteni A, Heffelfinger S, Moulder JE, et al. Potential deployment of angiotensin I converting enzyme inhibitors and of angiotensin II type 1 and type 2 receptor blockers in cancer chemotherapy. Anticancer Agents Med Chem. 2006;6:451–460. doi: 10.2174/187152006778226521. [DOI] [PubMed] [Google Scholar]
- 25.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration. Sutent Approval Report. Silver Spring, MD: U.S. Food and Drug Administration; 2006. [accessed July 15, 2009]. Available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021938s000_021968s000_Sutent.cfm. [Google Scholar]
- 27.Hsieh PC, MacGillivray C, Gannon J, et al. Local controlled intramyocardial delivery of platelet-derived growth factor improves postinfarction ventricular function without pulmonary toxicity. Circulation. 2006;114:637–644. doi: 10.1161/CIRCULATIONAHA.106.639831. [DOI] [PubMed] [Google Scholar]
- 28.Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: Enemy or ally? J Physiol. 2006;574:95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terai K, Hiramoto Y, Masaki M, et al. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554–9575. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyriakis JM, Force TL, Rapp UR, et al. Mitogen regulation of c-Raf-1 protein kinase activity toward mitogen-activated protein kinase-kinase. J Biol Chem. 1993;268:16009–16019. [PubMed] [Google Scholar]
- 31.Chen J, Fujii K, Zhang L, et al. Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK-ERK independent mechanism. Proc Natl Acad Sci U S A. 2001;98:7783–7788. doi: 10.1073/pnas.141224398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Neill E, Rushworth L, Baccarini M, et al. Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. Science. 2004;306:2267–2270. doi: 10.1126/science.1103233. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi O, Watanabe T, Nishida K, et al. Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J Clin Invest. 2004;114:937–943. doi: 10.1172/JCI20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris IS, Zhang S, Treskov I, et al. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation. 2004;110:718–723. doi: 10.1161/01.CIR.0000138190.50127.6A. [DOI] [PubMed] [Google Scholar]
- 35.Ewer MS, Lenihan DJ. Left ventricular ejection fraction and cardiotoxicity: Is our ear really to the ground? J Clin Oncol. 2008;26:1201–1203. doi: 10.1200/JCO.2007.14.8742. [DOI] [PubMed] [Google Scholar]
- 36.Altena R, Perik PJ, van Veldhuisen DJ, et al. Cardiovascular toxicity caused by cancer treatment: Strategies for early detection. Lancet Oncol. 2009;10:391–399. doi: 10.1016/S1470-2045(09)70042-7. [DOI] [PubMed] [Google Scholar]
- 37.Lester SJ, Tajik AJ, Nishimura RA, et al. Unlocking the mysteries of diastolic function: Deciphering the Rosetta Stone 10 years later. J Am Coll Cardiol. 2008;51:679–689. doi: 10.1016/j.jacc.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 38.Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–5212. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 39.Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults; A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 41.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 42.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 43.Ranpura V, Hapani S, Chuang J, et al. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis of randomized controlled trials. Acta Oncol. 2010;49:287–297. doi: 10.3109/02841860903524396. [DOI] [PubMed] [Google Scholar]
- 44.Wagner AD, Arnold D, Grothey AA, et al. Anti-angiogenic therapies for metastatic colorectal cancer. Cochrane Database Syst Rev. 2009;(3):CD005392. doi: 10.1002/14651858.CD005392.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 46.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 47.U.S. Food and Drug Administration. Nexavar Approval Report. Silver Spring, MD: U.S. Food and Drug Administration; 2005. [accessed July 15, 2009]. Available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021923_s000_NexavarTOC.cfm. [Google Scholar]
- 48.Choueiri TK, Schutz FA, Je Y, et al. Risk of arterial thromboembolic events with sunitinib and sorafenib: A systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28:2280–2285. doi: 10.1200/JCO.2009.27.2757. [DOI] [PubMed] [Google Scholar]
- 49.U.S. Food and Drug Administration. Avastin Approval Report. Silver Spring, MD: U.S. Food and Drug Administration; 2004. [accessed July 15, 2009]. Available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/STN_125085_Avastin.cfm. [Google Scholar]
- 50.Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA. 2008;300:2277–2285. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 51.Zangari M, Fink LM, Elice F, et al. Thrombotic events in patients with cancer receiving antiangiogenesis agents. J Clin Oncol. 2009;27:4865–4873. doi: 10.1200/JCO.2009.22.3875. [DOI] [PubMed] [Google Scholar]
- 52.Kuenen BC, Levi M, Meijers JC, et al. Potential role of platelets in endothelial damage observed during treatment with cisplatin, gemcitabine, and the angiogenesis inhibitor SU5416. J Clin Oncol. 2003;21:2192–2198. doi: 10.1200/JCO.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 53.Elice F, Jacoub J, Rickles FR, et al. Hemostatic complications of angiogenesis inhibitors in cancer patients. Am J Hematol. 2008;83:862–870. doi: 10.1002/ajh.21277. [DOI] [PubMed] [Google Scholar]
- 54.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 55.Allegra CJ, Yothers G, O'Connell MJ, et al. Initial safety report of NSABP C-08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009;27:3385–3390. doi: 10.1200/JCO.2009.21.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 58.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 59.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 60.Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: The BEAT study. Ann Oncol. 2009;20:1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 61.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 62.Gore ME, Szczylik C, Porta C, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: An expanded-access trial. Lancet Oncol. 2009;10:757–763. doi: 10.1016/S1470-2045(09)70162-7. [DOI] [PubMed] [Google Scholar]
- 63.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–2830. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 64.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-bind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 65.Stadler WM, Figlin RA, McDermott DF, et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer. 2010;116:1272–1280. doi: 10.1002/cncr.24864. [DOI] [PubMed] [Google Scholar]