Results of the Hermine study examining the use of trastuzumab for metastatic breast cancer patients in routine practice, including patients who received trastuzumab treatment beyond progression, are reported. The cardiac safety of trastuzumab in this setting is also reported.
Keywords: Human epidermal growth factor receptor 2, Metastatic breast cancer, Overall survival, Trastuzumab, Treatment beyond progression
Abstract
Background.
The Hermine study observed the use of trastuzumab for metastatic breast cancer (MBC) in routine practice, including patients who received trastuzumab treatment beyond progression (TBP).
Patients and Methods.
The study observed 623 patients for ≥2 years. Treatment was given according to oncologists' normal clinical practices. Endpoints included duration of treatment, efficacy, and cardiac safety. The TBP subanalysis compared overall survival (OS) in 177 patients who received first-line trastuzumab and either continued trastuzumab for ≥30 days following progression or stopped at or before progression.
Results.
The median treatment duration was 13.3 months. In the first-, second-, and third-line or beyond treatment groups, the median time to progression (TTP) were 10.3 months, 9.0 months, and 6.3 months, and the median OS times were 30.3 months, 27.1 months, and 23.2 months, respectively. Heart failure was observed in 2.6% of patients, although no cardiac-associated deaths occurred. In the TBP subanalysis, the median OS duration from treatment initiation and time of disease progression were longer in patients who continued receiving trastuzumab TBP (>27.8 months and 21.3 months, respectively) than in those who stopped (16.8 months and 4.6 months, respectively). However, the groups were not completely comparable, because patients who continued trastuzumab TBP had better prognoses at treatment initiation. The median TTP was longer in patients who continued trastuzumab TBP (10.2 months) than in those who stopped (7.1 months).
Conclusion.
The Hermine findings confirm that the pivotal trials of first-line trastuzumab treatment in MBC patients are applicable in clinical practice. The subanalysis suggests that trastuzumab TBP offers a survival benefit to MBC patients treated with first-line trastuzumab.
Introduction
The survival benefit from trastuzumab (Herceptin®; F. Hoffmann-La Roche Ltd, Basel, Switzerland), a recombinant monoclonal antibody, has been well established in clinical trials of patients with early breast cancer and metastatic breast cancer (MBC) that overexpress human epidermal growth factor receptor (HER)-2 [1–5]. Trastuzumab-based treatment is the standard of care for HER-2+ MBC patients [6], but MBC remains essentially incurable and patients ultimately experience progressive disease [1].
The Hermine study, an observational study conducted in France, was designed to evaluate outcomes in patients with MBC who received trastuzumab treatment in routine clinical practice. With >600 patients, the Hermine study is one of the largest studies of trastuzumab in MBC. Because trastuzumab has also been associated with cardiac events, particularly in patients with prior exposure to anthracyclines [7, 8], the Hermine study also aimed to examine the cardiac safety of trastuzumab in patients treated in routine practice.
This paper reports data collected for the overall study population, as well as according to the initial line of trastuzumab treatment. A subanalysis focused on patients who continued to receive trastuzumab after progression, presenting an opportunity to evaluate the efficacy of trastuzumab treatment beyond progression (TBP) in the largest patient series to date (n = 177).
Methods
Study Design and Patients
The Hermine trial was a French multicenter, pharmacoepidemiologic, retrospective and prospective, observational cohort study that was managed by an independent scientific committee that included three breast cancer specialists, a pathologist, and an epidemiologist. In accordance with French legislation regarding noninterventional studies, the study protocol was validated by the Comité Consultatif sur le Traitement de l'Information en Matière de Recherche dans le Domaine de la Santé and approved by the Commission Nationale de l'Informatique et des Libertés, which guarantees patient confidentiality.
Participating oncologists were drawn from universities, hospitals, and cancer treatment centers across France. In the absence of a national breast cancer oncologist database, the names of oncology specialists were obtained from a database managed by the sponsor (Roche SAS, Neuilly-sur-Seine, France). This database included 250 breast cancer specialists already prescribing trastuzumab. Specialists from the database were selected at random and invited to retrospectively include all women aged ≥18 years who had first received trastuzumab for MBC between January 1 and December 31, 2002; patients were identified via pharmacy records. All patients were informed of the study objectives. Once in the study, patients continued to be treated and assessed in accordance with their oncologists' normal clinical practices, with trastuzumab administered as an i.v. infusion either weekly or 3-weekly.
One-year follow-up data were collected retrospectively between November 2003 and April 2004. Final data (minimum patient follow-up ≥2 years) were collected prospectively in March 2005. All data were collected from patient files and reviewed by the independent scientific committee; any questions arising from this review were sent to the appropriate physicians for clarification.
A subanalysis of the Hermine study examined outcomes in patients with MBC treated initially with trastuzumab as first-line therapy who subsequently experienced disease progression and either continued trastuzumab TBP (for ≥30 days) or discontinued trastuzumab within 30 days of progression.
Study Endpoints
The primary endpoint was treatment duration with trastuzumab (defined as the time between first and last infusion, including beyond disease progression). Secondary endpoints included: combination therapies with trastuzumab; frequency of, and reasons for, stopping trastuzumab; time to progression (TTP); sites of disease progression; and overall survival (OS). Cardiac safety was also a secondary endpoint and included available left ventricular ejection fraction (LVEF) assessments and documented cardiac events. An LVEF ≥50% was considered normal.
The TBP subanalysis endpoints included duration of trastuzumab treatment, TTP from initiation of treatment, and OS from initiation of trastuzumab and from date of first progression.
Statistical Methods
In order to assess trastuzumab treatment duration to an accuracy of 15 days (using a two-sided 95% confidence interval [CI]), it was calculated that 454 patients would be required. This was based on the assumption of a mean treatment duration of 240 days, with a standard deviation of 163 days, as seen in a previous trial [5]. Allowing for 10% incomplete data, 500 patients were required. Each oncologist was expected to include at least five patients; it was assumed that 10% of oncologists would not complete the study, so 110 specialists were invited to enroll patients.
Statistical analyses were performed using SAS, version 8.2 (SAS Institute Inc., Cary, NC). Time-to-event data (treatment duration, TTP, OS) were analyzed using the Kaplan–Meier method both for the overall population and according to the initial line of trastuzumab treatment (first, second, and third or beyond). TTP was defined as time from initiation of trastuzumab to progression or disease-related death, and the OS time was defined as the time from initiation of treatment to death resulting from any cause. For patients without progression or disease-related death at the end of the follow-up, data were recorded on the date of the last known follow-up visit. The impact of prognostic factors—including patient demographics, tumor characteristics (hormone-receptor status, Scarff-Bloom-Richardson histologic grade), MBC history (number of metastatic sites, site localization, and disease-free interval, defined as time from breast cancer diagnosis to appearance of metastatic disease), mode of trastuzumab administration (monotherapy versus combination therapy), and previous treatment lines for metastatic disease on TTP and OS—was analyzed in an exploratory analysis using a Cox regression model. Identified factors are presented with a hazard ratio (HR) and 95% CI. All analyses were performed using a two-sided significance level of 5%.
The TBP subanalysis compared duration of trastuzumab treatment, TTP from initiation of treatment, and OS using a log-rank test. An exploratory analysis was performed, as in the whole-population analysis, to identify potential independent prognostic factors for OS.
The analysis of cardiac safety, evaluated by LVEF assessments, was descriptive only.
Results
Patient Characteristics
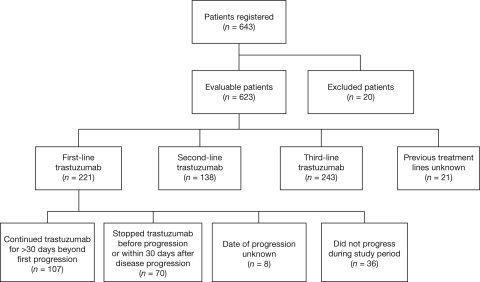
Overall, 116 oncologists agreed to participate in the study and 102 included at least one patient. In total, 643 patients were registered and, of these, 623 were evaluable (Fig. 1). Twenty patients were excluded because of protocol deviations: metastatic status not confirmed (n = 17), cancer other than breast (n = 1), missing file (n = 1), and treatment initiated before 2002 (n = 1).
Figure 1.
Consolidated Standards of Reporting Trials diagram of patients in the Hermine study.
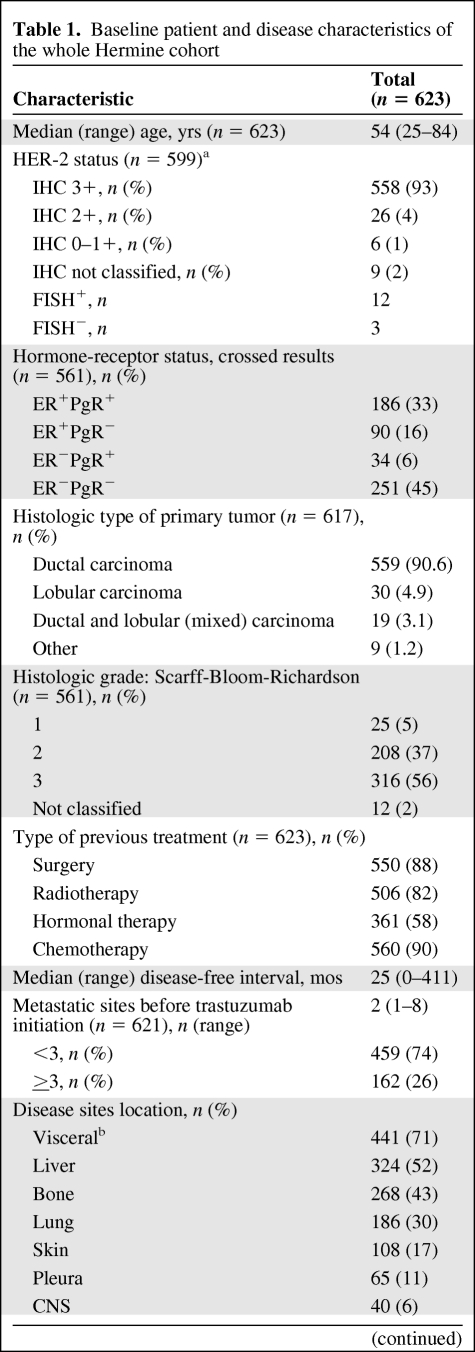
Patient characteristics are summarized in Table 1. HER-2 status was assessed locally by immunohistochemistry (IHC) for 599 (96%) patients; 93% had IHC 3+ tumors. Fluorescence in situ hybridization (FISH) was used alone for nine patients and in addition to IHC in six; overall, 12 patients had HER-2–amplified tumors as assessed by FISH (FISH+).
Table 1.
Baseline patient and disease characteristics of the whole Hermine cohort
Table 1.
(Continued)
aHER-2 status assessed by both IHC and FISH in six cases.
bLiver and/or lung and/or CNS.
Abbreviations: CNS, central nervous system; ER, estrogen receptor; FISH, fluorescence in situ hybridization; HER-2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; PgR, progesterone receptor.
The median time since diagnosis of the primary breast cancer and start of trastuzumab treatment was 2.9 years (range, 0–36.1); 20% of patients had MBC at first diagnosis. Prior anthracycline treatment had been received by 84% of the overall population.
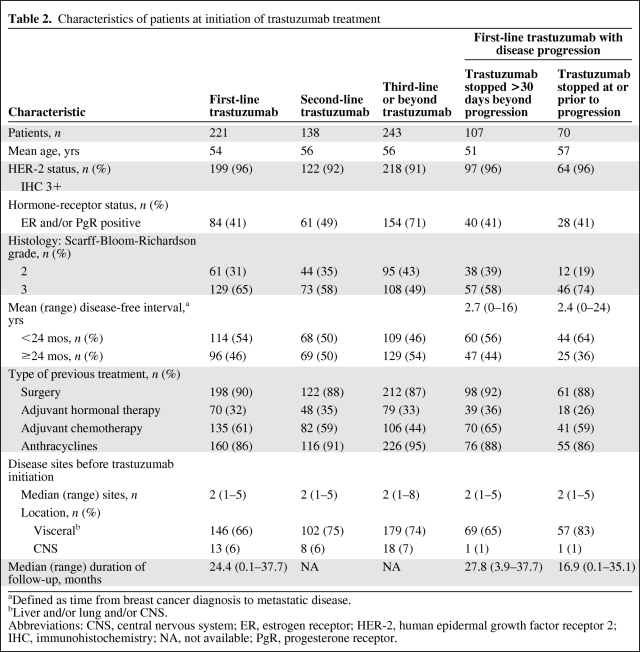
Trastuzumab was initially prescribed as first-, second-, or third-line or beyond treatment for MBC in 221 (36%), 138 (22%), and 243 (39%) patients, respectively (Table 2). Information on the number of previous treatment lines was missing for 21 patients (3%).
Table 2.
Characteristics of patients at initiation of trastuzumab treatment
aDefined as time from breast cancer diagnosis to metastatic disease.
bLiver and/or lung and/or CNS.
Abbreviations: CNS, central nervous system; ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; NA, not available; PgR, progesterone receptor.
Approximately one quarter of the patients had a history of cardiac problems before starting trastuzumab treatment, including arterial hypertension (17.1% of patients). Baseline LVEF was assessed in 357 patients (57%). The median LVEF was 63% (range, 23%–88%), with 96% of patients having a normal LVEF (≥50%); 2% had an LVEF <40%. A baseline electrocardiogram was assessed in 259 patients (44%) and was abnormal in 14 patients. Of these, two presented with ischemia, six with dysrhythmia, and six with conductive heart disorders.
TBP Subanalysis
From the total Hermine cohort, 177 patients experienced disease progression after receiving first-line trastuzumab and were evaluable in the TBP subanalysis (median follow-up, 24.4 months) (Fig. 1); 107 patients continued trastuzumab for >30 days beyond first progression and 70 stopped therapy before progression or within 30 days after disease progression.
Patient characteristics are presented in Table 2. More grade 3 tumors, a shorter disease-free interval, more visceral metastases, and less adjuvant hormonal therapy were observed in patients who did not receive trastuzumab after first progression. Patients who had brain metastases at the time of trastuzumab treatment initiation were rare in both groups. Patients receiving trastuzumab TBP had a longer median follow-up (27.8 months) than those for whom treatment was stopped at progression (16.9 months) (Table 2).
Exposure to Trastuzumab
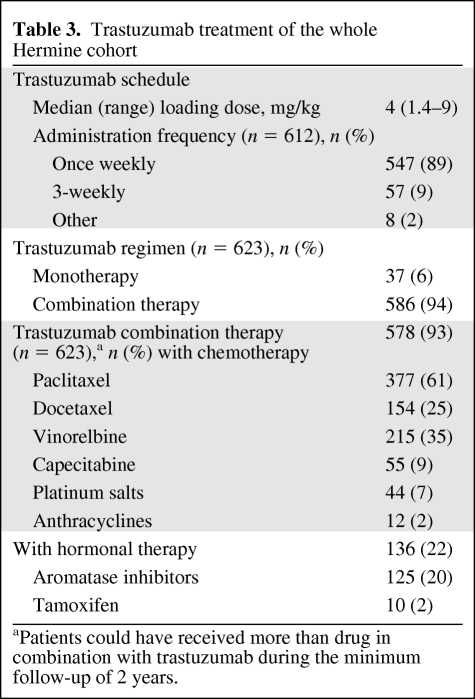
Most patients (89%) received once-weekly trastuzumab (Table 2). Trastuzumab was given as part of a combination regimen in 94% of patients, generally with chemotherapy (Table 3). Trastuzumab was combined with hormonal therapy (with or without chemotherapy) in 22% of patients (14.5%, 26.1%, and 23.5% of patients on first-, second-, and third-line treatment, respectively).
Table 3.
Trastuzumab treatment of the whole Hermine cohort
aPatients could have received more than drug in combination with trastuzumab during the minimum follow-up of 2 years.
Overall, the median treatment duration with trastuzumab was 13.3 months (range, 0–37.7 months). The median treatment durations in patients who initially received trastuzumab first line, second line, and third line or beyond were 16.1 months, 15.8 months, and 8.9 months, respectively.
Trastuzumab was temporarily discontinued on 411 occasions in 284 (46.1%) of the 616 patients for whom these data were recorded, for a median duration of 25 days (range, 8–644 days). After a median follow-up of 22.9 months (range, 0.1–37.7 months), 478 patients had definitively stopped trastuzumab, a rate of 75% at 2 years. Reasons for stopping trastuzumab within 1 year of initiation (n = 369) included clinical progression (n = 212), physician decision (n = 70), cardiac event (n = 37), and/or patient request (n = 13).
Efficacy
TTP
During follow-up, 545 progression events were recorded. Documented sites of first progression after initiation of trastuzumab in these patients were liver (33%), central nervous system (CNS; 28%), bone (25%), lung (17%), local-regional (9%), and/or pleura (6%).
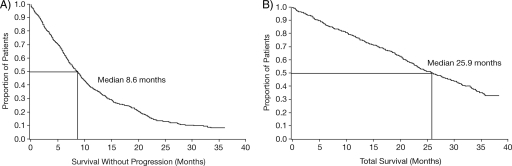
The median TTP was 8.6 months (95% CI, 7.6–9.3 months) (Fig. 2A) for the overall population and 10.3 months, 9.0 months, and 6.3 months, respectively, in the subgroups initially treated with trastuzumab first line, second line, and third line or beyond.
Figure 2.
Kaplan–Meier curve of time to progression (A) and overall survival (B) in the overall study population. Date of death was not recorded in five patients.
OS
There were 336 deaths, with a median OS time of 25.9 months (95% CI, 23.4–28.9 months) (Fig. 2B). The 2-year OS estimates varied with line of initial trastuzumab treatment: 58%, 55%, and 48% for first, second, and third line or beyond, respectively. The corresponding median OS times were 30.3 months, 27.1 months, and 23.2 months, respectively.
In an exploratory analysis, significant independent predictors of OS were: number of metastatic sites at treatment initiation (three or more: HR, 1.49; 95% CI, 1.16–1.91), mode of trastuzumab administration (combination: HR, 0.34; 95% CI, 0.23–0.51), CNS metastases at trastuzumab initiation (HR, 2.94; 95% CI, 2.02–4.28), visceral non-CNS metastases at trastuzumab initiation (presence of lung/liver metastases: HR, 1.64; 95% CI, 1.26–2.13), disease-free interval from breast cancer diagnosis to appearance of metastasis (>24 months: HR, 0.59; 95% CI, 0.48–0.74), and number of previous treatment lines for MBC (more than one: HR, 1.15; 95% CI, 1.01–1.31).
TBP Subanalysis
The first-line median TTP was longer for patients treated with trastuzumab after progression (10.2 months; 95% CI, 9.1–11.5 months) than for patients whose treatment was withdrawn at progression (7.1 months; 95% CI, 6.1, 7.9 months) (log-rank test p = .0215). Site of first progression in patients treated with first-line trastuzumab included the liver (34%), CNS (34%), bone (24%), and lungs (17%).
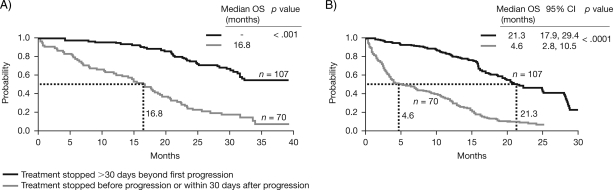
When analyzed from the initiation of trastuzumab, the OS time was significantly longer in patients who continued to receive trastuzumab treatment after progression than in patients whose treatment was withdrawn at progression (p < .001) (Fig. 3A), with a median OS time for patients who continued trastuzumab not reached after 27.8 months' follow-up and a median OS time of 16.8 months for patients who stopped trastuzumab at progression. Likewise, patients who continued trastuzumab after progression had a significantly longer median OS time from date of first progression (21.3 months) than patients whose trastuzumab treatment was stopped at progression (4.6 months) (p < .0001) (Fig. 3B).
Figure 3.
Kaplan–Meier plot comparing OS data from patients who continued trastuzumab beyond progression with that of patients who stopped trastuzumab treatment at or prior to progression from initiation of trastuzumab (A) and date of progression (B).
Abbreviations: CI, confidence interval; OS, overall survival.
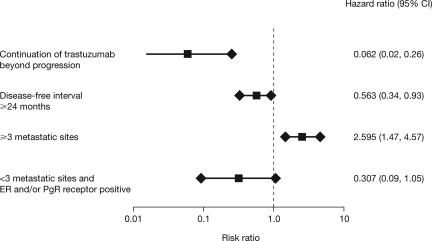
Exploratory subgroup analyses showed that continuing trastuzumab TBP was an independent prognostic factor for OS after first-line trastuzumab in MBC (Fig. 4).
Figure 4.
Independent prognostic survival factors for first-line trastuzumab in metastatic breast cancer.
Abbreviations: CI, confidence interval; ER, estrogen receptor; PgR, progesterone receptor.
Cardiac Safety
During the first year of follow-up, 468 patients (75%) underwent at least one LVEF assessment. Of these, 23% had an abnormal LVEF, with 4% having n LVEF <40%. At 1–2 years of follow-up, 192 patients (31%) had an LVEF assessment. The median LVEF in those patients was 62% (range, 26%–82%), with 12% having an abnormal value.
Fifty-four patients (9%) experienced at least one symptomatic cardiac adverse event (range, 1–3) during follow-up, 39 (6%) during trastuzumab treatment. Of these patients, 49 had previously received treatment with anthracyclines, 17 had a history of cardiac problems, and 12 had cardiovascular risk factors (hypertension, tobacco use, family history, diabetes, and hyperlipidemia). Of the 65 recorded events, the most common were heart failure (n = 16) and tachycardia/other arrhythmia (n = 11). New York Heart Association (NYHA) classification of heart failure was recorded for seven patients: I, n = 2; II, n = 3; and III, n = 2. Overall, 45 of the symptomatic events required medical treatment and 16 required hospitalization. The cardiac events resolved in 26 cases, improved in 21 cases, and were unchanged/outcome unrecorded in the remaining 18 cases. No cardiac-related deaths occurred.
TBP Subanalysis
Prior to starting trastuzumab treatment, LVEF measurements were collected in 96 patients (54%) in this subgroup, with a median value of 65% (range, 23%–81%). Six patients (7%) had an LVEF <50% and, of these, five had an LVEF <40%.
During the first year of follow-up, LVEF measurements were collected from 136 patients in this subgroup. LVEF was <50% in 12.7% of patients receiving trastuzumab after disease progression and in 24.4% of those who stopped trastuzumab after disease progression. The corresponding incidences of LVEF decline to <40% were 2.6% and 7.3%. An absolute decline in LVEF ≥15% was recorded in 20.5% and 21.7% of patients, respectively.
One patient from each of the two treatment groups developed congestive heart failure.
Discussion
The Hermine study was designed to evaluate use, efficacy, and safety outcomes with trastuzumab under noncontrolled, “real life” conditions with an unselected population of patients with HER-2+ MBC. In this pharmacoepidemiologic cohort of 623 patients initially treated with trastuzumab first line, second line, and third line or beyond, the median TTP was 8.6 months and median OS duration was 25.9 months. Results for TTP and OS seem to show clinical benefit irrespective of initial line of treatment, although the greatest benefits occurred in patients treated first line.
In those patients treated first line for MBC, the TTP was 10.3 months and the OS time was 30.3 months, outcomes that are at least comparable with those in the pivotal trials of first-line trastuzumab plus a taxane for HER-2+ MBC (TTP, 7.1 months and 11.7 months, respectively; OS, 25.0 months and 31.2 months, respectively) [4, 9]. Thus, the clinical benefits achieved with first-line trastuzumab for HER-2+ MBC in the controlled trial setting appear to be achieved in daily clinical practice.
The 2-year follow-up of patients who received first-line trastuzumab in routine practice allowed investigation of the benefit of continuing trastuzumab beyond disease progression. Those who continued with trastuzumab TBP had a significantly longer median OS time from the date of that progression than those who stopped trastuzumab at progression (21.3 months versus 4.6 months; p < .001). A significant difference in terms of OS was also apparent from the date of initiation of trastuzumab therapy. Despite the imbalance in prognostic factors between the two groups, an exploratory subgroup analysis identified continuing trastuzumab TBP as an independent prognostic survival factor after first-line trastuzumab treatment for MBC, suggesting that continuing trastuzumab TBP provides significant clinical benefit.
OS benefits were also associated with continuing trastuzumab TBP in Hermine patients who had initially received trastuzumab as second-line therapy for MBC (n = 121) [10].
The findings of the TBP subanalysis reflect those of previous smaller studies and are supported by studies in HER-2+ human xenograft models [11]. Numerous comparative and noncomparative studies have reported tumor response rates of 11%–50% to a second trastuzumab regimen, indicating that HER-2+ breast cancer is not refractory to further trastuzumab therapy after first progression [12–18]. In retrospective case analyses, a median TTP after a second trastuzumab-based regimen of 5–8 months has been reported [12–15, 19, 20]. In one case series [17], the OS time from the date of diagnosis of MBC was 62.4 months in patients who received at least two trastuzumab-based regimens (n = 23), compared with 38.5 months in those who received one regimen only (n = 113) (p = .01). In contrast, no difference in OS (calculated from the date of trastuzumab initiation) was documented in an Italian case series in which 40 patients had received trastuzumab TBP and 71 patients had stopped trastuzumab at progression (median, 30.1 months and 30.2 months, respectively) [15]. However, in our study, an imbalance was reported in the chemotherapy/hormonal therapy regimens between the two treatment groups.
Results from a phase III randomized study (German Breast Group [GBG]-26) comparing trastuzumab and capecitabine with capecitabine alone in 156 patients who had progressed during trastuzumab therapy also support our findings that trastuzumab is effective when continued as TBP [21]. Treatment with trastuzumab plus capecitabine doubled the overall response rate (48.1% versus 27.0%) and TTP (8.2 months versus 5.6 months; p = .034) compared with that seen with capecitabine alone, without any unexpected toxicity [21].
At the time of the Hermine study, there were no ongoing trials of lapatinib in France. However, second-line treatment with other HER-2–targeting therapies for advanced breast cancer has now been investigated in patients who progressed on first-line trastuzumab. In a phase III, randomized, open-label study, patients with HER-2+ locally advanced breast cancer or MBC, previously treated with an anthracycline, a taxane, and trastuzumab in the metastatic setting, received lapatinib plus capecitabine or capecitabine alone [22]. The primary endpoint of TTP was shorter with both lapatinib plus capecitabine (6.2 months) and capecitabine alone (4.3 months) than was observed in the Hermine study (10.2 months for patients receiving trastuzumab after progression and 7.1 months for patients who stopped trastuzumab at progression), but a cross-study comparison is not appropriate because of methodologic differences. The availability of lapatinib raises questions about the most suitable agent to maintain HER-2 suppression following disease progression, and this should be investigated in a randomized study. Preclinical evidence demonstrates that trastuzumab retains antitumor activity beyond progression as a result of continued suppression of HER-2 and activation of the immune system by antibody-dependent cellular cytotoxicity [23–25]. In addition, recent clinical trials have also demonstrated the benefit of continuing trastuzumab beyond disease progression [21, 26].
Differing protocols with regard to the monitoring, recording, and analysis of cardiac events make cross-study comparisons of cardiac safety problematic. However, the incidence of heart failure (2.6%) for patients enrolled in the Hermine study was similar to that in the pivotal first-line MBC M77001 trial evaluating trastuzumab plus docetaxel, which carefully screened patients for cardiac dysfunction prior to enrollment [4]. The incidence of symptomatic cardiac events in the Hermine study was also much lower than in the H0648g trial [5], despite 84% of patients having previously received anthracyclines. Furthermore, the majority of patients with symptomatic cardiac events subsequently recovered or improved. In another retrospective analysis, of patients who received trastuzumab for HER-2+ MBC (n = 173; median treatment duration, 21.3 months; range, 11.6–77.6 months), NYHA cardiac dysfunction (class I–IV) was reported in 31% of patients, compared with only 1.1% of patients in the Hermine study (class I–III; no class IV NYHA cardiac failure was reported) [27]. This difference may be a result of standardized cardiac monitoring, because the study was conducted at only one center, and emphasizes the importance of careful cardiac assessment. LVEF assessments confirmed that trastuzumab was also well tolerated when given beyond progression.
HER-2 overexpression is a recognized risk factor for CNS metastases [28]; however, patients with HER-2+ disease with CNS metastases treated with trastuzumab have a better survival outcome than patients with HER-2− disease and CNS metastases [29, 30]. Emerging evidence suggests that patients with CNS metastases should continue to receive trastuzumab along with established therapies for the CNS metastases [31].
Pharmacoepidemiologic studies such as the Hermine study have limitations and inherent biases, which were identified before the start of the study. The physician-selection and patient-inclusion criteria were chosen to minimize selection bias. After completion, the study design was rechecked to ensure the study was adequately controlled. Random selection of physicians from a comprehensive database of breast cancer specialists ensured that the oncologists participating in the study were representative of the database and the French oncologist population as a whole, in both geographic distribution and type of establishment. The selection criteria for patients also ensured that the Hermine study accurately reflected the true clinical use of trastuzumab in a heterogeneous population of breast cancer patients across France. Furthermore, patients were selected for inclusion after the date of first treatment. Patients were identified according to pharmacy records rather than by physician recommendation; 93% of eligible patients were actually registered in the study.
In the subanalysis of patients from the Hermine cohort who progressed after receiving first-line trastuzumab, there were some imbalances in terms of disease characteristics between patients who continued trastuzumab TBP and those who did not. Those who continued trastuzumab TBP had a better prognosis at treatment initiation, with the patients having lower Scarff-Bloom-Richardson grading, a longer disease-free interval, fewer visceral metastases, and more adjuvant hormonal therapy. This imbalance in prognosis between the groups is reflected in both the median TTP (10.2 months versus 7.1 months) and the median duration of treatment prior to disease progression (10.1 months versus 6.1 months). The TTP results in the Hermine study suggest that the patients who continued trastuzumab TBP may have belonged to a population with a more favorable prognosis than those who stopped trastuzumab at progression. However, it should be noted that the randomized phase III study GBG-26 showed similar TTP results for continued trastuzumab TBP [21].
Conclusions
In this large, observational study, the median trastuzumab treatment duration was 13.3 months. Trastuzumab combined with various chemotherapy and/or hormonal therapy options for MBC was associated with a TTP approaching 9 months and an OS time >2 years, despite >60% of the cohort receiving trastuzumab initially as second-line therapy or beyond.
The subanalysis of patients who progressed after first-line trastuzumab suggests that continuing trastuzumab TBP offers clinical benefit to patients with HER-2+ MBC treated initially with first-line trastuzumab. When calculated from the date of treatment initiation, the median OS time had not been reached after a follow-up of 27.8 months in patients who continued trastuzumab beyond first progression, compared with a median OS time of 16.8 months in patients who stopped trastuzumab at disease progression. A significantly longer median OS time (21.3 months) was also reached in those patients, compared with patients who stopped trastuzumab at disease progression (4.6 months), when assessed from the date of first progression.
Acknowledgments
The authors gratefully acknowledge all the investigators who participated in this study (listed in Appendix 1), Bérangère Vasseur (Roche) for scientific expertise, David Pau (Roche) for data management, Claire Cropet (Mapi-Naxis) for statistical analyses, and Katherine Wilson (Complete HealthVizion) for editorial assistance in preparing this manuscript.
This study was sponsored by Roche France. All patients were treated according to the physicians' decisions. As the sponsor, Roche France was involved in all steps of the study. The scientific committee reviewed all stages of the study from conception to data collection and analysis.
Appendix 1: Participating Investigators
Dr. Achour (St. Nazaire), Dr. Altwegg (Dijon), Dr. Amlaric (Marseille), Dr. Angellier (Le Coudray), Dr. Ardisson (Lyon), Dr. Arsène (Blois), Dr. Aubert (St. Quentin), Dr. Audhuy (Colmar), Dr. Avenin (Paris), Dr. Barbet (Mâcon), Dr. Barthier (Argenteuil), Dr. Bassot (Mainvilliers), Dr. Baticle (Montpellier), Dr. Berdah (Hyères), Dr. Besson (Quimper), Dr. Beuzboc (Paris), Dr. Bieber (Strasbourg), Professor Blay (Lyon), Dr. Boaziz (Ermont), Dr. Bouillet (Paris), Dr. Bourgeois (Poitiers), Professor Bougnoux (Tours), Dr. Bressac (Marseille), Dr. Cailleux (Tours), Dr. Cals (Toulon), Dr. Catimel (Annecy), Dr. Chaigneau (Besançon), Dr. Chevelle (Toulouse), Professor Chollet (Clermont-Ferrand), Dr. Combes (Le Mans), Dr. Coscas (Boulogne), Dr. Couderc (Tarbes), Dr. Coudert (Dijon), Dr. Cretin (Alès), Dr. Dalenc (Toulouse), Dr. Dalivoust (Aubagne), Dr. Dauba (Mont de Marsan), Dr. De Rauglaudre (Avignon), Dr. Debled (Rouen), Dr. Delaloge (Villejuif), Dr. Delcambre (Caen), Dr. Delecroix (St. Nazaire), Dr. Delozier (Caen), Dr. Dides (Mougins), Dr. Dohollou (Bordeaux), Dr. Dourthe (Metz), Dr. Dutel (Beauvais), Dr. Duvert (Lons-le-Saunier), Dr. Edel (Mulhouse), Dr. Eichler (Strasbourg), Dr. El Kouri (Nantes), Dr. Espie (Paris), Dr. Eychenne (Troyes), Dr. Favre (Orleans), Dr. Ferrero (Nice), Dr. Garnier (Grenoble), Dr. Garnier (Monaco), Dr. Geay (Paris), Dr. Gilly (Toulon), Dr. Gladieff (Toulouse), Dr. Goldschmidt (Villejuif), Dr. Gomez (Rouen), Dr. Gomez Abuin (Villejuif), Dr. Goubely (Avignon), Dr. Hardy Bessard (St Brieuc), Dr. Hocini (Paris), Dr. Honnadel (Beuvry), Dr. Jacquot (Montpellier), Professor Jasmin (Villejuif), Dr. Jaubert (Bordeaux), Dr. Kaluzinsky (Cherbourg), Dr. Kamonier (Trappes), Dr. Laplaige (Blois), Dr. Largillier (Nice), Dr. Lejeune (Marseille), Dr. Lesbats (St. Laurent du Var), Dr. Levy (Paris), Dr. Lotz (Paris), Dr. Martin (Lyon), Dr. Martinez (Toulouse), Professor Marty (Villejuif), Dr. Mille (St. Etienne), Dr. Monnier (Montbelliard), Dr. Montastruc (Muret), Dr. Montcuquet (Besançon), Dr. Moran (Harfleur), Dr. Moullet (Lyon), Professor Mousseau (La Tronche), Dr. Naman (Mougins), Dr. Netter (Meaux), Dr. Nicoara (Marseille), Dr. Noirclerc (Mulhouse), Dr. Nouyrigat (La Seyne-sur-Mer), Dr. Orfeuvre (Bourg en Bresse), Dr. Otmezguine (Boulogne), Dr. Palangie (Paris), Dr. Panis (Amiens), Dr. Paraiso (Mainvilliers), Dr. Piprot (Amiens), Professor Pivot (Besançon), Dr. Provencal (Annecy), Dr. Pujade Lauraine (Paris), Dr. Regal (Montpellier), Dr. Remy (Bayonne), Dr. Reyes (Toulouse), Dr. Romieu (Montpellier), Dr. Ronchin (Mougins), Dr. Rosset Bons (Nimes), Dr. Rotarski (Bayonne), Dr. Sillet Bach (Brive), Dr. Simon (Brest), Dr. Stein (Dole), Dr. Teissier (Mougins), Dr. Tigaud (Lyon), Dr. Topard (Nimes), Professor Tubiana (Limoges), Dr. Valenza (Draguignan), Dr. Vanlemmens (Lille), Dr. Vannetzel (Neuilly-sur-Seine), Dr. Vanoli (St Rémy de Provence), Dr. Veyret (Rouen), Dr. Vuillemin (Vannes), Dr. Walter (Metz), Dr. Wendling (Hyères), Dr. Yakendji (Créteil).
Author Contributions
Conception/Design: Moïse Namer, Eric C. Antoine, Anne Vincent-Salomon, Loïc Bergougnoux, Frank Campana, Jean-Marc Extra
Financial support: Loïc Bergougnoux, Frank Campana
Administrative support: Loïc Bergougnoux, Frank Campana, Céline Remblier
Provision of study material or patients: Thierry Delozier, Pierre Kerbrat, Anne Bethune-Volters, Jean-Paul Guastalla, Marc Spielmann, Louis Mauriac, Jean-Louis Misset, Daniel Serin, Mario Campone, Christophe Hebert
Collection and/or assembly of data: Loïc Bergougnoux, Frank Campana, Christophe Hebert, Céline Remblier
Data analysis and interpretation: Moïse Namer, Eric C. Antoine, Anne Vincent-Salomon, Loïc Bergougnoux, Frank Campana, Jean-Marc Extra
Manuscript writing: Loïc Bergougnoux, Frank Campana, Christophe Hebert
Final approval of manuscript: Moïse Namer, Eric C. Antoine, Anne Vincent-Salomon, Thierry Delozier, Pierre Kerbrat, Anne Bethune-Volters, Jean-Paul Guastalla, Marc Spielmann, Louis Mauriac, Jean-Louis Misset, Daniel Serin, Mario Campone, Christophe Hebert, Jean-Marc Extra
Other: Céline Remblier, study logistics.
References
- 1.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 2.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 3.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 4.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.Goldhirsch A, Coates AS, Gelber RD, et al. First—select the target: Better choice of adjuvant treatments for breast cancer patients. Ann Oncol. 2006;17:1772–1776. doi: 10.1093/annonc/mdl398. [DOI] [PubMed] [Google Scholar]
- 7.Cook-Bruns N. Retrospective analysis of the safety of Herceptin® immunotherapy in metastatic breast cancer. Oncology. 2001;61(suppl 2):58–66. doi: 10.1159/000055403. [DOI] [PubMed] [Google Scholar]
- 8.Suter TM, Cook-Bruns N, Barton C. Cardiotoxicity associated with trastuzumab (Herceptin®) therapy in the treatment of metastatic breast cancer. Breast. 2004;13:173–183. doi: 10.1016/j.breast.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Smith IE. Efficacy and safety of Herceptin® in women with metastatic breast cancer: Results from pivotal clinical studies. Anticancer Drugs. 2001;12(suppl 4):S3–S10. doi: 10.1097/00001813-200112004-00002. [DOI] [PubMed] [Google Scholar]
- 10.Antoine EC, Extra JM, Vincent-Salomon A, et al. Multiple lines of trastuzumab provide a survival benefit for women with metastatic breast cancer: Results from the Hermine cohort study [abstract 2099] Eur J Cancer. 2007;43(suppl 5):213. [Google Scholar]
- 11.Pietras RJ, Pegram MD, Finn RS, et al. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene. 1998;17:2235–2249. doi: 10.1038/sj.onc.1202132. [DOI] [PubMed] [Google Scholar]
- 12.Bartsch R, Wenzel C, Hussian D, et al. Analysis of trastuzumab and chemotherapy in advanced breast cancer after the failure of at least one earlier combination: An observational study. BMC Cancer. 2006;6:63. doi: 10.1186/1471-2407-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fountzilas G, Razis E, Tsavdaridis D, et al. Continuation of trastuzumab beyond disease progression is feasible and safe in patients with metastatic breast cancer: A retrospective analysis of 80 cases by the Hellenic Cooperative Oncology Group. Clin Breast Cancer. 2003;4:120–125. doi: 10.3816/cbc.2003.n.017. [DOI] [PubMed] [Google Scholar]
- 14.Gelmon KA, Mackey J, Verma S, et al. Use of trastuzumab beyond disease progression: Observations from a retrospective review of case histories. Clin Breast Cancer. 2004;5:52–58. doi: 10.3816/cbc.2004.n.010. discussion 59–62. [DOI] [PubMed] [Google Scholar]
- 15.Montemurro F, Donadio M, Clavarezza M, et al. Outcome of patients with HER2-positive advanced breast cancer progressing during trastuzumab-based therapy. The Oncologist. 2006;11:318–324. doi: 10.1634/theoncologist.11-4-318. [DOI] [PubMed] [Google Scholar]
- 16.Morabito A, Longo R, Gattuso D, et al. Trastuzumab in combination with gemcitabine and vinorelbine as second-line therapy for HER-2/neu overexpressing metastatic breast cancer. Oncol Rep. 2006;16:393–398. [PubMed] [Google Scholar]
- 17.Stemmler HJ, Kahlert S, Siekiera W, et al. Prolonged survival of patients receiving trastuzumab beyond disease progression for HER2 overexpressing metastatic breast cancer (MBC) Onkologie. 2005;28:582–586. doi: 10.1159/000088296. [DOI] [PubMed] [Google Scholar]
- 18.Tripathy D, Slamon DJ, Cobleigh M, et al. Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol. 2004;22:1063–1070. doi: 10.1200/JCO.2004.06.557. [DOI] [PubMed] [Google Scholar]
- 19.Bartsch R, Wenzel C, Altorjai G, et al. Capecitabine and trastuzumab in heavily pretreated metastatic breast cancer. J Clin Oncol. 2007;25:3853–3858. doi: 10.1200/JCO.2007.11.9776. [DOI] [PubMed] [Google Scholar]
- 20.García-Sáenz JA, Martín M, Puente J, et al. Trastuzumab associated with successive cytotoxic therapies beyond disease progression in metastatic breast cancer. Clin Breast Cancer. 2005;6:325–329. doi: 10.3816/CBC.2005.n.035. [DOI] [PubMed] [Google Scholar]
- 21.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: A German Breast Group 26/Breast International Group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 22.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 23.Shirane M, Fujimoto-Ouchi K, Sekiguchi F, et al. Preclinical study of continuous administration of trastuzumab as combination therapy after disease progression with trastuzumab monotherapy [abstract 411] Eur J Cancer. 2005;3(suppl):115. doi: 10.1007/s00280-009-1160-0. [DOI] [PubMed] [Google Scholar]
- 24.Scaltriti M, Verma C, Guzman M, et al. Lapatinib induces HER2 stabilization and enhances the antitumour activity of trastuzumab in vivo [abstract 2559]. Presented at the American Association of Cancer Research Annual Meeting; April 12–14; San Diego, California. 2008. [Google Scholar]
- 25.Friess T, Scheuer W, Hasmann M. Combinations of trastuzumab with either pertuzumab or bevacizumab show higher efficacy than lapatinib-based combinations against HER2-positive breast cancer xenografts progressing on trastuzumab monotherapy [poster B102]. Presented at the American Association of Cancer Research–National Cancer Institute–European Organization for Research and Treatment of Cancer International Conference: Molecular Targets and Cancer Therapeutics; October 22–26; San Francisco, California. 2007. [Google Scholar]
- 26.O'Shaughnessy J, Blackwell KL, Burstein H, et al. A randomized study of lapatinib alone or in combination with trastuzumab in heavily pretreated HER2+ metastatic breast cancer progressing on trastuzumab therapy [abstract 1015] J Clin Oncol. 2008;26(15 suppl):44s. [Google Scholar]
- 27.Guarneri V, Lenihan DJ, Valero V, et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: The M.D. Anderson Cancer Center experience. J Clin Oncol. 2006;24:4107–4115. doi: 10.1200/JCO.2005.04.9551. [DOI] [PubMed] [Google Scholar]
- 28.Pestalozzi BC, Zahrieh D, Price KN, et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17:935–944. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 29.Kirsch DG, Ledezma CJ, Mathews CS, et al. Survival after brain metastases from breast cancer in the trastuzumab era [letter] J Clin Oncol. 2005;23(suppl 1):2114–2116. doi: 10.1200/JCO.2005.05.249. author reply 2116–2117. [DOI] [PubMed] [Google Scholar]
- 30.Sawrie SM, Meredith RF, Spencer SA, et al. Her2-neu status as a predictor of survival in patients with brain metastases from primary breast adenocarcinoma. Poster 1016 presented at the 43rd ASCO Annual Meeting; June 1–5; Chicago, Illinois, USA. 2007. [Google Scholar]
- 31.Bartsch R, Rottenfusser A, Wenzel C, et al. Trastuzumab prolongs overall survival in patients with brain metastases from Her2 positive breast cancer. J Neurooncol. 2007;85:311–317. doi: 10.1007/s11060-007-9420-5. [DOI] [PubMed] [Google Scholar]