Reported are the results of an open-label, randomized, prospective trial aimed at testing the efficacy and safety of treatment with oral lactoferrin versus i.v. iron, both combined with recombinant human erythropoietin, for the treatment of anemia in a population of advanced cancer patients undergoing chemotherapy.
Keywords: Cancer-related anemia, Erythropoietin, Iron, Lactoferrin, Advanced cancer patients, Cachexia
Abstract
Advanced-stage cancer patients often suffer from anemia that closely resembles the anemia of chronic inflammatory diseases characterized by specific changes in iron homeostasis and absorption. i.v. iron improves the efficacy of recombinant human erythropoietin (rHuEPO) in anemic cancer patients undergoing chemotherapy. We report the results of an open-label, randomized, prospective trial aimed at testing the efficacy and safety of treatment with oral lactoferrin versus i.v. iron, both combined with rHuEPO, for the treatment of anemia in a population of 148 advanced cancer patients undergoing chemotherapy. All patients received s.c. rHuEPO-β, 30,000 UI once weekly for 12 weeks, and were randomly assigned to ferric gluconate (125 mg i.v. weekly) or lactoferrin (200 mg/day). Both arms showed a significant hemoglobin increase. No difference in the mean hemoglobin increase or the hematopoietic response, time to hematopoietic response, or mean change in serum iron, C-reactive protein, or erythrocyte sedimentation rate were observed between arms. In contrast, ferritin decreased in the lactoferrin arm whereas it increased in the i.v. iron arm. In conclusion, these results show similar efficacy for oral lactoferrin and for i.v. iron, combined with rHuEPO, for the treatment of anemia in advanced cancer patients undergoing chemotherapy.
Introduction
Cancer patients undergoing chemotherapy, and especially those with advanced-stage disease, often suffer from anemia [1]. In fact, chemotherapy-induced anemia is often associated with the anemia typical of advanced-stage disease, and in this case, antiblastic treatments can waken dormant anemia or worsen it [2]. Therefore, there is a substantial difference between patients with early-stage disease and patients with advanced-stage disease. Indeed, if, during adjuvant chemotherapy, anemia should be seen as a treatment side effect occurring in a “healthy individual,” the anemia that accompanies antiblastic therapy in advanced cancer patients should be considered part of a more complex syndrome of metabolic disorders that are able, by themselves, to induce anemia [3]. The etiology of this particular form of anemia, called “anemia of cancer,” is considered multifactorial, and the triggering factors include: the release of iron-binding protein from macrophages in response to inflammation, the production of myelosuppressive factors by the tumor and/or activated immune cells, and poor nutritional status [4]. Furthermore, circulating levels of erythropoietin (EPO) are significantly lower in anemic cancer patients than in individuals with a similar degree of anemia resulting from isolated iron deficiency [5, 6].
The incidence of anemia during antiblastic treatment varies, and it may occur in up to 75% of patients [2]. Predictors of anemia are administration of regimens based on platinum and anthracyclines, advanced-stage disease, and the hemoglobin (Hb) level before treatment. Thus, in the majority of advanced cancer patients, chemotherapy-induced anemia is associated with, or may waken, dormant anemia, the incidence and severity of which correlate with stage of disease and prognosis [7]. This type of anemia occurs in the absence of bleeding, hemolysis, cancer infiltration of bone marrow, and renal and/or liver failure. It is caused by the chronic inflammatory state and oxidative stress resulting from the action of proinflammatory cytokines released by the activated immune system and the tumor itself [3].
Several pieces of evidence attribute a central role to inflammatory mediators in the etiopathogenesis of cancer anemia [8, 9]. Indeed, cytokines induce changes in iron balance, proliferation of erythroid progenitors, EPO production, survival of circulating erythrocytes, and alterations in energy metabolism per se, each capable of inducing anemia. Patients with cancer anemia may have low or normal serum iron levels, but their ferritin levels are increased and the bone marrow is rich in iron. Therefore, it has been suggested that there is a possible flaw in iron use, rather than a shortage of iron, a condition called “functional iron deficiency.” Changes in iron homeostasis cause a shift in iron from the circulation to deposits and limited availability to erythroid progenitors, thereby reducing erythropoiesis. In chronic inflammation, iron entry into macrophages and reticuloendothelial cells is increased primarily through erythrophagocytosis. Moreover, proinflammatory stimuli, by reducing ferroportin expression, block iron excretion, thus increasing its accumulation [10].
More recently, the identification of hepcidin has enabled a better understanding of the relationship among the immune system, iron homeostasis, and anemia of chronic inflammatory diseases [11]. Hepcidin, whose synthesis by the liver is strongly induced by interleukin 6, is specifically involved in the diversion of iron traffic through duodenal absorption and blocks its release from macrophages [12].
In cancer patients, mainly with advanced-stage disease, these observations are probably the most plausible explanation for the better results obtained with erythropoiesis-stimulating agents (ESAs) plus i.v. iron than with ESAs alone or with ESAs plus oral iron in the treatment of chemotherapy-induced anemia [13].
Recent observations show a growing interest in lactoferrin, a specific protein involved in iron transport mechanisms, for the treatment of particular forms of iron-related anemia. Lactoferrin is an 80-kDa iron-binding protein of the transferrin family, abundantly expressed in most biological fluids, which plays an important role in host defense against infection and excessive inflammation [14, 15].
We present the results of an open-label, randomized, prospective trial comparing the efficacy and safety of oral lactoferrin with those of i.v iron supplementation combined with recombinant human EPO (rHuEPO) therapy in a population of previously untreated advanced cancer patients with anemia undergoing chemotherapy.
Patients and Methods
Study Design
This was an open-label, randomized, controlled, prospective study comparing the efficacy and safety of rHuEPO combined with oral lactoferrin (Lattoglobina®; Grunenthal-Formenti, Milan, Italy) or i.v. iron supplementation in advanced cancer patients with anemia undergoing chemotherapy. The protocol was approved by the institutional ethics committee. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
Patients
Patients were recruited from January to August 2009 at the Department of Medical Oncology, Sirai Hospital, Carbonia, Italy, the Medical Oncology Unit, “N.S. Bonaria” Hospital, San Gavino, Italy, and the Department of Medical Oncology, University of Cagliari, Cagliari, Italy.
Patients were eligible for the treatment protocol if they were ≥18 years old, had a histological diagnosis of a solid tumor at an advanced disease stage (stage III–IV), had an Hb level ≤10 g/dl (in accordance with the latest international guidelines for the ESA treatment of anemia in cancer patients) [16], had an Eastern Cooperative Oncology Group performance status (ECOG PS) score ≤2, had no previous treatment, and were receiving first-line chemotherapy while on study. Patients were also required to have a serum ferritin level ≥100 ng/ml and ≤800 mg/dl and/or a transferrin saturation >15%, a life expectancy of ≥6 months, and adequate renal and hepatic function. Patients with anemia attributable to factors other than cancer and chemotherapy (i.e., B12 or folate deficiency, hemolysis, gastrointestinal bleeding, myelodysplastic syndrome, or bone marrow metastases) were not eligible. Other exclusion criteria included a prior transfusion, ESA or i.v. iron therapy within 4 weeks of enrolment, am allergy or intolerance to iron and/or rHuEPO, active infection, absolute iron deficiency, pregnancy, breastfeeding, inadequate birth control measures, a history of seizure disorders, active cardiac disease, thromboembolic disease, and uncontrolled hypertension.
Protocol
This was a prospective, open-label, randomized, controlled study. Written informed consent was provided by all patients. Clinical assessment included patient characteristics, tumor site, tumor stage, ECOG PS score, current chemotherapy regimen, physical examination, and vital signs. Laboratory tests included measurements of the blood cell count, Hb, reticulocyte count, serum iron, serum ferritin, transferrin saturation, C-reactive protein (CRP), and the erythrocyte sedimentation rate (ESR), as well as a chemical profile. These parameters were assessed at baseline before starting rHuEPO treatment as well as at diagnosis before starting chemotherapy.
Patients eligible for the treatment protocol were then randomized in a 1:1 ratio to either (a) 125 mg of ferric gluconate i.v. once weekly or (b) two tables of lactoferrin daily (i.e., 200 mg/day). Oral lactoferrin was dispensed weekly, with adherence monitored via tablet count.
All patients received rHuEPO-β, 30,000 UI s.c. weekly. rHuEPO treatment was continued for 12 weeks or until achievement of an Hb level >12 g/dl. RHuEPO dose escalation or reduction was not permitted to avoid confounding the iron response data.
All patients received an adequate dose of low molecular weight heparin s.c. during rHuEPO treatment because they were affected by advanced cancer and related chronic inflammation, which are well recognized procoagulant conditions. Patients were excluded (or treatment was interrupted) if they had a high risk for bleeding (international normalized ratio >1.3 or platelet count <150 × 109/l).
Patients were not allowed to take any vitamin, mineral, or herbal supplements containing >27 mg/day iron or >100 mg/day vitamin C during the study. Blood transfusion were allowed at the investigators’ discretion if the Hb level decreased to <8 g/dl. Changes to the chemotherapy plan were allowed.
Study Endpoints
The primary efficacy variable was defined as the change in Hb from baseline (i.e., before starting rHuEPO treatment).
Secondary efficacy variables included the hematopoietic response, time to hematopoietic response, time-adjusted area under the Hb–time curve between week 0 and week 12 (Hb area under the curve, AUC0–12), and change from baseline in other laboratory parameters (serum iron, serum ferritin, CRP, and ESR). A hematopoietic response to rHuEPO was defined as an increase in Hb of ≥2 g/dl (Hb response) or achievement of the target Hb level of ≥12 g/dl (Hb correction) without transfusion use at any time point during the study. The Hb AUC was recently considered to be a clinically meaningful alternative measure to assess the overall efficacy of ESAs [17]. Efficacy variables were measured at baseline and weekly throughout treatment.
Safety
The safety profile of treatment was evaluated by weekly monitoring of the incidence of adverse events, changes from starting levels in the serum analysis and chemistry profile, and vital signs. Adverse events were classified in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0. The nature, frequency, and severity of all adverse events and their relationship to treatment were assessed.
Statistical Analysis
Results form previous clinical trials were used to determine the sample size, based on a two-sided t-test to detect noninferiority in Hb change from baseline between the lactoferrin and i.v. iron arms. On the basis of previous clinical investigations in cancer patients, it was anticipated that the i.v. iron arm would have a 2.5-g/dl greater mean change in Hb from baseline than the no i.v. iron comparator and that the expected standard deviation (SD) would be 1.5. Using these calculations, and considering an α error of 0.01 and a β error of 0.05 (power of 95%), a sample size of 73 patients per arm was needed to detect a mean Hb change ≥2.5 g/dl (assuming an SD of 1.0 g/dl) in each treatment arm. Continuous variables are summarized using means and SDs, and discrete variables are summarized using frequencies and percentages. Distributions of continuous variables were checked for linearity. For quantitative variables, the mean change in Hb (and in secondary endpoints) from baseline to the end of treatment between treatment groups was compared using the Student’s t-test for independent data. Moreover, data on Hb and the iron index and proinflammatory marker profiles over time were analyzed by means of a repeated measures analysis of variance. The hypothesis of no difference in the response rate (i.e., hematopoietic response) between treatment arms was tested by the χ2 statistic. Differences were considered significant if p < .05. All analyses were performed using SPSS, Version 15.0 (SPSS Inc., Chicago, IL).
Results
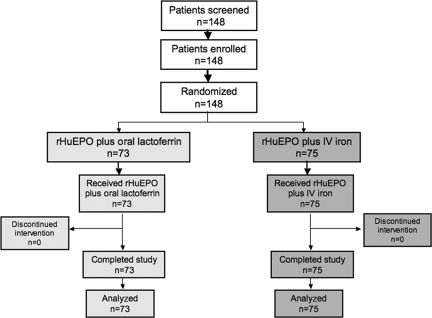
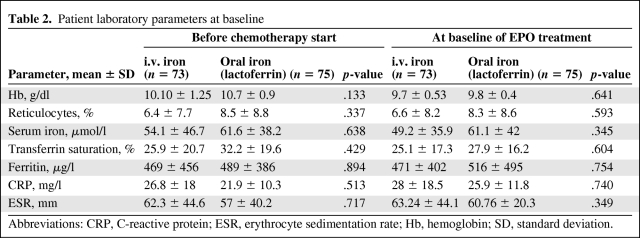
In total, 148 patients were enrolled and underwent random assignment to receive either rHuEPO plus i.v. iron (n = 73) or rHuEPO plus oral lactoferrin (n = 75). All patients were evaluable for efficacy and safety endpoints (Fig. 1) . Patients were well balanced between the two groups in terms of age, sex, tumor site, and tumor stage. Patients’ clinical characteristics are listed in Table 1. Baseline laboratory parameters were also superimposable between arms (Table 2). It is relevant to note that, at diagnosis, patients had a mean Hb level <11 g/dl accompanied by low iron, high ferritin, and high CRP and ESR levels (Table 2), thus defining the picture of “anemia of cancer” [10]. In fact, 80% of patients were already anemic and had started rHuEPO after a mean of one cycle of chemotherapy when their Hb level had fallen to <10 g/dl (threshold for initiating ESA therapy according to international guidelines) [16].
Figure 1.
Consort diagram.
Abbreviation: rHuEPO, recombinant human erythropoietin.
Table 1.
Patient clinical characteristics
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; SD, standard deviation.
Table 2.
Patient laboratory parameters at baseline
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, hemoglobin; SD, standard deviation.
No patient discontinued the study as a result of adverse events, death, protocol violation, or other reasons. No patient was excluded for hypersensitivity to lactoferrin or i.v. iron.
Efficacy Evaluations
Primary Endpoint
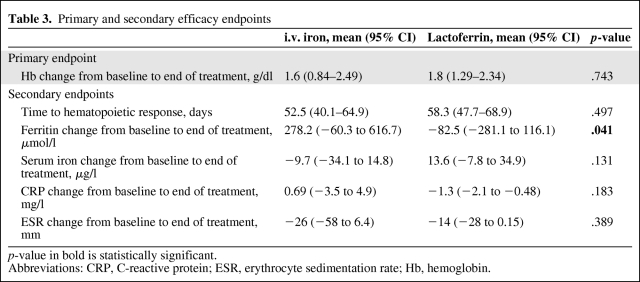
Mean Hb levels before rHuEPO treatment were 9.7 ± 0.5 g/dl in the i.v. iron arm and 9.8 ± 0.4 g/dl in the lactoferrin arm. After rHuEPO treatment, the mean Hb levels were 11.4 ± 1.6 g/dl in the i.v. iron arm and 11.6 ± 1.2 g/dl in the lactoferrin arm (p = 0.036 and p < .001, in comparison with baseline, respectively). The mean Hb change from baseline to the end of treatment was not significantly different between treatment arms (+1.6 ± 1.4 g/dl for i.v. iron versus +1.8 ± 1.2 g/dl for lactoferrin; p = .743) (Table 3). The mean Hb change also was not significantly different between treatment arms after 4 weeks (+0.55 ± 1 g/dl for i.v. iron versus +0.9 ± 0.9 g/dl for lactoferrin; p = 0.300) and after 8 weeks (+1.12 ± 1 g/dl for i.v. iron versus +1.6 ± 0.9 g/dl for lactoferrin; p = .132) of treatment.
Table 3.
Primary and secondary efficacy endpoints
p-value in bold is statistically significant.
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, hemoglobin.
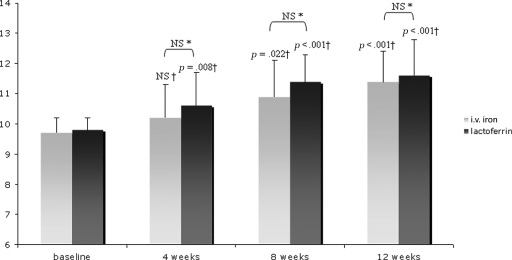
Over time, the Hb level increased significantly in both arms (p = 0.001 in the i.v. iron arm and p < .001 in the lactoferrin arm) (Fig. 2).
Figure 2.
Hemoglobin (Hb) levels from baseline to the end of the study at week 12. Bars represent mean Hb levels. Hb changes (g/dl) from baseline were not significantly different between arms (*p calculated by Student’s t-test for independent data). Hb levels over time increased significantly in both treatment groups after 4, 8, and 12 weeks versus baseline (†p calculated by analysis of variance).
Abbreviation: NS, not significant.
Secondary Endpoints
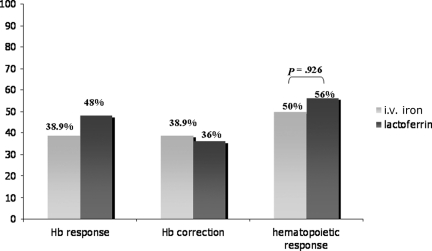
As for the hematopoietic response rate, 50% of patients in the i.v. iron arm and 56% of patients in the lactoferrin arm achieved a hematopoietic response (p = .926 between arms) (Fig. 3). The times to hematopoietic response (mean ± SD) were similar in the two treatment arms: 52.5 ± 17.9 days for i.v. iron arm and 58.3 ± 18.7 days for the lactoferrin arm (p = .497) (Table 3). The time-adjusted Hb AUC0–12 was significantly higher in the lactoferrin arm than in the i.v. iron arm (p = .005; 95% confidence interval [CI], 0.28–1.52).
Figure 3.
Percentages of hemoglobin (Hb) responders in the two treatment arms. Responders were patients who achieved the target Hb level of ≥12 g/dl (Hb correction) or an increase in Hb of ≥2 g/dl (Hb response) without transfusion use at any time point during the study. The p-value was calculated using the χ2 test.
As for the iron indexes, the ferritin change was significantly different between arms (p = .041): patients in the i.v. iron arm achieved a 287.2-μmol/l ferritin increase (95% CI, −60.3 to 616.7 μmol/l) whereas those in the lactoferrin arm had a 82.5-μmol/l decrease (95% CI, −281.1 to 116.1 μmol/l) (Table 3). In contrast, the serum iron change was not significantly different between the two arms (Table 3). Considering the profiles over time, neither ferritin nor serum iron changed significantly during treatment in either arm.
The assessment of proinflammatory parameters showed that the mean changes in the ESR and CRP levels were not significantly different between arms (Table 3). The profile over time demonstrated a decrease in the ESR in the lactoferrin arm after 4, 8, and 12 weeks of treatment (p = .02, p = .004, and p = .05, respectively) as well as a significant decrease in CRP level in the lactoferrin arm after 12 weeks of treatment (p = .028), in comparison with baseline.
Safety
Both i.v. iron and lactoferrin were well tolerated. Most adverse events were deemed by the investigators to be unrelated to i.v. iron or lactoferrin and were attributable to chemotherapy or the underlying malignancy. The most frequently reported adverse events in the i.v. iron and lactoferrin arms were, respectively, grade 1 nausea/vomiting (38.1% versus 35.21%), grade 1 asthenia (41.3% versus 42.6%), grade 2 diarrhea (22.2% versus 21.3%), and grade 2 leukopenia (20.4% versus 19%). No severe side effects (grade 3 or 4 toxicities) related to iron infusion or lactoferrin oral consumption were observed. No cardiovascular and thromboembolic events were observed in either arm.
Discussion
In recent years, an increasing number of articles in the literature have shown that ESAs are more effective when associated with i.v. iron in the treatment of chemotherapy-induced anemia in patients with cancer at different stages [18–21]. The present study, starting from this evidence, assessed the efficacy of rHuEPO-β combined with a compound (lactoferrin) specific for its ability to selectively modulate iron homeostasis in the treatment of anemia in advanced cancer patients undergoing chemotherapy. So far, clinical trials that have assessed the efficacy of ESAs, combined or not with iron, administered orally or i.v., for the treatment of chemotherapy-induced anemia have studied heterogeneous patient samples. Indeed, the majority of published studies included both patients undergoing adjuvant chemotherapy, and therefore with early-stage disease, and advanced patients undergoing several chemotherapy regimens. These studies basically considered anemia as having a single nature, that is, a result of antineoplastic treatment. This assumption may be partially correct for patients undergoing adjuvant chemotherapy. However, the same cannot be said for advanced cancer patients, for whom the tumor and the associated chronic inflammation can, by themselves, induce anemia and thus worsen or make overt the chemotherapy anemia-inducing action. Moreover, it should be considered that all antiblastic treatments can induce symptoms that, by themselves, can lead to anemia. For instance, vomiting and diarrhea are able to induce anemia from lack of iron, folates, and amino acids [22]. Anemia in cancer patients should thus be considered not just as a side effect of antiblastic therapy but also a specific symptom of neoplastic disease, able to affect survival, treatment efficacy, disease progression [7], and, above all, patient quality of life [23]. The response to antiblastic treatment or disease progression can also radically change ESA efficacy [24]. A study by Ray-Coquard et al. [25] on cancer patients undergoing different chemotherapy regimens showed that low baseline Hb levels, a poor PS score, and a reduced absolute lymphocyte count are independent predictive factors of severe anemia. However, studies published so far did not make a distinction between patients undergoing adjuvant chemotherapy and advanced cancer patients, patients with inflammatory state and those without, or (disease) responders and nonresponders, nor did they take into account the incidence of anemia-inducing side effects and levels of Hb at the start of antiblastic treatment.
There is no doubt that, in light of these possible variables, which are likely to characterize, at least in part, the course of neoplastic disease in patients included in the previous studies, the gold standard for the treatment of chemotherapy-induced anemia in patients with cancer at different stages is an ESA plus i.v. iron (which is able to correct every condition that changes normal iron metabolism).
Starting from this premise and to better clarify the role of altered iron metabolism in a selected group of cancer patients undergoing chemotherapy, namely, those with advanced-stage disease with an inflammatory state who were already anemic at the start of the chemotherapy regimen, we compared the efficacy of rHuEPO-β combined with i.v. iron with the efficacy of rHuEPO-β combined with lactoferrin, a specific modulator of iron metabolism also capable of immune-modulating and antioxidant activities. The results of our study also confirm the good efficacy of an ESA plus i.v. iron in the treatment of anemia in this selected group of patients. Surprisingly, the combination of rHuEPO-β plus lactoferrin also showed similar efficacy. Starting from the first week of treatment with rHuEPO-β plus lactoferrin, Hb levels increased rapidly and in a statistically significant way, and at the end of treatment the mean Hb changes were superimposable in the two arms. Moreover, the percentage of responders as well as the time to hematopoietic response were comparable between the two arms and with rates reported in a previous study using an ESA plus i.v. iron [21]. Noteworthy, among the secondary parameters of efficacy, the Hb AUC0–12 was better in the lactoferrin arm than in the i.v. iron arm. One of the most interesting findings is the evidence that, in both arms, serum iron levels were absolutely superimposable throughout treatment. In contrast, the levels of ferritin in the rHuEPO-β plus lactoferrin arm decreased significantly and progressively during therapy. These data seem to confirm the particular lactoferrin capacity to modulate iron homeostasis, and explain the maintenance of physiological levels of iron in the blood and suggest that the iron provided by lactoferrin is likely to be well used in human adults. Indeed, serum ferritin is inversely correlated with iron adsorption, and a beneficial effect of lactoferrin on iron acquisition in the gut is well documented [26].
Although the inflammation parameters assessed (i.e., CRP and ESR) did not show substantial differences at the end of treatment, their immediate decrease in the lactoferrin group should be highlighted. Additionally, rHuEPO-β plus lactoferrin, like ESAs plus i.v. iron, was also shown to be safe and without any side effects.
However, the present study has some limitations. Indeed, it is a noninferiority trial, and therefore the finding that the two interventions are therapeutically equivalent should be considered tentative and needs replication in future large, randomized trials.
The present results, although preliminary and needing future research, introduce an innovative therapeutic approach to anemia in advanced cancer patients undergoing chemotherapy and, importantly, also suggest a novel way to deal with this issue.
Indeed, they underline how selective modulation of iron metabolism, in patients whose iron metabolism is altered for reasons specifically correlated with the development of neoplastic disease, is able to better support the efficacy of ESAs.
Moreover, the use of an orally administered compound gives further unquestionable advantages both in terms of patient compliance, because there is no need for hospitalization, and also in terms of cost savings. Indeed, it is well known that i.v. iron administration, aside from the general anaphylactic risks, also requires specifically trained medical staff and therapeutic support centers.
In our opinion, not less important are the particular pharmacological characteristics of lactoferrin. Apart from having specific and established immune-modulating activities [27, 28], it also has specific antineoplastic actions in vitro and in vivo [29]. The latter should not be underestimated, especially in light of recent reports [30–34] of a possible proneoplastic effect of ESAs, which could thus be partly counteracted or balanced. More research on this topic is warranted.
Acknowledgments
This work was supported by the Associazione Sarda per la ricerca nell’Oncologia Ginecologica-ONLUS.
Author Contributions
Conception/Design: Antonio Macciò, Clelia Madeddu, Giovanni Mantovani
Provision of study material or patients: Antonio Macciò, Giulia Gramignano, Carlo Mulas, Eleonora Sanna
Collection and/or assembly of data: Antonio Macciò, Clelia Madeddu, Giulia Gramignano, Carlo Mulas, Eleonora Sanna
Data analysis and interpretation: Antonio Macciò, Clelia Madeddu, Giulia Gramignano, Carlo Mulas
Manuscript writing: Antonio Macciò, Clelia Madeddu, Giovanni Mantovani
Final approval of manuscript: Antonio Macciò, Clelia Madeddu, Giulia Gramignano, Carlo Mulas, Eleonora Sanna, Giovanni Mantovani
References
- 1.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: A systematic review of the literature. Am J Med. 2004;116(suppl 7A):11S–26S. doi: 10.1016/j.amjmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: Incidence and treatment. J Natl Cancer Inst. 1999;91:1616–1634. doi: 10.1093/jnci/91.19.1616. [DOI] [PubMed] [Google Scholar]
- 3.Macciò A, Madeddu C, Massa D, et al. Hemoglobin levels correlate with interleukin-6 levels in patients with advanced untreated epithelial ovarian cancer: Role of inflammation in cancer-related anemia. Blood. 2005;106:362–367. doi: 10.1182/blood-2005-01-0160. [DOI] [PubMed] [Google Scholar]
- 4.Spivak JL. The anaemia of cancer: Death by a thousand cuts. Nat Rev Cancer. 2005;5:543–555. doi: 10.1038/nrc1648. [DOI] [PubMed] [Google Scholar]
- 5.Miller CB, Jones RJ, Piantadosi S, et al. Decreased erythropoietin response in patients with the anemia of cancer. N Engl J Med. 1990;322:1689–1692. doi: 10.1056/NEJM199006143222401. [DOI] [PubMed] [Google Scholar]
- 6.Means RT, Jr, Krantz SB. Progress in understanding the pathogenesis of the anemia of chronic disease. Blood. 1992;80:1639–1647. [PubMed] [Google Scholar]
- 7.Caro JJ, Salas M, Ward A, et al. Anemia as an independent prognostic factor for survival in patients with cancer: A systemic, quantitative review. Cancer. 2001;91:2214–2221. [PubMed] [Google Scholar]
- 8.Raj DS. Role of interleukin-6 in the anemia of chronic disease. Semin Arthritis Rheum. 2009;38:382–388. doi: 10.1016/j.semarthrit.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Buck I, Morceau F, Grigorakaki C, et al. Linking anemia to inflammation and cancer: The crucial role of TNFα. Biochem Pharmacol. 2009;77:1572–1579. doi: 10.1016/j.bcp.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 11.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 12.Andrews NC. Anemia of inflammation: The cytokine-hepcidin link. J Clin Invest. 2004;113:1251–1253. doi: 10.1172/JCI21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auerbach M, Ballard H, Glaspy J. Clinical update: Intravenous iron for anaemia. Lancet. 2007;369:1502–1504. doi: 10.1016/S0140-6736(07)60689-8. [DOI] [PubMed] [Google Scholar]
- 14.González-Chávez SA, Arévalo-Gallegos S, Rascón-Cruz Q. Lactoferrin: Structure, function and applications. Int J Antimicrob Agents. 2009;33(301):e1–e8. doi: 10.1016/j.ijantimicag.2008.07.020. 301. [DOI] [PubMed] [Google Scholar]
- 15.Baker HM, Baker EN. Lactoferrin and iron: Structural and dynamic aspects of binding and release. Biometals. 2004;17:209–216. doi: 10.1023/b:biom.0000027694.40260.70. [DOI] [PubMed] [Google Scholar]
- 16.Rizzo JD, Somerfield MR, Hagerty KL, et al. American Society of Clinical Oncology; American Society of Hematology. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26:132–149. doi: 10.1200/JCO.2007.14.3396. [DOI] [PubMed] [Google Scholar]
- 17.Duh MS, Lefebvre P, Fastenau J, et al. Assessing the clinical benefits of erythropoietic agents using area under the hemoglobin change curve. The Oncologist. 2005;10:438–448. doi: 10.1634/theoncologist.10-6-438. [DOI] [PubMed] [Google Scholar]
- 18.Auerbach M, Ballard H, Trout JR, et al. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: A multicenter, open-label, randomized trial. J Clin Oncol. 2004;22:1301–1307. doi: 10.1200/JCO.2004.08.119. [DOI] [PubMed] [Google Scholar]
- 19.Henry DH, Dahl NV, Auerbach M, et al. Intravenous ferric gluconate significantly improves response to epoetin alpha versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. The Oncologist. 2007;12:231–242. doi: 10.1634/theoncologist.12-2-231. [DOI] [PubMed] [Google Scholar]
- 20.Bastit L, Vandebroek A, Altintas S, et al. Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alpha administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. J Clin Oncol. 2008;26:1611–1618. doi: 10.1200/JCO.2006.10.4620. [DOI] [PubMed] [Google Scholar]
- 21.Pedrazzoli P, Farris A, Del Prete S, et al. Randomized trial of intravenous iron supplementation in patients with chemotherapy-related anemia without iron deficiency treated with darbepoetin alpha. J Clin Oncol. 2008;26:1619–1625. doi: 10.1200/JCO.2007.12.2051. [DOI] [PubMed] [Google Scholar]
- 22.Rodgers GM, 3rd, Becker PS, Bennett CL, et al. ; National Comprehensive Cancer Network. Cancer- and chemotherapy-induced anemia. J Natl Compr Canc Netw. 2008;6:536–564. doi: 10.6004/jnccn.2008.0042. [DOI] [PubMed] [Google Scholar]
- 23.Cella D, Viswanathan HN, Hays RD, et al. Development of a fatigue and functional impact scale in anemic cancer patients receiving chemotherapy. Cancer. 2008;113:1480–1488. doi: 10.1002/cncr.23698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beguin Y. Prediction of response and other improvements on the limitations of recombinant human erythropoietin therapy in anemic cancer patients. Haematologica. 2002;87:1209–1221. [PubMed] [Google Scholar]
- 25.Ray-Coquard I, Le Cesne A, Rubio MT, et al. Risk model for severe anemia requiring red blood cell transfusion after cytotoxic conventional chemotherapy regimens. The Elypse 1 Study Group. J Clin Oncol. 1999;17:2840–2846. doi: 10.1200/JCO.1999.17.9.2840. [DOI] [PubMed] [Google Scholar]
- 26.Lönnerdal B, Bryant A. Absorption of iron from recombinant human lactoferrin in young US women. Am J Clin Nutr. 2006;83:305–309. doi: 10.1093/ajcn/83.2.305. [DOI] [PubMed] [Google Scholar]
- 27.Artym J, Zimecki M, Kuryszko J, et al. Lactoferrin accelerates reconstitution of the humoral and cellular immune response during chemotherapy-induced immunosuppression and bone marrow transplant in mice. Stem Cells Dev. 2005;14:548–555. doi: 10.1089/scd.2005.14.548. [DOI] [PubMed] [Google Scholar]
- 28.Wolf JS, Li G, Varadhachary A, et al. Oral lactoferrin results in T cell-dependent tumor inhibition of head and neck squamous cell carcinoma in vivo. Clin Cancer Res. 2007;13:1601–1610. doi: 10.1158/1078-0432.CCR-06-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varadhachary A, Wolf JS, Petrak K, et al. Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy. Int J Cancer. 2004;111:398–403. doi: 10.1002/ijc.20271. [DOI] [PubMed] [Google Scholar]
- 30.Bohlius J, Schmidlin K, Brillant C, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: A meta-analysis of randomised trials. Lancet. 2009;373:1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- 31.Handland BK, Longmore GD. Erythroid-stimulating agents in cancer therapy: Potential dangers and biologic mechanisms. J Clin Oncol. 2009;27:4217–4226. doi: 10.1200/JCO.2008.21.6945. [DOI] [PubMed] [Google Scholar]
- 32.Henke M, Laszig R, Rb̈e C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 33.Leyland-Jones B, O'Shaughnessy JA. Erythropoietin as a critical component of breast cancer therapy: Survival, synergistic, and cognitive applications. Semin Oncol. 2003;30(suppl 16):174–184. doi: 10.1053/j.seminoncol.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 34.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]