The evidence supporting adjuvant therapy in early-stage non-small cell lung cancer patients is reviewed and key mitigating factors in providing treatment, such as stage of disease and the impact of the new seventh edition of the tumor–node–metastasis classification system, are discussed.
Keywords: Non-small cell lung cancer, Adjuvant chemotherapy, Early-stage non-small cell lung cancer, Personalized medicine, Postoperative radiotherapy
Abstract
The cornerstone of treatment for early-stage non-small cell lung cancer (NSCLC) has long been surgical resection. Over the past few years, there has been a paradigm shift to provide adjuvant platinum-based chemotherapy for patients with completely resected stage II–IIIA NSCLC founded on large randomized clinical trials demonstrating longer overall survival with this treatment. Reassuringly, the National Cancer Institute of Canada Cancer Therapeutics Group JBR.10 trial recently reported a continued survival advantage for patients treated with adjuvant chemotherapy after >9 years of median follow-up. In contrast, the gains from using this approach for stage IB disease are less clear, although data from an unplanned subgroup analysis suggest benefit for patients with tumors ≥4 cm. Herein, we review the evidence supporting adjuvant therapy in early-stage NSCLC patients before discussing key mitigating factors in providing treatment, such as stage of disease and the impact of the new seventh edition of the tumor–node–metastasis classification system. Criteria such as patient age and performance status, as well as the value of appropriate chemotherapy selection, are highlighted as measures to help guide management. The role of postoperative radiotherapy and the future landscape of early-stage NSCLC research are also explored; namely, therapeutic strategies exploiting pharmacogenomic and gene-expression profiling, in an attempt to personalize care, and the integration of novel targeted therapies into adjuvant clinical trials.
Introduction
Lung cancer is a strikingly prevalent malignancy worldwide, accounting for 12.3% of all new diagnoses, and it remains the leading cause of cancer-related death for both men and women in the U.S. [1]. Non-small cell lung cancer (NSCLC) represents 80%–85% of all lung cancers, and in its early stages, surgical resection offers the best opportunity for long-term survival. Despite complete (R0) resection, generally consisting of a lobectomy with adequate mediastinal lymph node dissection, there remains a high risk for developing recurrent disease. The 5-year overall survival (OS) rate ranges from 73% for patients with pathologic stage IA NSCLC to only 25% for those with stage IIIA disease [2, 3]. Over the past few years, one of the most significant advances in NSCLC research is the clear demonstration of longer survival with adjuvant chemotherapy for early-stage disease. Herein, the evidence for adjuvant therapy in NSCLC and opportunities for using clinical and molecular selection criteria to optimize therapeutic benefit are explored.
Evidence for Adjuvant Chemotherapy
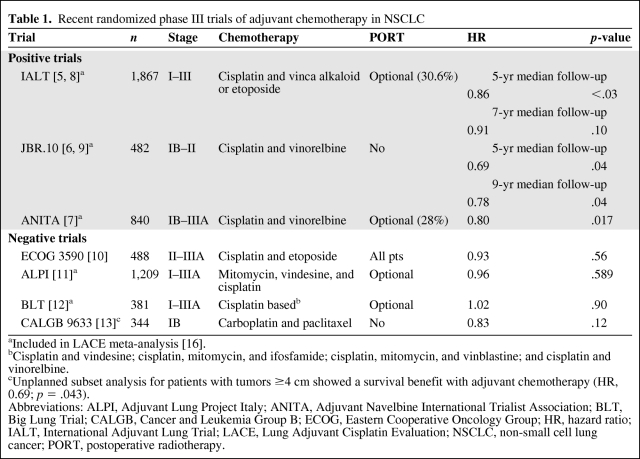
In 1995, the Non-Small Cell Lung Cancer Collaborative Group (NSCLCCG) published a meta-analysis showing a strong trend in favor of adjuvant cisplatin-based chemotherapy (hazard ratio [HR], 0.87; 95% confidence interval [CI], 0.74–1.02; p = .08), with an absolute improvement in the 5-year survival rate of 5% [4]. Although the benefit was not statistically significant, this observation prompted additional clinical trials to address the appropriate role of adjuvant chemotherapy in patients with resected NSCLC (Table 1).
Table 1.
Recent randomized phase III trials of adjuvant chemotherapy in NSCLC
aIncluded in LACE meta-analysis [16].
bCisplatin and vindesine; cisplatin, mitomycin, and ifosfamide; cisplatin, mitomycin, and vinblastine; and cisplatin and vinorelbine.
cUnplanned subset analysis for patients with tumors ≥4 cm showed a survival benefit with adjuvant chemotherapy (HR, 0.69; p = .043).
Abbreviations: ALPI, Adjuvant Lung Project Italy; ANITA, Adjuvant Navelbine International Trialist Association; BLT, Big Lung Trial; CALGB, Cancer and Leukemia Group B; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; IALT, International Adjuvant Lung Trial; LACE, Lung Adjuvant Cisplatin Evaluation; NSCLC, non-small cell lung cancer; PORT, postoperative radiotherapy.
Three randomized phase III trials have demonstrated a survival advantage with adjuvant chemotherapy: the International Adjuvant Lung Trial (IALT), National Cancer Institute of Canada Cancer Therapeutics Group JBR.10 trial, and Adjuvant Navelbine International Trialist Association (ANITA) trial [5–7]. Although these studies differed in key aspects, such as stage of patients enrolled and the use of postoperative radiotherapy (PORT), they also shared common features. Most importantly, they employed cisplatin-based chemotherapy regimens. Cisplatin plus vinorelbine was the relatively modern regimen of choice in the JBR.10 and ANITA trials, whereas a significant proportion (26%) received this combination in the IALT. The survival advantage for adjuvant therapy was in the range of 4.1%–15% in these studies. The broad range of benefit is speculated to be related to the variability of patients enrolled and, possibly, the use of PORT.
The IALT, initially presented in 2003, demonstrated that chemotherapy resulted in an approximately 4% higher survival rate at 5 years (44.5% versus 40.4%; HR, 0.56; p = .03), but a recent update found no OS advantage after 7 years of follow-up (HR, 0.91; p = .10) [5, 8]. This late loss of survival benefit appeared to develop because of an excess of non–cancer related deaths occurring in the chemotherapy arm. In contrast, the updated survival analysis of the JBR.10 trial, presented at the 2009 American Society of Clinical Oncology (ASCO) Annual Meeting, reported an enduring survival advantage for patients treated with adjuvant chemotherapy with >9 years of follow-up [9]. Similarly, in the ANITA study, the OS rate at 5 years was 8.6% higher in the chemotherapy arm, and this survival advantage was maintained at 7 years (8.4%) [7]. Compared with the IALT, a significantly higher incidence of non–lung cancer deaths or second primary malignancies was not observed in the JBR.10 trial.
Notably, the positive adjuvant therapy trials were preceded by three negative randomized trials completed shortly after the 1995 NSCLCCG meta-analysis, which all failed to show a benefit with adjuvant cisplatin-based chemotherapy. The Eastern Cooperative Oncology Group (ECOG) 3590 trial randomized completely resected stage II and IIIA patients to PORT or chemotherapy plus PORT [10]. The inclusion of PORT, which has been associated with greater mortality in stage II NSCLC patients, may have negated any potential benefit of adjuvant chemotherapy. The Adjuvant Lung Project Italy (ALPI) randomly allocated patients to observation or three cycles of mitomycin, vindesine, and cisplatin, an antiquated chemotherapy regimen [11]. High early death rates and poor compliance with adjuvant chemotherapy were known shortcomings of that study. Finally, the Big Lung Trial (BLT), a randomized trial conducted in the U.K., was not sufficiently powered to detect clinically significant differences in survival [12]. Also, a significant proportion of patients (15%) had microscopically incomplete surgically resected disease.
The Cancer and Leukemia Group B (CALGB) 9633 trial was unique in focusing exclusively on stage IB (T2N0) resected NSCLC patients who were randomized to adjuvant carboplatin plus paclitaxel chemotherapy or observation [13]. That trial accrued 344 patients prior to early closure, when an interim analysis suggested a significantly higher survival rate with adjuvant chemotherapy (12% at 4 years; HR, 0.62). An updated report with more mature results, however, revealed that the OS advantage was no longer statistically significant, although the HR (0.83) was of a magnitude similar to those seen in the IALT (HR, 0.86) and the ANITA trial (HR, 0.80) [14]. A number of plausible explanations for the negative results of the CALGB 9633 trial can be proposed. The lack of benefit with a carboplatin-based regimen as opposed to a cisplatin backbone is one hypothesis, especially given the cisplatin versus carboplatin meta-analysis demonstrating carboplatin regimens to be inferior to cisplatin-based chemotherapy in advanced NSCLC [15]. It is also widely believed that carboplatin is substandard when treating other curable malignancies, such as germ cell tumors. Additional explanations include a true lack of benefit in patients with stage IB disease, because no other positive adjuvant therapy trial has shown a significant survival benefit for this substratum of patients, and, finally, a lack of statistical power. The CALGB 9633 trial was initially designed to detect a 13% higher 5-year survival rate (HR, 0.67), with an accrual goal of 500 patients. Because of slow enrollment and early closure, this goal was not reached and the resulting small sample size and low event rate contributed to the inability to detect a statistically significant survival advantage.
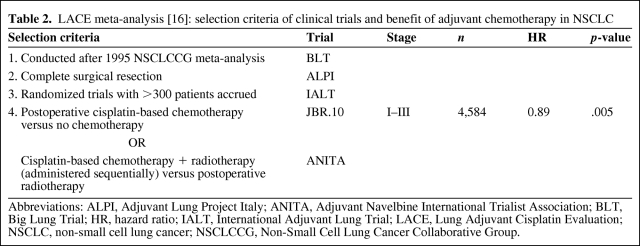
The Lung Adjuvant Cisplatin Evaluation (LACE) was a pooled analysis incorporating data from the five largest adjuvant cisplatin chemotherapy trials completed after the 1995 meta-analysis (BLT, ALPI, IALT, JBR.10, ANITA) (Table 2) [16]. The LACE meta-analysis included 4,584 patients and showed significantly better OS (HR, 0.89; 95% CI, 0.82–0.96; p = .005) and disease-free survival (DFS) (HR, 0.84; 95% CI, 0.78–0.91; p < .001) outcomes were conferred with adjuvant chemotherapy; the absolute survival benefit at 5 years was 5.4%. An excess of non–lung cancer deaths, primarily resulting from pulmonary/cardiovascular disease, occurred within 6 months of random assignment (HR, 2.41; 95% CI, 1.64–3.55; p < .001), but this finding did not extend past this point (HR, 1.06; 95% CI, 0.83–1.37; p for interaction < .001). Finally, an update of the 1995 NSCLCCG meta-analysis presented at the 2007 ASCO Annual Meeting demonstrated a conclusive and consistent benefit with adjuvant chemotherapy for surgically resected NSCLC (HR, 0.86; 95% CI, 0.81–0.93; p < .000001) [17].
Table 2.
LACE meta-analysis [16]: selection criteria of clinical trials and benefit of adjuvant chemotherapy in NSCLC
Abbreviations: ALPI, Adjuvant Lung Project Italy; ANITA, Adjuvant Navelbine International Trialist Association; BLT, Big Lung Trial; HR, hazard ratio; IALT, International Adjuvant Lung Trial; LACE, Lung Adjuvant Cisplatin Evaluation; NSCLC, non-small cell lung cancer; NSCLCCG, Non-Small Cell Lung Cancer Collaborative Group.
Clearly, the survival benefit with adjuvant therapy in early-stage NSCLC has been established and it is the standard of care. Japanese investigators have focused their research on providing adjuvant uracil/tegafur, but considering that it is not available in North America, data supporting its use in the adjuvant setting are not further reviewed here. Attention has now shifted to developing an approach to select patients most likely to benefit from adjuvant therapy. This approach should incorporate key practical issues such as stage of disease, patient age and performance status, selection of the appropriate chemotherapy regimen, and toxicity risks.
Fundamental Determinants for Providing Adjuvant Chemotherapy in Early-Stage NSCLC
Stage of Disease
The adjuvant chemotherapy trials discussed above were not uniform in terms of their eligibility criteria for stage of disease, and this variability has contributed to the ongoing debate of determining which early-stage NSCLC patient should be considered for adjuvant chemotherapy. However, the available evidence allows for certain conclusions to be made.
Stage IA
A relatively small number of patients with stage IA disease have been included in adjuvant randomized control trials. The LACE meta-analysis showed that the benefit of adjuvant chemotherapy varied considerably by stage, with a potential detriment for those with stage IA NSCLC (HR, 1.40; 95% CI, 0.95–2.06) [16]. Currently, adjuvant chemotherapy is not recommended for stage IA NSCLC patients.
Stage II
A consistent benefit for adjuvant chemotherapy has been established in stage II NSCLC. To date, the JBR.10 trial has shown the most striking survival benefit in this subset of patients (HR, 0.59; 95% CI, 0.42–0.85; p = .004), with an absolute 20% higher 5-year survival rate [6]. Similarly, the ANITA trial showed a 13% higher 5-year survival rate (HR, 0.71; 95% CI, 0.49–1.03), and the LACE study revealed a 10% higher 5-year survival rate (HR, 0.83; 95% CI, 0.73–0.95) [7, 16].
Stage IIIA
For completely resected stage IIIA NSCLC, adjuvant chemotherapy is clearly beneficial; a 16% absolute higher 5-year survival rate was seen in the ANITA trial (HR, 0.69; 95% CI, 0.53–0.90) [7]. In the IALT, the greatest benefit was observed in stage IIIA patients (HR, 0.79; 95% CI, 0.66–0.95) [5]. Finally, the LACE meta-analysis data support the use of adjuvant chemotherapy for patients with stage IIIA disease (HR, 0.83; 95% CI, 0.72–0.95), translating to a 13% absolute higher 5-year survival rate [16].
Stage IB
The benefit of adjuvant chemotherapy in stage IB NSCLC is less apparent. There is a lack of robust evidence from any of the adjuvant randomized trials or the LACE meta-analysis to advocate adjuvant chemotherapy. Controversy exists as to whether a subset of stage IB patients, defined by tumor size, benefit from adjuvant chemotherapy. An unplanned subset analysis of the CALGB 9633 data revealed that, in patients with tumors ≥4 cm in diameter, a statistically significant DFS and OS benefit was observed (HR, 0.69; 95% CI, 0.48–0.99; p = .043) [13]. Updated results of the JBR.10 trial, reported at the 2009 ASCO Annual Meeting, showed that, in a similar subset analysis, motivated by the CALGB 9633 trial data, patients with stage IB NSCLC and tumors ≥4 cm showed a nonsignificant trend in favor of adjuvant therapy (HR, 0.66; 95% CI, 0.39–1.14; p – .13) [9]. Although these data suggest clinical benefit with adjuvant chemotherapy for tumors ≥4 cm, caution should be exercised in placing emphasis on these results, given the nature of unplanned subgroup analyses. The de facto practice is to consider these patients for adjuvant therapy, in an individualized manner, after a thorough discussion of the risks and perceived benefits.
Impact of Recent Changes to Tumor–Node–Metastasis Staging Classification
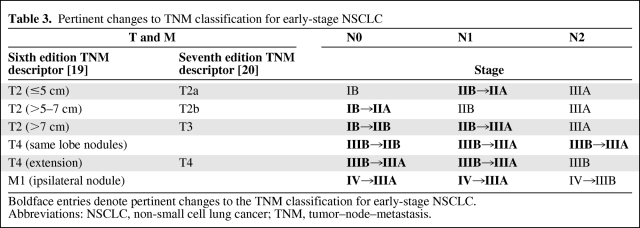
The evidence supporting adjuvant chemotherapy in resected NSCLC is derived from trials that staged patients based on the fifth edition of tumor–node–metastasis (TNM) Classification of Malignant Tumors (1997) [18]. No changes were made in 2002 for the sixth edition; however, the recently implemented seventh edition contains important changes that are impacting treatment recommendations [19, 20]. The key modifications are summarized in Table 3 and include amendments based on tumor size and location of additional pulmonary nodules.
Table 3.
Pertinent changes to TNM classification for early-stage NSCLC
Boldface entries denote pertinent changes to the TNM classification for early-stage NSCLC. %Abbreviations: NSCLC, non-small cell lung cancer; TNM, tumor–node–metastasis.
Considering that there are no prospectively validated data supporting a survival benefit with adjuvant chemotherapy in stage IB patients, the shift of T2(>5–7 cm)N0 and T2(>7 cm)N0 lesions into the category of stage II disease is potentially problematic. However, by accepting the contention that patients with tumors ≥4 cm benefit from adjuvant therapy, then these patients should reasonably be offered adjuvant chemotherapy as per other stage II NSCLC patients.
The revision to stage based on the location of separate pulmonary nodules is somewhat more challenging. Tumors with satellite nodules within the lobe of the primary tumor previously were classified as T4 lesions and, thus, patients were denoted to have stage IIIB NSCLC. The seventh edition of the TNM classification downstages these lesions to stage IIIA (T3N1 or T3N2) disease. Adjuvant early-stage NSCLC chemotherapy trials have all excluded patients with T4 lesions from accrual. Despite the lack of evidence to provide specific recommendations for or against adjuvant therapy in this subset of patients, given the known high risk for recurrence, even with node-negative disease, it is reasonable to offer adjuvant chemotherapy for appropriately resected patients.
Age as a Consideration for Treatment
The median age for developing lung cancer is 70 years, with >60% of this population aged >65 years. In contrast, the median age for study participants in the LACE meta-analysis was 59 years, with >71% aged <65 years and only 9% aged ≥70 years [21].
Conclusions regarding the benefit of adjuvant chemotherapy in elderly patients are hampered by the lack of consensus on the definition of “elderly.” In a separate analysis of the JBR.10 trial, Pepe et. al. [22] classified patients aged <65 years as “young” and those aged ≥65 years as “elderly”. A similarly designed examination of the LACE meta-analysis defined “elderly” as ≥70 years old [21].
Although these two analyses differ in their definitions, they illustrate similar findings and allow for certain assumptions regarding the role of adjuvant chemotherapy in NSCLC patients aged 65–75 years. Elderly patients were more prone to have squamous cell histology and less likely to have an ECOG performance status (PS) score of 0. Furthermore, they received a significantly lower total dose of cisplatin and fewer cycles of chemotherapy. In the JBR.10 trial, this subpopulation more often discontinued chemotherapy because of patient refusal. Consequently, an excess in grade 3 or 4 toxicity or treatment-related mortality was not seen. Most importantly, elderly patients enjoyed survival benefits similar to those of the younger cohort. It is reasonable to conclude that NSCLC patients aged 65–75 years can tolerate adjuvant cisplatin-doublet chemotherapy without excess toxicity and with a survival benefit similar to that of younger patients. However, a cautious approach needs to be applied when considering adjuvant cisplatin-doublet chemotherapy for those aged >75 years. In an analysis of the elderly in the JBR.10 clinical trial, chemotherapy was associated with significantly shorter OS and disease-specific survival times, although only 23 patients in that trial were aged >75 years. Underrepresentation of this age group in the LACE elderly analysis (1.3%) prevents the formation of adequate conclusions from the LACE data.
PS
It is well recognized that patients enrolled in adjuvant trials are generally those with an excellent PS (ECOG PS score, 0 or 1). For example, the JBR.10 trial excluded patients with a PS score ≥2 and, in the LACE pooled analysis, only 4% of patients had a PS score of 2. Interestingly, a significant interaction between chemotherapy effect and PS was seen (test for trend, p = .009 for OS and p = .01 for DFS). Adjuvant chemotherapy appeared to have a detrimental effect in patients with a PS score of 2. Given these findings, it would be prudent to offer adjuvant chemotherapy only to patients with a PS score of 0 or 1.
Selection of Adjuvant Chemotherapy Regimen
The majority of randomized clinic trials since the 1995 meta-analysis incorporated a cisplatin-based treatment regimen [4]. Three of the positive trials used cisplatin-doublet chemotherapy, and the ANITA and JBR.10 trials specifically used cisplatin plus vinorelbine [6, 7]. The LACE meta-analysis found a trend for longer survival with cisplatin plus vinorelbine; however, this may be explained by the higher planned doses of cisplatin in the cisplatin- and vinorelbine-treated patients [16]. Interestingly, no randomized trial has been reported assessing adjuvant chemotherapy with a cisplatin doublet combined with a more contemporary cytotoxic (gemcitabine, docetaxel, pemetrexed). The prevailing assumption is that the equivalence of modern cisplatin-doublet chemotherapy in advanced NSCLC will translate to similar equivalence in the adjuvant setting. Indeed, the current ECOG-sponsored Intergroup trial, ECOG 1505, incorporates standard adjuvant chemotherapy regimens of cisplatin combined with vinorelbine, gemcitabine, docetaxel, or pemetrexed.
Based strictly on the available evidence, carboplatin plus paclitaxel chemotherapy cannot be advocated for routine use in the adjuvant setting. The Cancer Care Ontario/ASCO Joint Expert Panel recommended against the use of carboplatin-based adjuvant regimens, because of the negative CALGB 9633 trial results [13, 23]. Critics may argue that this was not related to the choice of chemotherapy, but rather to a lack of statistical power to detect a significant benefit in a relatively good-risk population of node-negative NSCLC patients. The HR of 0.83, although not statistically significant, was in favor of the chemotherapy arm, and a subset analysis demonstrated that, in the higher risk strata of patients with tumors ≥4 cm in size, adjuvant chemotherapy appeared beneficial. Nevertheless, in routine practice, cisplatin-based doublet adjuvant chemotherapy should be considered standard of care. For patients at significant risk for relapse (stage II/IIA) but with contraindications to cisplatin chemotherapy, such as those with hearing deficits or renal insufficiency, carboplatin-based doublet chemotherapy could be considered a reasonable alternative.
Toxicity and Quality of Life
A complete discussion of NSCLC adjuvant therapy involves a description of not only its benefits but also its potential for harm, such as the risk for death related to treatment, effect of treatment on quality of life (QOL), and potential for long-term side effects.
The reported toxicity rates from the adjuvant NSCLC clinical trial are highly variable, likely related not only to the patient population studied and chemotherapy regimen employed but also to differences in the methods of toxicity surveillance and data collection. The general applicability of the toxicity data is limited, in particular, by the younger population accrued within the clinical trials.
The LACE meta-analysis reported a chemotherapy-related death rate of 0.9% [16]. There was an excess of non–lung cancer deaths in the first 6 months postrandomization related to chemotherapy toxicity and pulmonary/cardiovascular disease. The overall rate of grade 3 or 4 toxicity was 66%, predominantly a result of neutropenia. In the JBR.10 and ANITA trials, the febrile neutropenia rates were 7% and 9%, respectively [6, 7].
The JBR.10 clinical trial was the only adjuvant study formally reporting QOL by implementation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 [24]. Although patients receiving adjuvant therapy reported worsening in certain aspects of QOL from baseline to 3 months of follow-up, likely reflecting the toxicities of chemotherapy, by 9 months, QOL scores were comparable with those of the observation arm. Hearing loss was a notable long-term adverse event and sensory neuropathy could be detected 30 months after randomization.
Evidence for PORT in NSCLC
Although adjuvant systemic therapy for resected NSCLC has become the standard of care during the past decade, the role of PORT remains controversial. As detailed in a recent review, several trials have shown a lower local recurrence rate but no consistent difference in OS [25]. A 1998 meta-analysis of nine randomized trials, some beginning as early as 1965, demonstrated an overall detrimental effect of PORT, with a 2-year OS rate of 48% in patients who received PORT versus 55% in patients who did not receive PORT [26]. In particular, worse outcomes were seen in patients with N0 and N1 disease, whereas an adverse effect of PORT in patients with N2 disease was not shown. This meta-analysis has been widely criticized for the inclusion of trials employing older radiation techniques such as cobalt machines, radiation fields inferior to current standards, and high overall doses and fraction sizes. These factors call into question the applicability of the results in a modern setting.
An analysis from the Surveillance, Epidemiology, and End Results (SEER) database of 7,465 patients with stage II or III resected NSCLC with a median follow-up of 3.5 years did not show a significant impact of PORT on survival in a multivariate analysis [27]. Subgroup analyses showed worse survival outcomes for patients with N0 or N1 disease who received PORT, a result consistent with the PORT meta-analysis. However, in contrast, patients in the SEER database analysis with N2 disease who received PORT had a statistically significant survival benefit, compared with those who did not receive PORT (HR, 0.855; 95% CI, 0.762–0.959; p = .0077).
More recently, the impact of PORT in the ANITA randomized trial was reported [28]. PORT was recommended, in a nonrandomized fashion, for patients with node-positive disease but was not mandated by the protocol. If PORT was used, the recommended dose and fractionation were 45–60 Gy in 2 Gy fractions over 5 weeks. Of the 840 total patients accrued, 232 received PORT. One third of those were in the observation arm and 21.6% were in the chemotherapy arm. For patients with N2 disease, survival was longer for patients receiving PORT in both the observation and chemotherapy arms. However, in patients with N1 disease, PORT was advantageous in the observation arm but detrimental in the chemotherapy arm. Although the use of PORT in the ANITA trial was not randomized, these findings add to the signal of a potential benefit for PORT in N2 disease. Furthermore, this benefit was found in the setting of adjuvant chemotherapy administration.
PORT has been shown to lead to a lower local recurrence rate, but demonstration of a survival benefit in randomized trials is lacking. Most of the evidence suggesting a potential benefit for PORT in patients with N2 disease is retrospective and nonrandomized. A more definitive answer is needed. The ongoing phase III Lung Adjuvant Radiotherapy Trial is comparing three-dimensional conformal PORT with no PORT in patients with completely resected NSCLC and N2 nodal staging and is powered to detect a 10% difference in the 3-year DFS rate, its primary endpoint, with a targeted accrual of 700 patients [29]. The results of trials evaluating PORT in the setting of modern radiotherapy techniques and adjuvant chemotherapy are highly anticipated.
Future Directions: Personalized Medicine and Targeted Therapies
The cornerstone of treatment for early-stage NSCLC continues to be surgical resection. The shift to provide adjuvant platinum-based chemotherapy is founded on clinical trials demonstrating a survival benefit with this treatment. Despite the disconcerting loss of the OS benefit in the long-term follow-up analysis of the IALT, the ANITA and JBR.10 trial data reassuringly indicate a persistent advantage with adjuvant chemotherapy. Current research efforts are attempting to improve upon these gains by adopting pharmacogenomic approaches to identify, among heterogeneous patient populations, those individuals who are most likely to benefit from chemotherapy based on predictive biomarkers. For example, given the marginal benefit of adjuvant chemotherapy for patients with tumors ≥4 cm in stage IB NSCLC, the ability to define biologically distinct patient subsets within this group would be of particular value in risk stratification—those with an excellent prognosis not requiring adjuvant chemotherapy and others with a relatively poor prognosis, but for whom biomarkers would predict a benefit from platinum-based chemotherapy [30]. Differential expression of excision repair cross-complementation group 1 (ERCC1) and/or ribonucleotide reductase M1 (RRM1) is emerging as a promising strategy to molecularly define these patients. ERCC1 plays a key role in DNA repair after cisplatin damage, whereas RRM1, inhibited by gemcitabine, provides deoxyribonucleotides not only for de novo DNA synthesis but also for DNA repair. Using a recent innovation in methodology for quantitative protein expression (automated quantitative analysis), patients with a high expression level of both genes had longer survival times than patients in other groups [31]. An ongoing Southwest Oncology Group clinical trial (S0720) is evaluating the prognostic and predictive role of ERCC1 and RRM1 in selecting patients with stage I (primary tumor ≥2 cm) NSCLC for adjuvant platinum-based chemotherapy. In that trial, patients with completely resected stage IA–IB NSCLC with high expression levels of ERCC1 and RRM1 are assigned to observation alone, because of a good prognosis, whereas those with low levels of either biomarker are assigned to cisplatin–gemcitabine adjuvant therapy, given the poorer expected prognosis and predicted benefit of platinum-based chemotherapy [32]. The primary objective is to test the feasibility of such an approach, defined by the percentage of patients who can be assigned to treatment appropriately, reflecting the adequacy of tumor specimen collection and analysis [30, 33].
The C30506 study is a CALGB trial for patients with resected stage I NSCLC, 2–6 cm in size, who are not candidates for the ongoing E1505 Intergroup trial and who do not routinely receive adjuvant chemotherapy. The premise for this trial is founded on a genomics prognostic model known as the lung metagene score (LMS) to select patients for adjuvant chemotherapy based on published preliminary data [34]. Patients are randomized to either adjuvant cisplatin-based doublet chemotherapy or standard observation and are stratified according to the LMS. The key primary objectives of this unique trial are to validate the positive prognostic value for survival of patients with a low LMS, who are believed to be at a low risk for recurrence, and to determine whether a survival advantage is associated with adjuvant chemotherapy in patients with an unfavorably high LMS [30, 35]. Fresh tissue collection is required at the time of surgical resection for RNA microarray analysis and determination of an LMS.
In a move toward personalized care, many European groups are focusing on biomarker-driven adjuvant therapy. In an ongoing phase III International Tailored Chemotherapy Adjuvant trial, patients with completely resected stage II–III NSCLC are treated with standard adjuvant chemotherapy or a tailored regimen based on thymidylate synthase and ERCC1 gene expression levels. The Tailored Post-Surgical Therapy in Early Stage trial is randomizing patients with stage II–IIIA (non-N2) resected nonsquamous NSCLC to a noncustomized adjuvant chemotherapy arm and a genotypic arm, in which patients with known epidermal growth factor receptor (EGFR)-activating mutations receive erlotinib for 1 year, while those who have wild-type EGFR receive chemotherapy based on the level of ERCC1 expression. Finally, the Spanish Customized Adjuvant Trial is looking at assigning adjuvant chemotherapy after determining the level of BRCA1 mRNA expression, which predicts response or lack of response to cisplatin and docetaxel, in surgically resected stage II–IIIA NSCLC patients, compared with a standard arm of cisplatin plus docetaxel for all patients. Indeed, the identification of predictive biomarkers and the development of genomic and pharmacogenomic models to “personalize” the adjuvant treatment of early-stage NSCLC patients is an active area of current, and undoubtedly, future research.
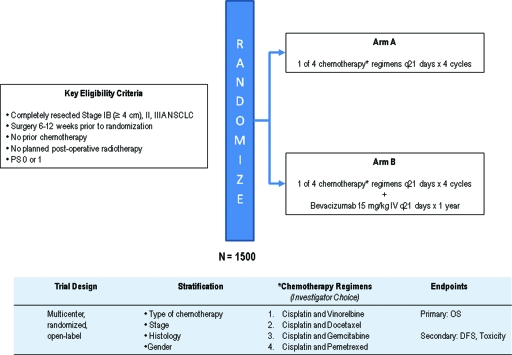
Integrating novel biological therapeutics into standard treatment paradigms for advanced NSCLC patients has been successful, and these are now being investigated in the early-stage setting within the context of large, phase III, randomized clinical trials. Bevacizumab, a recombinant, humanized monoclonal antibody against vascular endothelial growth factor, inhibits angiogenesis and is the first targeted therapy in NSCLC to improve clinical outcomes when given in combination with chemotherapy [36, 37]. Whether its role is limited to advanced NSCLC or its benefits can extend to earlier stage disease is the focus of an ongoing ECOG adjuvant therapy trial (the E1505 trial) (Fig. 1) In that trial, patients are randomized to receive adjuvant platinum-based doublet chemotherapy, either with or without bevacizumab, for resected stage IB (≥4 cm), II, and IIIA NSCLC.
Figure 1.
ECOG 1505: Phase III trial of adjuvant chemotherapy with or without bevacizumab for completely resected stage IB–IIIA NSCLC.
Abbreviations: DFS, disease-free survival; ECOG, Eastern Cooperative Oncology Group; NSCLC, non-small cell lung cancer; OS, overall survival; PS, performance status; q21, every 21.
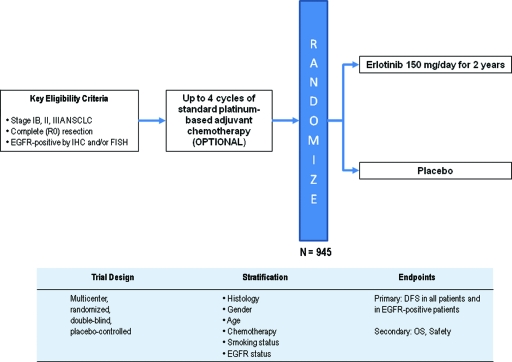
Inhibition of EGFR with the tyrosine kinase inhibitor erlotinib also represents a reasonable therapeutic target for adjuvant therapy clinical trials. The Randomized Double-Blind Trial in Adjuvant NSCLC with Tarceva® is a phase III trial for patients with resected stage IB–IIIA NSCLC who are EGFR+ by immunohistochemistry and/or fluorescent in situ hybridization (Fig. 2) The target accrual is 945 patients, who are being randomized 2:1 to receive erlotinib or placebo for 2 years after surgical resection. Patients may receive up to four cycles of adjuvant chemotherapy, but administration of adjuvant chemotherapy is not required. Although this trial is designed to select patients with EGFR expression, a key question for future trials will be to define the optimal adjuvant therapy for patients who possess EGFR mutations.
Figure 2.
RADIANT: Phase III trial of adjuvant erlotinib after surgical resection of stage IB–IIIA EGFR+ NSCLC.
Abbreviations: DFS, disease-free survival; EGFR, epidermal growth factor receptor; FISH, fluorescent in situ hybridization; IHC, immunohistochemistry; NSCLC, non-small cell lung cancer; OS, overall survival; RADIANT, Randomized Double-Blind Trial in Adjuvant NSCLC with Tarceva®.
The recombinant melanoma-associated antigen (MAGE)- A3 vaccine is also being investigated in NSCLC. Its immunogenicity has been documented in melanoma and lung cancer, and many CD4 and CD8 T-cell epitopes have been mapped to this target protein [38]. Based on encouraging phase II data, a phase III efficacy trial, MAGE-A3 Adjuvant Non-Small Cell Lung Cancer Immunotherapy, was launched for patients with surgically resected stage IB–III NSCLC that expresses MAGE-A3 [39]. It is hypothesized that postoperative MAGE-A3 immunization will result in a longer DFS interval, the primary endpoint of the study.
Conclusion
This review summarizes the evidence underlying adjuvant therapy for patients with early-stage NSCLC. The standard of care for stage II–IIIA NSCLC patients is adjuvant cisplatin-based doublet chemotherapy after appropriate surgical resection to improve OS. The benefit for patients with stage IB NSCLC is less apparent, likely because of the heterogeneity of this population. The latest revisions to the TNM staging criteria should assist in risk stratification.
Although introduction of adjuvant therapy represents one of the most significant breakthroughs in NSCLC management over the last decade, considerable gains have yet to be made. The landscape for treating NSCLC patients is in flux, with emerging strategies using pharmacogenomic and gene-expression profiling to attempt personalized care and to obtain the maximal therapeutic benefit. Moreover, as research efforts continue to unravel the molecular pathways behind lung tumorigenesis, novel targets are being exposed, forming the rationale for modern adjuvant therapy clinical trials integrating biological agents such as bevacizumab and erlotinib and the vaccine to MAGE-A3. Whether these approaches will substantially improve upon the current standard of adjuvant cisplatin-based doublet chemotherapy remains to be seen.
Author Contributions
Conception/Design: Randeep Sangha, Charles A. Butts
Manuscript writing: Randeep Sangha, Julie Price, Charles A. Butts
Final approval of manuscript: Randeep Sangha, Julie Price, Charles A. Butts
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 3.Besse B, Le Chevalier T. Adjuvant or induction cisplatin-based chemotherapy for operable lung cancer. Oncology (Williston Park) 2009;23:520–527. [PubMed] [Google Scholar]
- 4.Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: A meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 5.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 6.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 7.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 8.Arriagada R, Dunant A, Pignon JP, et al. Long-term results of the International Adjuvant Lung Cancer Trial evaluating adjuvant cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28:35–42. doi: 10.1200/JCO.2009.23.2272. [DOI] [PubMed] [Google Scholar]
- 9.Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small cell lung cancer: Updated survival analysis of JBR.10. J Clin Oncol. 2010;28:29–34. doi: 10.1200/JCO.2009.24.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller SM, Adak S, Wagner H, et al. A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non-small-cell lung cancer. Eastern Cooperative Oncology Group. N Engl J Med. 2000;343:1217–1222. doi: 10.1056/NEJM200010263431703. [DOI] [PubMed] [Google Scholar]
- 11.Scagliotti GV, Fossati R, Torri V, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell lung cancer. J Natl Cancer Inst. 2003;95:1453–1461. doi: 10.1093/jnci/djg059. [DOI] [PubMed] [Google Scholar]
- 12.Waller D, Peake MD, Stephens RJ, et al. Chemotherapy for patients with non-small cell lung cancer: The surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg. 2004;26:173–182. doi: 10.1016/j.ejcts.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 13.Strauss GM, Herndon JE, 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strauss G, Herndon J, Maddaus M, et al. Adjuvant chemotherapy in stage IB non-small cell lung cancer (NSCLC): Update of Cancer and Leukemia Group B (CALGB) protocol 9633. J Clin Oncol. 2006;24(18 suppl):7007. [Google Scholar]
- 15.Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: An individual patient data meta-analysis. J Natl Cancer Inst. 2007;99:847–857. doi: 10.1093/jnci/djk196. [DOI] [PubMed] [Google Scholar]
- 16.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 17.Stewart LA, Burdett S, Tierney JF, et al. Surgery and adjuvant chemotherapy compared to surgery alone in non-small cell lung cancer: A meta-analysis using individual patient data from randomized clinical trials. J Clin Oncol. 2007;25(18 suppl):7552. [Google Scholar]
- 18.Fleming ID, Cooper JS, Hensen DE, et al., editors. Fifth Edition. Philadelphia: Lippincott-Raven; 1997. AJCC Cancer Staging Manual; pp. 1–294. [Google Scholar]
- 19.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. Sixth Edition. New York: Springer: 2002. pp. 1–435. [Google Scholar]
- 20.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. Seventh Edition. New York: Springer: 2010. pp. 1–730. [Google Scholar]
- 21.Früh M, Rolland E, Pignon JP, et al. Pooled analysis of the effect of age on adjuvant cisplatin-based chemotherapy for completely resected non-small-cell lung cancer. J Clin Oncol. 2008;26:3573–3581. doi: 10.1200/JCO.2008.16.2727. [DOI] [PubMed] [Google Scholar]
- 22.Pepe C, Hasan B, Winton TL, et al. Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. J Clin Oncol. 2007;25:1553–1561. doi: 10.1200/JCO.2006.09.5570. [DOI] [PubMed] [Google Scholar]
- 23.Pisters KM, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol. 2007;25:5506–5518. doi: 10.1200/JCO.2007.14.1226. [DOI] [PubMed] [Google Scholar]
- 24.Bezjak A, Lee CW, Ding K, et al. Quality-of-life outcomes for adjuvant chemotherapy in early-stage non-small-cell lung cancer: Results from a randomized trial, JBR.10. J Clin Oncol. 2008;26:5052–5059. doi: 10.1200/JCO.2007.12.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogart JA, Aronowitz JN. Localized non-small cell lung cancer: Adjuvant radiotherapy in the era of effective systemic therapy. Clin Cancer Res. 2005;11:5004s–5010s. doi: 10.1158/1078-0432.CCR-05-9010. [DOI] [PubMed] [Google Scholar]
- 26.PORT Meta-analysis Trialists Group. Postoperative radiotherapy in non-small-cell lung cancer: Systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet. 1998;352:257–263. [PubMed] [Google Scholar]
- 27.Lally BE, Zelterman D, Colasanto JM, et al. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2006;24:2998–3006. doi: 10.1200/JCO.2005.04.6110. [DOI] [PubMed] [Google Scholar]
- 28.Douillard JY, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: The adjuvant Navelbine International Trialist Association (ANITA) randomized trial. Int J Radiat Oncol Biol Phys. 2008;72:695–701. doi: 10.1016/j.ijrobp.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 29.Le Péchoux C, Dunant A, Pignon JP, et al. Need for a new trial to evaluate adjuvant postoperative radiotherapy in non-small-cell lung cancer patients with N2 mediastinal involvement. J Clin Oncol. 2007;25:e10–e11. doi: 10.1200/JCO.2006.09.6263. [DOI] [PubMed] [Google Scholar]
- 30.Calhoun R, Jablons D, Lau D, et al. Adjuvant treatment of stage IB NSCLC: The problem of stage subset heterogeneity. Oncology (Williston Park) 2008;22:511–516. discussion 516, 521–523. [PubMed] [Google Scholar]
- 31.Zheng Z, Chen T, Li X, et al. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med. 2007;356:800–808. doi: 10.1056/NEJMoa065411. [DOI] [PubMed] [Google Scholar]
- 32.Gautschi O, Mack PC, Davies AM, et al. Pharmacogenomic approaches to individualizing chemotherapy for non-small-cell lung cancer: Current status and new directions. Clinical Lung Cancer. 2008;9(suppl 3):S129–S138. doi: 10.3816/CLC.2008.s.019. [DOI] [PubMed] [Google Scholar]
- 33.Sangha R, Lara PN, Jr, Mack PC, et al. Beyond antiepidermal growth factor receptors and antiangiogenesis strategies for nonsmall cell lung cancer: Exploring a new frontier. Curr Opin Oncol. 2009;21:116–123. doi: 10.1097/CCO.0b013e3283210489. [DOI] [PubMed] [Google Scholar]
- 34.Potti A, Mukherjee S, Petersen R, et al. A genomic strategy to refine prognosis in early-stage non-small-cell lung cancer. N Engl J Med. 2006;355:570–580. doi: 10.1056/NEJMoa060467. [DOI] [PubMed] [Google Scholar]
- 35.Wakelee H, Langer C, Vokes E, et al. Cooperative group research efforts in lung cancer: Focus on early-stage non-small-cell lung cancer. Clin Lung Cancer. 2008;9:9–15. doi: 10.3816/CLC.2008.n.002. [DOI] [PubMed] [Google Scholar]
- 36.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 37.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 38.Romero P. Current state of vaccine therapies in non-small-cell lung cancer. Clin Lung Cancer. 2008;9(suppl 1):S28–S36. doi: 10.3816/clc.2008.s.005. [DOI] [PubMed] [Google Scholar]
- 39.Vansteenkiste J, Zielinski M, Linder A, et al. Final results of a multi-center, double-blind, randomized, placebo-controlled Phase II study to assess the efficacy of MAGE-A3 immunotherapeutic as adjuvant therapy in stage IB/II non-small cell lung cancer (NSCLC) J Clin Oncol. 2007;25(18 suppl):7554. [Google Scholar]