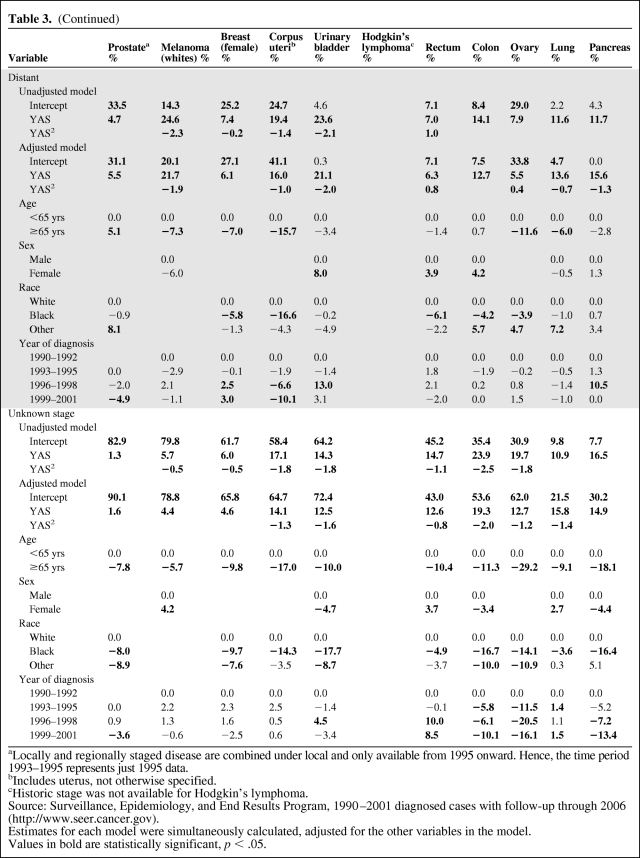
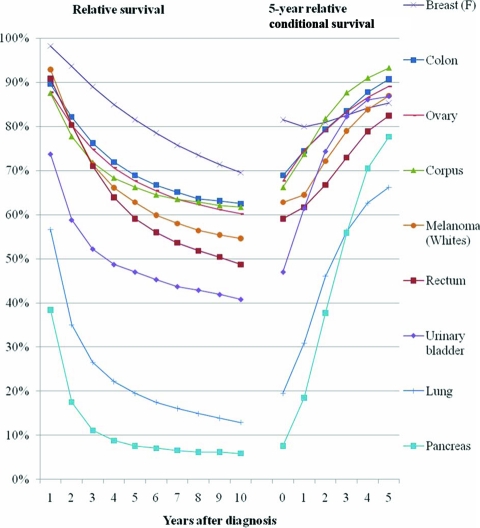
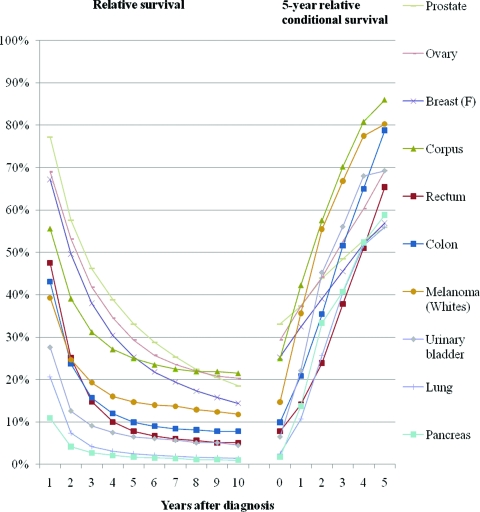
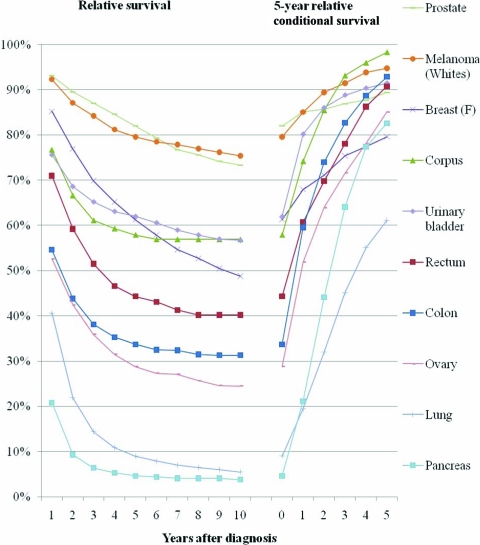
The study reports 5-year relative cancer survival probabilities conditional on having already survived ≥1 years after the initial diagnosis for 11 cancer sites, for patients diagnosed during 1990–2001 and followed through 2006.
Keywords: Cancer, Conditional survival, Observed survival, Population-based, Prognosis, Relative survival, SEER
Abstract
Purpose.
To report 5-year relative cancer survival probabilities conditional on having already survived ≥1 years after the initial diagnosis for 11 cancer sites, diagnosed during 1990–2001 and followed through 2006.
Methods.
Analyses are based on 1,151,496 cancer cases in population-based cancer registries in the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute.
Results.
The 5-year relative conditional survival probability tended to improve with each year already survived. Improvement was greatest for more lethal cancers (e.g., lung or pancreas) and for cases with a more advanced stage at diagnosis. The 5-year relative survival probability conditional on already having survived 5 years exceeded 90% for locally staged prostate cancer, melanoma (whites only), breast cancer (females only), corpus uteri cancer, urinary bladder cancer, Hodgkin’s lymphoma, rectal cancer, colon cancer, ovary cancer, and pancreatic cancer. Only lung cancer did not reach 90%. For these cancer sites combined, 5-year relative survival probability conditional on already having survived 5 years averaged about 85% for regionally staged disease, 68% for distant staged disease, and 87% for unknown staged disease. The 5-year relative conditional survival probability tended to be significantly lower among patients diagnosed at older ages, among males, among nonwhites, and among those diagnosed during 1990–1995 compared with later years.
Conclusion.
Conditional survival probability estimation provides further useful prognostic information to cancer patients, tailored to the time already survived since diagnosis.
Introduction
It was estimated that 1,479,350 cases of cancer would be diagnosed during 2009 [1]. When one of these patients is diagnosed with cancer, the physician can supply the patient with data on their prognosis, depending on factors such as the patient’s age and stage at diagnosis. However, as a cancer patient lives beyond the initial date of diagnosis, their estimated survival period generally improves. Conditional survival data allow those with cancer to update their prognosis and can help to assess the confidence with which one can determine that a patient is “cured” of cancer [2].
Most of the previously published studies on the conditional survival probability of cancer patients have focused on one or a few cancer sites [3–14]. Some of these studies have determined that conditional survival rates tend to equilibrate over time [3, 12, 13], some have identified age as the most important prognostic factor [7, 10], and a string of studies has focused on the substantial gains in conditional survival estimates for patients with distant stage cancer who survive >1 year [4, 5, 11]. Typically, these studies have used data from the U.S. Surveillance, Epidemiology, and End Results (SEER) Program [4–6, 9–15], whereas a few have used European or Asian data [3, 7, 8].
Each of these studies that estimate the conditional survival probability for specific cancer sites aids the patient with updated prognoses as the patient continues to survive. What they do not allow for, however, is a means to compare cancers across multiple sites, and in essence, track cancer survival in general. By providing estimates of conditional survival for many cancer sites, we can readily see whether a trend in conditional survival is unique to a single cancer site or whether it plays across to a region of the body, or to all cancers. An inquiry into multiple sites can also identify if certain global trends are found only in men or women, specific age groups, or patients with a specific stage of cancer.
In order to estimate conditional survival rates, long-term follow-up is required. Without such, it is difficult to accurately update 5-year survival likelihoods for the population as a whole as these patients continue to live years after their initial diagnosis. SEER data facilitate the compiling of extensive conditional survival tables for the U.S. population. In this paper, we present 5-year relative conditional survival probabilities for patients diagnosed with selected types of invasive cancer in 1990–2001, and followed through 2006 for vital status and cause of death. The effects of age, sex, race, year of diagnosis, and years already survived on the relative conditional survival estimates are also assessed.
Materials and Methods
Analyses are based on 1,151,496 cancer cases diagnosed during 1990–2001 and actively followed through 2006. Data were collected from medical records at hospitals and other facilities by population-based cancer registries in the SEER Program of the National Cancer Institute [16]. The SEER Program was established in response to the National Cancer Act of 1971 that mandated public health surveillance of cancer in the U.S. for use in the prevention, diagnosis, and treatment of cancer. The SEER Program began collecting data on cancer cases on January 1, 1973, and its current areas of inclusion cover 26% of the U.S. population (23% of African Americans, 40% of Hispanics, 42% of American Indians and Alaska Natives, and 59% of the Asian/Pacific Islander population) [17, 18].
The tumor registries participating in the SEER Program routinely abstract the records of all cancer patients in hospitals, clinics, nursing homes, and other health service units that provide diagnostic or treatment services; from private pathology laboratories and radiotherapy units; and from death certificates. Data collected by the tumor registries include patient demographics, tumor characteristics, morphology, diagnostic information, extent of disease, first course of treatment, and active patient follow-up of vital status including cause of death. Cancers are coded according to the International Classification of Disease for Oncology Second Edition [19]. The current study uses the SEER historic stage classification.
Survival probabilities were calculated using the SEER Survival System (SEER*Stat) [20]. Observed survival estimates (proportion of cancer patients surviving for a specified time period) were calculated by the life table method. Relative survival probability estimates were then obtained by adjusting the observed survival for expected mortality [21]. Relative survival probability estimates compare survival in the patient cohort with the expected survival of the general population having the same characteristics as the patient population with respect to age, sex, race, and calendar period. They provide a net survival measure representing cancer survival in the absence of other causes of death. The use of relative survival circumvents the problem associated with tumor registries of inaccurate or unavailable death certificates and the uncertainty about cause of death [21–24]. Therefore, with relative survival, cause of death is not required and the relative survival probability measure indicates the excess mortality experienced by cancer patients; that is, the excess in death is directly or indirectly caused by their cancer. Relative survival probabilities are larger than observed survival rates because they estimate only the effect of the cancer. However, relative survival probability estimates for lung cancer underestimate the effect of lung cancer alone because there is a higher percentage of smokers among lung cancer patients than in the general population, and smokers tend to be at a higher risk for other chronic diseases such as heart disease and stroke.
Eleven major cancer types were considered in this study: prostate, melanoma (whites only), breast (females only), corpus uteri, urinary bladder, Hodgkin’s lymphoma, rectum, colon, ovary, lung, and pancreas. Standard case selection criteria employed by the SEER Program were used. Specifically, cases were selected if they were actively followed and had malignant behavior and a known age. Cases were excluded if they were a second or later primary. Death certificate only and autopsy only cases were also excluded.
Relative conditional survival estimates were derived from the cumulative relative survival estimates. For example, the 5-year relative survival probability conditional on having already survived 1 year after diagnosis was obtained by dividing the cumulative relative survival estimate through 6 years by the 1-year cumulative relative survival estimate; the 5-year relative survival probability conditional on having already survived 2 years after diagnosis was calculated by dividing the cumulative relative survival estimate through 7 years by the 2-year cumulative relative survival estimate; and so on.
Trends in 5-year relative survival estimates conditional on already having survived 0–5 years from diagnosis were modeled using regression modeling [25]. The model regressed stage- and site-specific relative conditional survival estimates on age, sex, and race, year of diagnosis, as well as on linear and quadratic time variables reflecting years already having survived since diagnosis. The quadratic term was selected because of the tendency for the trends to increase at a decreasing rate. Tests of significance were based on two-sided hypotheses at the 0.05 level. Analyses were performed using Statistical Analysis System software, Version 9.2 (SAS Institute Inc., Cary, NC).
Results
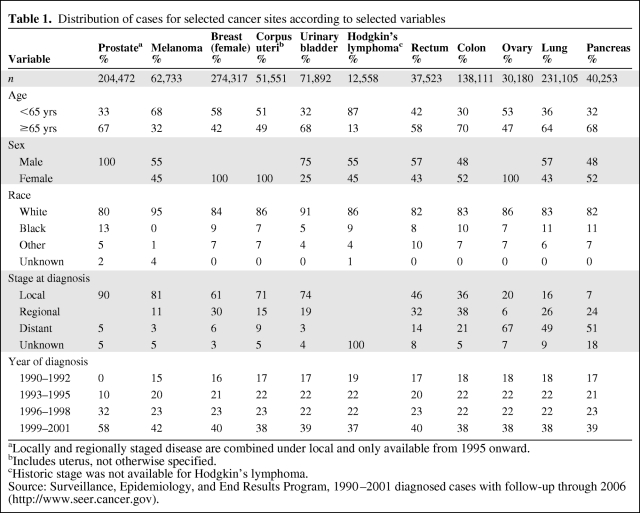
The distribution of site-specific cancer cases is presented according to selected variables in Table 1. The stage of disease at diagnosis varied considerably by cancer site, with prostate cancer, melanoma skin cancer, female breast cancer, corpus uterine cancer, and urinary bladder cancer cases showing the best stage at diagnosis, rectal and colon cancer cases showing a moderate stage at diagnosis, and ovarian cancer, lung cancer, and pancreatic cancer cases showing the worst stage at diagnosis. The majority of cancers of the prostate, urinary bladder, rectum, colon, lung, and pancreas were diagnosed at age ≥65 years. Besides those cancers unique to either men or women, males were more likely to be diagnosed with cancers involving melanoma of the skin, the urinary bladder, the rectum, and the lung. The distribution of cancer involving blacks varied by cancer site, being the highest for malignancies involving the prostate, lung, or pancreas and lowest for malignancies involving the ovaries, the urinary bladder, or melanoma. In general, the number of blacks in the distribution increased with later and unknown stage at diagnosis (data not shown).
Table 1.
Distribution of cases for selected cancer sites according to selected variables
aLocally and regionally staged disease are combined under local and only available from 1995 onward.
bIncludes uterus, not otherwise specified.
cHistoric stage was not available for Hodgkin’s lymphoma.
Source: Surveillance, Epidemiology, and End Results Program, 1990–2001 diagnosed cases with follow-up through 2006 (http://www.seer.cancer.gov).
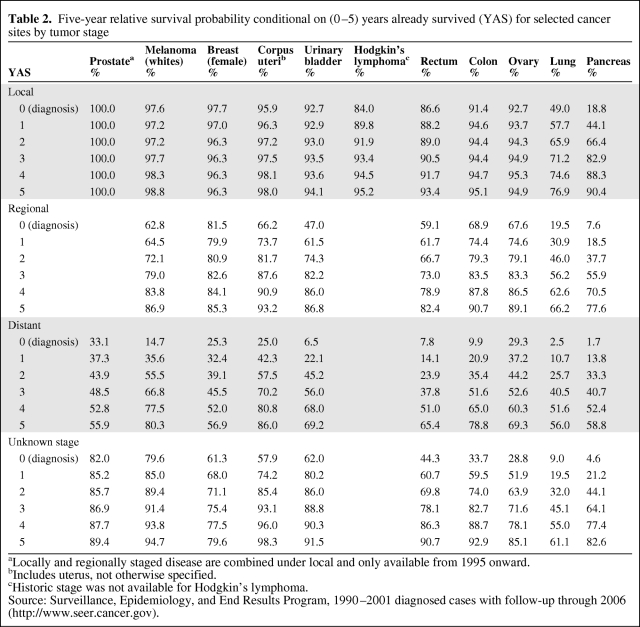
The 5-year relative survival probability estimates at diagnosis and conditional on having already survived 1–5 years after diagnosis are presented in Table 2.
Table 2.
Five-year relative survival probability conditional on (0–5) years already survived (YAS) for selected cancer sites by tumor stage
aLocally and regionally staged disease are combined under local and only available from 1995 onward.
bIncludes uterus, not otherwise specified.
cHistoric stage was not available for Hodgkin’s lymphoma.
Source: Surveillance, Epidemiology, and End Results Program, 1990–2001 diagnosed cases with follow-up through 2006 (http://www.seer.cancer.gov).
Generally, the 5-year relative survival probabilities tended to increase when conditional on more years already survived, albeit at a decreasing rate. The improvement in 5-year relative survival probability when conditional on more years already survived was greatest for later staged disease and for the more lethal types of cancer (i.e., lung and pancreatic cancers). The 5-year relative conditional survival probability only remained level for early-stage prostate cancer and colon cancer and was only 100% when conditional on having already survived 5 years among prostate cancer patients.
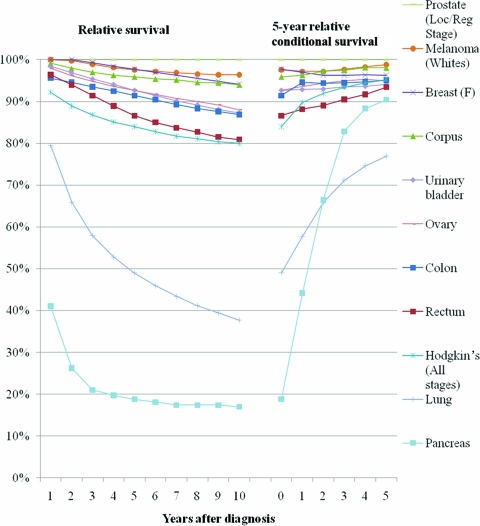
The relative survival and 5-year relative conditional survival probabilities are presented graphically in Figures 1 (local stage), 2(regional stage), 3(distant stage), and 4 (unknown stage). Site- and stage-specific trends in relative conditional survival probabilities tended to significantly increase and, for some cancer sites, at a decreasing rate. However, trends were insignificant for early-stage prostate cancer and for locally staged colon cancer. For locally staged breast cancer, the trend decreased. After adjusting for age, sex, race, and year of diagnosis using regression modeling, positive trends in locally staged urinary bladder cancer and ovarian cancer became insignificant, the trend in locally staged colon cancer became significantly positive, and the trend in regionally staged female breast cancer became significantly negative.
Figure 1.
Relative survival and 5-year relative survival probabilities conditional on years already survived after diagnosis: locally staged disease at diagnosis.
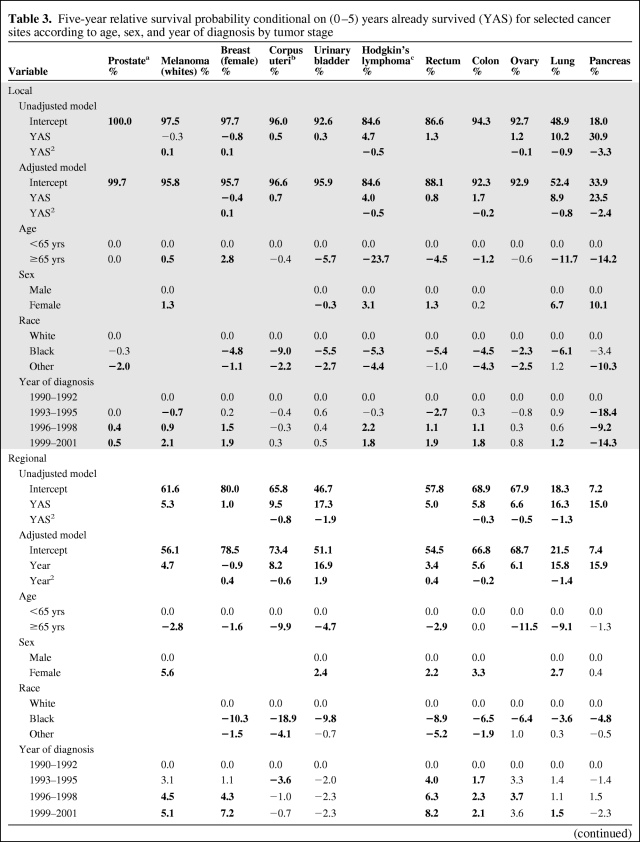
The 5-year relative conditional survival probabilities were modeled according to age, sex, race, year of diagnosis, and years survived after diagnosis (0–5), for each of the cancer sites and tumor stage classifications (Table 3. For models involving female breast cancer, Hodgkin's lymphoma, colon cancer, lung cancer, and pancreatic cancer, a significant quadratic term was added. The 5-year relative conditional survival probability tended to be significantly lower among patients diagnosed at older ages, among males, among nonwhites, and among those diagnosed during 1990–1995 compared with later years. For patients with locally staged disease at diagnosis, exceptions included a better relative conditional survival probability for older aged female breast cancer patients and for whites diagnosed with melanoma. For urinary bladder cancer, females had a slightly poorer relative conditional survival probability, and for pancreatic cancer the relative conditional survival probability was best in the years 1990–1992. For patients with distant staged disease, older age was positively associated with a better 5-year relative conditional survival probability only among prostate cancer patients; other races, compared with whites, had significantly better 5-year relative conditional survival probabilities among patients with cancers of the prostate, colon, ovaries, or lungs; and the 5-year relative conditional survival probability significantly decreased with later year of diagnosis for cases with prostate cancer or corpus uterine cancer.
Table 3.
Five-year relative survival probability conditional on (0–5) years already survived (YAS) for selected cancer sites according to age, sex, and year of diagnosis by tumor stage
Table 3.
(Continued)
aLocally and regionally staged disease are combined under local and only available from 1995 onward. Hence, the time period 1993–1995 represents just 1995 data.
bIncludes uterus, not otherwise specified.
cHistoric stage was not available for Hodgkin’s lymphoma.
Source: Surveillance, Epidemiology, and End Results Program, 1990–2001 diagnosed cases with follow-up through 2006 (http://www.seer.cancer.gov).
Estimates for each model were simultaneously calculated, adjusted for the other variables in the model.
Values in bold are statistically significant, p < .05.
Figure 2.
Relative survival and 5-year relative survival probabilities conditional on years already survived after diagnosis: regionally staged disease at diagnosis.
Figure 3.
Relative survival and 5-year relative survival probabilities conditional on years already survived after diagnosis: distant staged disease at diagnosis.
Figure 4.
Relative survival and 5-year relative survival probabilities conditional on years already survived after diagnosis: unstaged disease at diagnosis.
Discussion
This study presents 5-year relative conditional survival probability estimates for 11 major cancer sites. The results may serve as a more comprehensive guide for both patients and physicians who are seeking to update a cancer prognosis. For all of the locally staged cancers considered except lung cancer, the 5-year relative survival probability conditional on already having survived 5 years exceeded 90%, thus nearing the survival of the general population. For all cancer sites combined, the 5-year relative survival probability conditional on already having survived 5 years averaged about 85% for regionally staged disease, 68% for distant staged disease, and 87% for unknown staged disease. Therefore, the 5-year relative survival probability estimates conditional on having already survived 5 years did not reach the survival of the general population, although they came closest among those diagnosed with locally staged disease. The only exception is among prostate cancer patients, for whom the 5-year relative survival probability for locally/regionally staged cases remains at about 100% through 10 years of follow-up.
The results also show that the 5-year relative conditional survival probability tended to be significantly lower among patients diagnosed at older ages, among males, among nonwhites, and among those diagnosed during 1990–1995 compared with later years. After adjusting for these factors, the 5-year relative conditional survival probability continued to significantly improve for patients who had already survived 1–5 years after diagnosis for most cancer sites, particularly in the later stage categories. In addition, the significant decrease persisted in the adjusted model in the 5-year relative conditional survival trend for locally staged breast cancer patients.
In a recent study, Janssen-Heijnen and colleagues presented conditional survival probabilities for patients with multiple cancer sites, based on data from the Netherlands [15]. For colon, rectal, and lung cancer, our results are nearly identical to theirs. However, the relative conditional survival rates are approximately 10% higher using SEER data for female breast cancer, prostate cancer, and melanoma in males. The difference in survival estimates for melanoma may be because our study looked at melanomas among whites only, but for the other cancer sites, possible explanations for the discrepancy may be better access to treatment for these cancers or just a difference that arises as a result of time trends (the Janssen-Heijnen et al. [15] data set runs for 1980–2004, whereas our data set runs for 1995–2004, followed through to 2006 for vital status and cause of death).
Janssen-Heijnen and colleagues also provide 5-year relative survival probabilities conditional on having survived 1, 2, and 3 years after diagnosis for female breast cancer and colon cancer patients. The current results for colon cancer are similar for early-stage but lower for later-stage disease. This was also observed for female breast cancer. Because of the differing staging criteria and years of diagnosis used in the studies, it is difficult to determine the cause of this discrepancy. It is possible that shorter wait times for oncological specialists in the U.S. allow for greater detection of cancer among patients who were not aware of their cancer until they were terminally ill, which in turn decreases the survival estimates of the later-stage population.
Another study reported conditional relative survival rates for eight cancer sites (colon, rectum, corpus uteri and uterus, female breast, lung, ovary, prostate, and urinary bladder) by sex based on SEER data for 1990–1999 [18]. Our updated results show similar patterns for each of the cancer sites, with the exception that the 5-year relative survival and conditional survival estimates for ovarian cancer patients are lower in the current study. This difference may be partly because the earlier study involved nine SEER areas in their survival analysis, whereas the current study includes 17 SEER areas.
A finding of note is that the 5-year relative survival estimates decrease with older age for each of the cancer sites, except for distant staged prostate cancer and locally staged female breast cancer. A recent study by Lin et al. [25] corroborates our findings regarding prostate cancer in young men, demonstrating that men diagnosed with advanced prostate cancer at age of 35–44 have a poor prognosis when compared with older men. Studies also confirm the finding that young women tend to have poorer survival with breast cancer. In regard to both prostate and breast cancer, the exact mechanisms leading to the poorer prognoses are unknown, although a likely explanation is the lack of competing morbidities among younger patients [26–29].
The results advocate the potential benefits of early screening and diagnosis of cancer. For example, the 5-year relative survival rates at diagnosis for the locally, regionally, and distantly staged cases of colon cancer are 91%, 69%, and 10%, respectively. Even after surviving for 5 years from the initial time of diagnosis, a substantial gap still exists in the 5-year relative survival probability for patients with colon cancer (95%, 91%, and 79%, respectively). Possible explanations for the continued gap include recurrence of cancer at the same site and the introduction of cancer into new sites as later-stage cancer metastasizes. Note that even the 5-year relative survival probability conditional on having already survived 5 years among locally staged patients remained 5% below the survival probability for the general population.
Relative conditional survival improves the most over time for those with the poorest initial prognosis. This may be because as time passes, there is a natural selection effect on the initial population. In other words, the patients at greatest risk die early on, and as time progresses, we are left with a healthier population of patients.
In general, relative conditional survival can be useful at both the individual patient level and at the aggregate level. For example, an individual patient being seen in follow-up a few years after diagnosis can receive an updated prognosis. This conditional survival information could also be used to assist clinicians in determining how prognosis related to each cancer site changes over time. Conditional survival could also be used when designing clinical trials, to determine the appropriate length of follow-up needed before drawing conclusions. In evaluating new treatments, these data assist researchers and clinicians in establishing benchmarks of the natural trends of cancer survivorship against which the efficacy of new treatments can be measured.
Conclusion
Cancer surveillance statistics provide a foundation for measuring a patient’s likely prognosis. Long-term SEER data provide the means to calculate both long-term survival estimates and also long-term relative conditional survival estimates. Relative survival provides a net survival measure representing cancer survival in the absence of other causes of death. Conditional survival provides further useful prognostic information, tailored to the time a person has already survived with the cancer. The 5-year relative conditional survival probability tended to improve with each year already survived, even after adjusting for age at diagnosis, sex, race, and year of diagnosis. An exception involves a significant decrease in the 5-year relative conditional survival trend for locally staged female breast cancer patients. Improvement in the relative survival probability conditional on more years already survived was greatest for the more lethal cancers and for cases with a more advanced stage at diagnosis. The 5-year relative survival probability estimates conditional on having already survived 5 years did not reach the survival rate of the general population, although they came closest among those diagnosed with locally staged disease, then regionally staged, and then distantly staged disease. An exception involves prostate cancer, for which the 5-year relative survival probability for locally/regionally staged cases remained at 100%. The 5-year relative conditional survival probability was generally significantly lower among patients diagnosed at older ages, among males, among nonwhites, and among those diagnosed during 1990–1995 compared with later years. These results may serve as a more comprehensive guide for both patients and physicians who are seeking to update a cancer prognosis.
Author Contributions
Conception/Design: Ray M. Merrill
Financial support: Ray M. Merrill
Collection and/or assembly of data: Ray M. Merrill
Data analysis and interpretation: Ray M. Merrill, Bradley D. Hunter
Manuscript writing: Ray M. Merrill, Bradley D. Hunter
Final approval of manuscript: Ray M. Merrill, Bradley D. Hunter
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Donovan RJ, Carter OB, Byrne MJ. People’s perceptions of cancer survivability: Implications for oncologists. Lancet Oncol. 2006;7:668–675. doi: 10.1016/S1470-2045(06)70794-X. [DOI] [PubMed] [Google Scholar]
- 3.Yang YH, Liu SH, Ho PS, et al. Conditional survival rates of buccal and tongue cancer patients: How far does the benefit go? Oral Oncol. 2009;45:177–183. doi: 10.1016/j.oraloncology.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Wang SJ, Emery R, Fuller CD, et al. Conditional survival in gastric cancer: A SEER database analysis. Gastric Cancer. 2007;10:153–158. doi: 10.1007/s10120-007-0424-9. [DOI] [PubMed] [Google Scholar]
- 5.Choi M, Fuller CD, Thomas CR, Jr, et al. Conditional survival in ovarian cancer: Results from the SEER dataset 1988–2001. Gynecol Oncol. 2008;109:203–209. doi: 10.1016/j.ygyno.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 6.Fuller CD, Wang SJ, Thomas CR, Jr, et al. Conditional survival in head and neck squamous cell carcinoma: Results from the SEER dataset 1973–1998. Cancer. 2007;109:1331–1343. doi: 10.1002/cncr.22563. [DOI] [PubMed] [Google Scholar]
- 7.Moller MB, Pedersen NT, Christensen BE. Conditional survival of patients with diffuse large B-cell lymphoma. Cancer. 2006;106:2165–2170. doi: 10.1002/cncr.21877. [DOI] [PubMed] [Google Scholar]
- 8.Skuladottir H, Olsen JH. Conditional survival of patients with the four major histologic subgroups of lung cancer in Denmark. J Clin Oncol. 2003;21:3035–3040. doi: 10.1200/JCO.2003.04.521. [DOI] [PubMed] [Google Scholar]
- 9.Kato I, Severson RK, Schwartz AG. Conditional median survival of patients with advanced carcinoma: Surveillance, Epidemiology, and End Results data. Cancer. 2001;92:2211–2219. doi: 10.1002/1097-0142(20011015)92:8<2211::aid-cncr1565>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 10.Davis FG, McCarthy BJ, Freels S, et al. The conditional probability of survival of patients with primary malignant brain tumors: Surveillance, Epidemiology, and End Results (SEER) data. Cancer. 1999;85:485–491. [PubMed] [Google Scholar]
- 11.Wang SJ, Fuller CD, Emery R, et al. Conditional survival in rectal cancer: A SEER database analysis. Gastrointest Cancer Res. 2007;1:84–89. [PMC free article] [PubMed] [Google Scholar]
- 12.Alanee S, Shukla A. Paediatric testicular cancer: An updated review of incidence and conditional survival from the Surveillance, Epidemiology and End Results database. BJU Int. 2009;104:1280–1283. doi: 10.1111/j.1464-410X.2009.08524.x. [DOI] [PubMed] [Google Scholar]
- 13.Merrill RM, Henson DE, Barnes M. Conditional survival among patients with carcinoma of the lung. Chest. 1999;116:697–703. doi: 10.1378/chest.116.3.697. [DOI] [PubMed] [Google Scholar]
- 14.Merrill RM, Henson DE, Ries LA. Conditional survival estimates in 34,963 patients with invasive carcinoma of the colon. Dis Colon Rectum. 1998;41:1097–1106. doi: 10.1007/BF02239430. [DOI] [PubMed] [Google Scholar]
- 15.Janssen-Heijnen ML, Houterman S, Lemmens VE, et al. Prognosis for long-term survivors of cancer. Ann Oncol. 2007;18:1408–1413. doi: 10.1093/annonc/mdm127. [DOI] [PubMed] [Google Scholar]
- 16.Hankey BF, Ries LA, Edwards BK. The Surveillance, Epidemiology and End Results Program: A national resource. Cancer Epidemiol Biomarkers Prev. 1999;8:1117–1121. [PubMed] [Google Scholar]
- 17.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. [accessed August 2, 2009]. Available at http://www.seer.cancer.gov.
- 18.Gloeckler Ries LA, Reichman ME, Lewis DR, et al. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) Program. The Oncologist. 2003;8:541–552. doi: 10.1634/theoncologist.8-6-541. [DOI] [PubMed] [Google Scholar]
- 19.Percy C, Van Holten V, Muir C, editors. Second Edition. Geneva: World Health Organization; 1990. International Classification of Disease for Oncology; pp. 1–144. [Google Scholar]
- 20.National Cancer Institute, U.S. National Institutes of Health. Surveillance Epidemiology and End Results. SEER*Stat software, version 6.6.1. Available at http://seer.cancer.gov/seerstat.
- 21.Ries LAG, Kosary CL, Lyles BA. Cancer patient survival: Why use the relative survival rate. The Abstract. 1995;21:28–30. [Google Scholar]
- 22.Henson DE, Ries LA. The relative survival rate. Cancer. 1995;76:1687–1688. doi: 10.1002/1097-0142(19951115)76:10<1687::aid-cncr2820761002>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.Percy CL, Miller BA, Ries LA. Effect of changes in cancer classification and the accuracy of cancer death certificates on trends in cancer mortality. In: Davis DL, Hoel D, editors. Trends in Cancer Mortality in Industrial Countries. New York: New York Academy of Sciences; 1990. pp. 87–99. [DOI] [PubMed] [Google Scholar]
- 24.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: A statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 25.Lin DW, Porter M, Montgomery B. Treatment and survival outcomes in young men diagnosed with prostate cancer: A population-based cohort study. Cancer. 2009;115:2863–2871. doi: 10.1002/cncr.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickman PW, Sloggett A, Hills M, et al. Regression models for relative survival. Stat Med. 2004;23:51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 27.de la Rochefordiere A, Asselain B, Campana F, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993;341:1039–1043. doi: 10.1016/0140-6736(93)92407-k. [DOI] [PubMed] [Google Scholar]
- 28.Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000;355:1869–1874. doi: 10.1016/s0140-6736(00)02292-3. [DOI] [PubMed] [Google Scholar]
- 29.Fredholm H, Eaker S, Frisell J, et al. Breast cancer in young women: Poor survival despite intensive treatment. PLoS One. 2009;4:e7695. doi: 10.1371/journal.pone.0007695. [DOI] [PMC free article] [PubMed] [Google Scholar]